Abstract
Roses (Rosa spp.) are widely used as ornamental plants and cut flowers and in perfumes and cosmetics; therefore, they have extremely high ornamental and economic value. Improving ornamental qualities (color, aroma, flower shape, plant architecture, petal senescence), agronomic traits (disease resistance, stress tolerance), and other traits would add value to cultivated roses. However, the lack of an efficient genetic transformation system limits functional genetic studies of roses. Therefore, we aimed to develop a simple, high-throughput Agrobacterium rhizogenes-mediated hairy root transformation system to analyze gene function in rose. We explored the factors affecting the induction and transformation of hairy root cultures in rose by screening different types of explants, Rosa genotypes, Agrobacterium strains, media, and infection and co-cultivation times for genetic transformation under aseptic conditions. We established an A. rhizogenes-mediated hairy root transformation system in rose using A. rhizogenes strains MSU440 and Ar Qual, successfully obtained transgenic hairy roots, and conducted preliminary experiments to examine their regeneration by employing the ultraviolet light (UV)-visible reporter eYGFPuv as a marker. Finally, we established a simple hairy root transformation process under non-aseptic conditions. The rapid and efficient transformation system developed here will enable efforts to investigate gene function in roses and engineer improved rose varieties. Refining the transformation protocol to work under aseptic and non-aseptic conditions will allow researchers to use this system efficiently and broadly.
1. Introduction
The genus Rosa belongs to the Rosaceae family [1,2,3] and comprises approximately 200 species, among which only ~20 species have contributed to the genetic makeup of our present cultivars, namely, Rosa × hybrida [4,5,6,7]. Roses are highly prized as ornamental and cut flowers. Furthermore, roses are in high demand for the perfume and cosmetic industries. Contemporary rose cultivars display a vast array of characteristics, including diverse flower shapes, colors, and fragrances. Improving key ornamental and agronomic traits of roses, such as color, fragrance, plant architecture (number of flowers, plant shape), and disease resistance, could enhance their value. For example, suppressing petal senescence could enhance the shelf life of cut roses; in contrast, inducing complete petal senescence (termed “cleaning”) produces garden varieties that do not require deadheading [8,9]. In addition, roses are susceptible to a number of diseases, such as black spot and botrytis [10,11]; identifying resistance genes from across Rosaceae could improve efforts to engineer resistant varieties.
Improving agronomic and ornamental traits in roses by transgenic technology or gene editing requires an understanding of gene function and the effects of expressing genes from other species [12,13,14]. Genetic transformation is an important tool for analyzing gene function, but many rose cultivars are recalcitrant to regeneration and hence to genetic transformation owing to their long growth cycle and high heterozygosity [15,16,17]. Indeed, a limited number of successful rose transformation protocols using somatic embryos have been reported [18,19,20]. In addition, virus-induced gene silencing (VIGS) technology and transient transformation techniques are commonly used to verify gene function and in biochemical experiments [21,22,23,24]. However, the recalcitrance of rose hampers the high-throughput characterization of gene function, emphasizing the need for a rapid, simple, highly efficient transformation protocol.
Agrobacterium rhizogenes-mediated hairy root transformation is useful for species recalcitrant to transformation by Agrobacterium tumefaciens [25,26]. Compared to A. tumefaciens-mediated transformation, A. rhizogenes-mediated hairy root transformation is more rapid and has a higher transformation frequency, because transgenic hairy roots grow rapidly without requiring a complex culture process to regenerate plantlets [27,28]. Therefore, A. rhizogenes-mediated transformation constitutes a simple, rapid, efficient method for the production of metabolites and the study of gene function in plants [29]. To date, successful A. rhizogenes-mediated transformation has been reported for several crops, such as grape (Vitis vinifera), peach (Amygdalus persica), litchi (Litchi chinensis), cucumber (Cucumis sativus), sweet potato (Ipomoea batatas), and soybean (Glycine max) [30,31,32,33,34]. However, there are few reports on the induction of transgenic hairy roots of roses mediated by A. rhizogenes [35,36]. In addition, genetic transformation of roses often relies on tissue culture techniques, and there is currently no direct method for inducing transgenic hairy roots in rose through in vitro infection under non-aseptic conditions.
Here, we screened rose genotypes for efficient genetic transformation under aseptic and non-aseptic conditions. We also developed an optimized transformation procedure for rose using the UV-visible reporter green fluorescent protein (eYGFPuv) as a marker [37]. We established an A. rhizogenes-mediated hairy root transformation system for rose, successfully obtained transgenic hairy roots, and conducted preliminary analysis of their regeneration, providing a foundation for the subsequent transformation of genes (from roses or other sources) and the investigation of their effects in roses. Finally, we demonstrated the potential of the hairy root transgenic system under non-aseptic conditions, a rapid process with a high transformation frequency.
2. Methods
2.1. Plant Materials
Seedlings of Rosa chinensis “Sichun” and “Old Blush”, Rosa rugosa “Purple branch”, Rosa multiflora “Grevillei”, and Rosa hybrida “Suehime”, “Hiogi”, “Pink Moscow”, and “Samantha” were used as starting materials for propagation under aseptic conditions, as we previously reported [23,24]. The materials were repeatedly subcultured every 3–4 weeks on proliferation medium comprising MS salts (HB8469-8, HaiBo, Qingdao, China) + 1.5 mg/L 6-Benzylaminopurine (6-BA, A430059, Sangon Biotech, Shanghai, China) + 0.1 mg/L 1-naphthaleneacetic acid (NAA, A600729, Sangon Biotech, Shanghai, China) + 30 g/L sucrose + 6.5 g/L agar, pH 5.75. They were maintained in a growing room with controlled conditions (22 °C and 250 μmol·m−2·s−1) under LD (16:8 h, light:dark).
Rosa multiflora var. Spineless, Rosa luciae Franch. & Roch. ex Crép., Rosa chinensis “Yue Yue Hong”, Rosa persetosa Rolfe, Rosa pseudobanksiae T. T. Yu & T. C. Ku, Rosa multiflora var. multiflora, R. rugosa “Plena”, R. hybrida “Pink mooscoe”, R. hybrida “Samantha”, and R. chinensis “Old Blush” were used to test the regenerative ability for different Rosa cultivars to regenerate hairy roots in vivo. Then, the two candidate cultivars (“Pink mooscoe” and “Samantha”) were used for further research. In vitro-grown seedlings of R. hybrida “Pink mooscoe”, R. hybrida “Samantha”, and R. chinensis “Old Blush” were used as candidate materials for transformation via cutting under non-aseptic conditions. These plants were grown in a plant incubator with controlled conditions (25 °C, 40% relative humidity and 250 μmol·m−2·s−1).
2.2. Agrobacterium Strains and Vectors
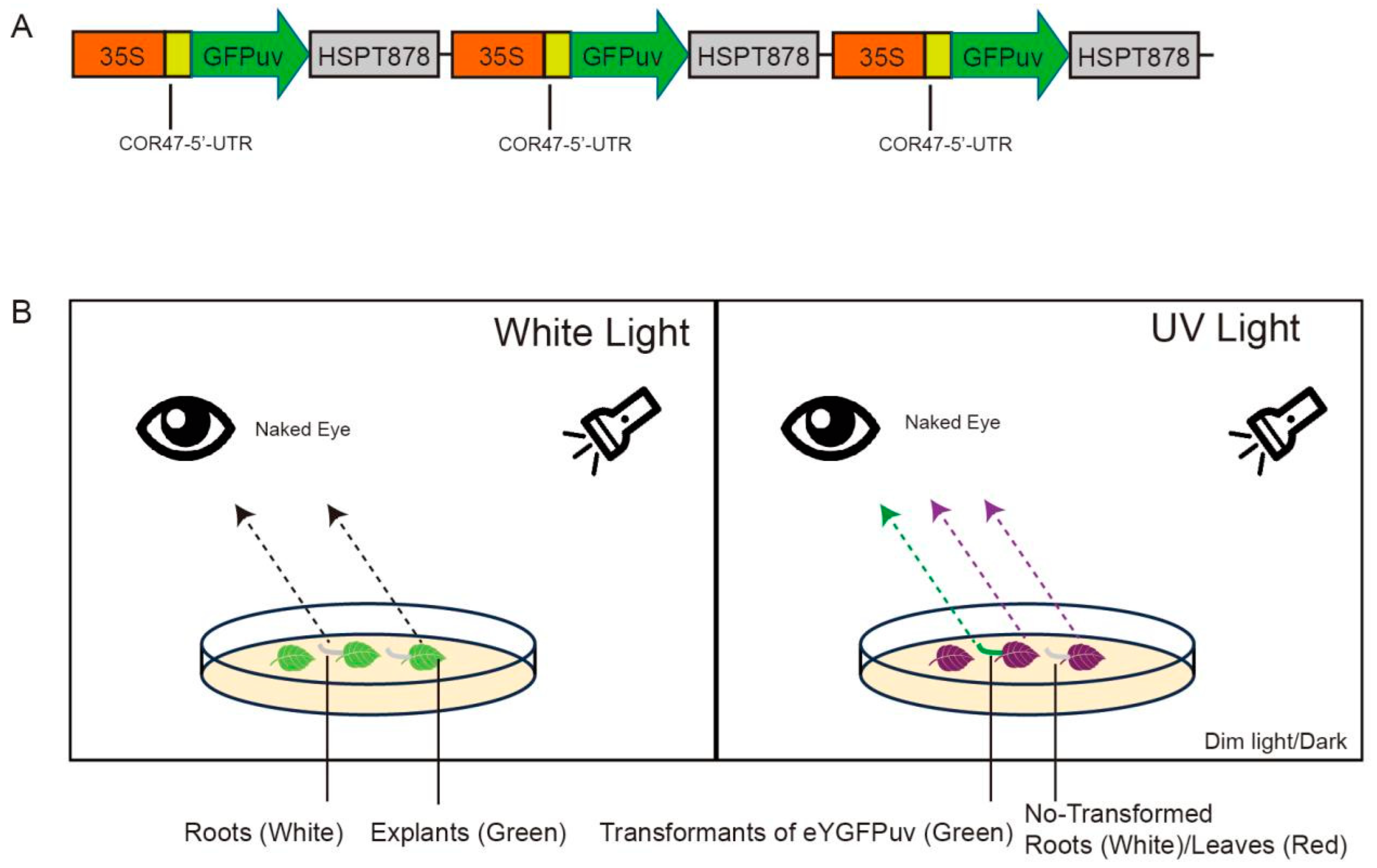
A. rhizogenes strains MSU440 (AC1070, WEIDI), Ar Qual (AC1060, WEIDI), and Ar1193 (AC1090, WEIDI) were used as candidate strains to induce hairy root. The UV-visible reporter green fluorescent protein (eYGFPuv) was chosen as a marker (Figure 1 and Figure S1) [35]. Vectors containing GFPuv were introduced into the different A. rhizogenes strains by the freeze–thaw method [38].
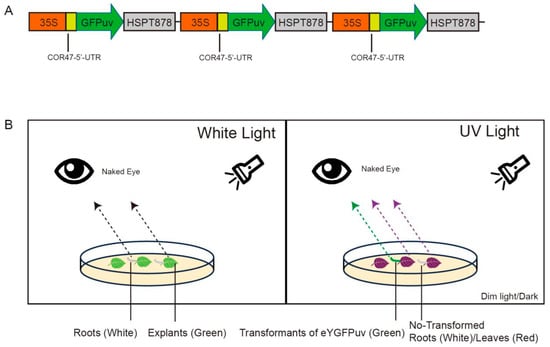
Figure 1.
Visualization of eYGFPuv in leaf and root tissue during transient expression. (A) Illustration of the plasmid of 3× eYGFPuv; (B) diagram showing how eYGFPuv-expressing samples can be visualized using UV light. Under 365 nm UV light, eYGFPuv-expressing hairy roots exhibit green fluorescence, whereas non-transformed leaves (explants) exhibit red autofluorescence.
2.3. A. rhizogenes-Mediated Hairy Root Transformation
A. rhizogenes strains MSU440, Ar Qual, and Ar1193 harboring the eYGFPuv plasmid were cultured in 800 μL YEB medium (5 g L−1 Lab-Lemco Power (LP0029, OXOID), 1 g L−1 Yeast Extract Power (LP0021, OXOID), 5 g L−1 tryptone (LP0042, OXOID), 5 g L−1 sucrose, 0.5 g L−1 Mg2SO4) containing 50 mg L−1 kanamycin sulfate (A506636, Sangon Biotech, Shanghai, China). A large volume (50–200 mL) of medium inoculated with a 1:50 dilution of overnight culture was incubated to logarithmic phase (OD600 = 0.5) on a rotary shaker at 200 rpm. The Agrobacterium solutions were centrifuged at 5000 g for 10 min and the were pellets resuspended at different concentrations (OD600 = 0.4, 0.8, 1.2, 1.6) in liquid medium containing half-strength MS salts (1/2 MS) containing different concentrations of acetosyringone (AS, A601111, Sangon Biotech, Shanghai, China). The resulting bacterial suspensions were used for transformation.
For regeneration under aseptic conditions, different explants from test seedlings were infected two different ways: “dip”, in which the seedlings were directly dipped into the Agrobacterium culture, and “immersion”, in which the seedlings were submerged into Agrobacterium suspensions (OD600 = 0.8–1.0) and incubated for different amounts of time in a 200 mL wide-mouth bottle. The excess solution was removed by blotting on sterile paper. The infected explants were transferred onto solid medium without hormones (containing 50 mg L−1 Timentin) and co-cultivated in the dark for different durations. After co-cultivation, the infected rose explants were washed with sterile water and transferred onto media containing different MS salt concentrations, hormone treatments, and carbon sources (3% glucose or sucrose, containing 500 mg L−1 Timentin)). The explants were maintained at 25 °C in the dark for hairy root regeneration.
For regeneration under non-aseptic conditions, the leaves, hypocotyls, or seedlings of R. multiflora were directly dipped into Agrobacterium cultures prior to cutting. A. rhizogenes strain MSU440 harboring the eYGFPuv plasmid was cultured in YEB medium to a given concentration (OD600 = 0.8~1.0). The stems were submerged in bacterial solution for different lengths of time prior to cutting. Aseptically grown seedlings grown in tissue culture were infected using three different methods: “dip”, in which the seedlings were directly dipped into the Agrobacterium culture; “immersion”, in which the seedlings were submerged several times in bacterial solution; and “vacuum”, in which the seedlings were submerged in bacterial solution and vacuum-infiltrated at 0.5 MPa for 2 min in a sealed vacuum suction container. Then, the explants or seedings were planted in substrate consisting of a mixture of roseite, perlite, and peat soil in a 4:1:1 ratio (v/v/v). Then, they were maintained in a plant incubator with controlled condition (25 °C, 40% relative humidity and 250 μmol·m−2·s−1).
Jasmonic acid (JA) treatment for in vitro-grown rose seedlings was performed every 8 h in the first 3 days after cutting by spraying the plants with 100 mg L−1 JA (392707, Sigma-Aldrich, City of Saint Louis, MO, USA) solution. Furthermore, the seedlings were irrigated with bacterial solution at different frequencies (0, 2, 5, 10 d) to improve hairy root regeneration.
Different treatments are shown in Table 1.

Table 1.
Different types of treatments of the induction of hairy roots under aseptic conditions.
2.4. Fluorescence Assay of Regenerated Hairy Roots
Fluorescence in transgenic hairy roots was detected using a handheld UV flashlight (UV302D, LIGHTFE, Shenzhen, China). Green fluorescence could be observed with the naked eye: The eYGFPuv-expressing roots showed green fluorescence, whereas non-transformed roots showed red autofluorescence (Figure 1).
2.5. Statistical Analysis
Each experiment was conducted at least three times independently. For one transformation experiment under aseptic conditions, we transformed over 10 Petri plates, and each Petri plate contained 8–10 explants. For one transformation experiment under non-aseptic conditions, we transformed over 50 explants at one time. The percentage data of rooting plants compared to total transgenic plants represent the rooting rate. The percentage data of fluorescence-positive plants compared to total transgenic plants represent the fluorescence-positive rate. Statistical significance was determined using the unpaired two-sample t-test.
3. Results
3.1. Effect of Explant Type and Infection Methods on the Induction of Hairy Roots
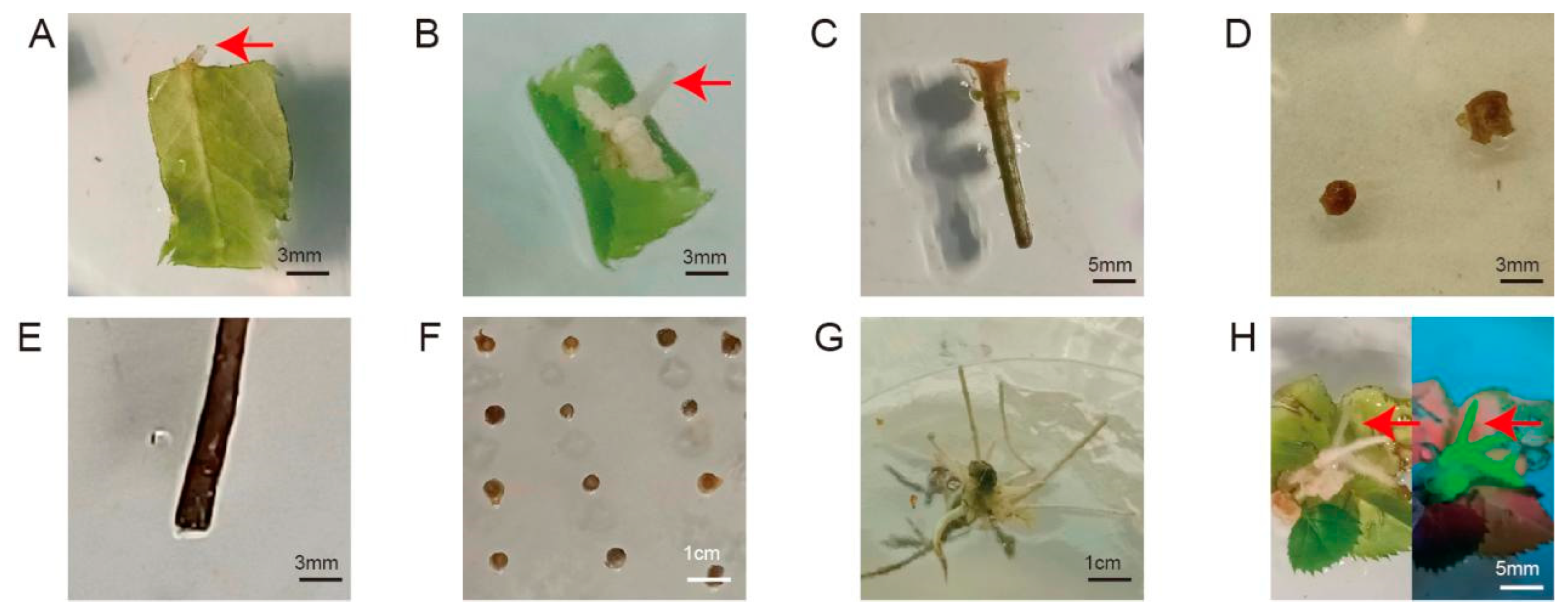
To construct the A. rhizogenes-mediated genetic transformation system, we screened eight types of explants and different infection methods. We then recorded the rooting rate and fluorescence-positive rate of each combination at 1 month after infection and recorded the growth status of the explants. As shown in Figure 2, after infection with A. rhizogenes, four types of explants successfully produced roots from the wounding sites: leaf discs, leaf discs with petiole segments, rootless seedlings, and compound leaves. However, four other explant types, including petioles, ovaries, stem segments, and transverse slices of stems, showed different degrees of browning and then died after infection and co-cultivation. Leaf discs and leaf discs with petioles took a long time to produce roots and had extremely low rooting rates, with no fluorescent signal; therefore, we excluded these explant types from further analysis. Rootless seedlings were also excluded from further analysis because the morphology of the regenerated roots was consistent with that of non-transformed roots, and no fluorescent signal was detected, indicating that the regenerated roots were adventitious roots.
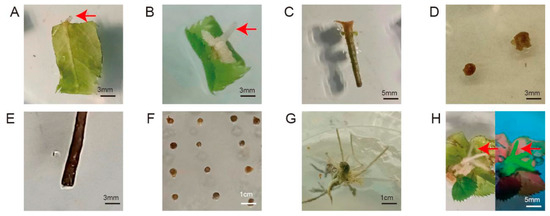
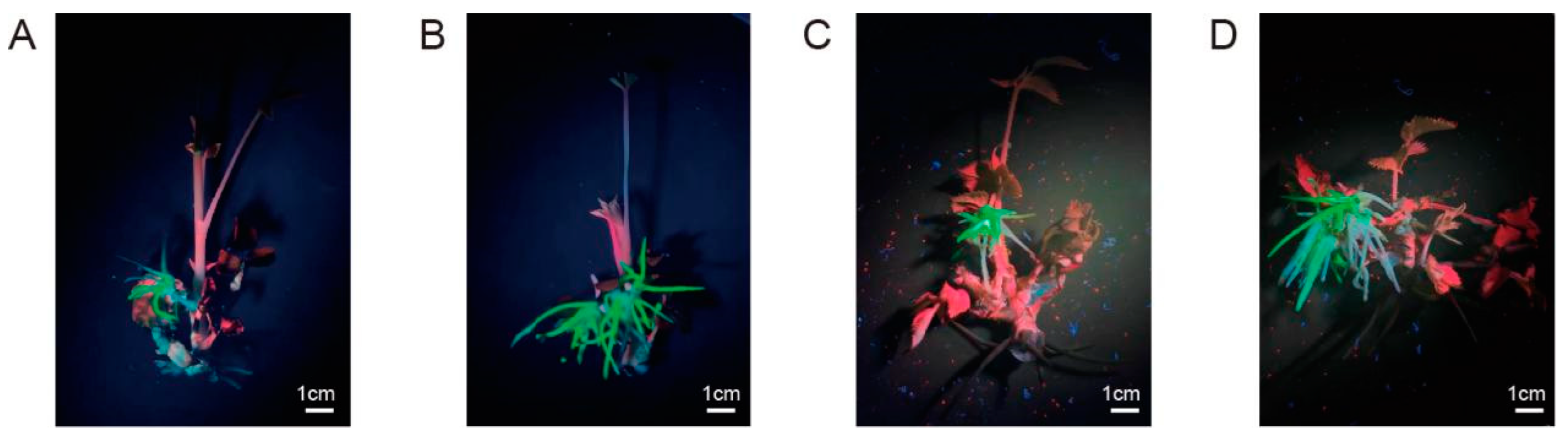
Figure 2.
Hairy root induction and transformation status of different explants. (A) leaf discs; (B) leaf discs with petiole segments; (C) petiole; (D) ovaries; (E) stem segment; (F) transverse slices of stems; (G) rootless seedlings; (H) compound leaves (left, under white light; right, under UV light). Red arrow, root.
Dipping the wounds of compound leaves into the bacterial solution was better than immersing the explant in bacterial solution owing to the lower browning and contamination rates (contamination: Agrobacterium overgrowth and inability to be suppressed) (Figure S2). Notably, the rooting rate of infected compound leaves was 20%, with a relatively low browning rate, and their morphology conformed to the characteristics of hairy roots. The overall rate of explants bearing transformed hairy roots was 15%, as determined by visualizing GFP fluorescence (Table S1). Therefore, we chose compound leaves as explants to construct a hairy root induction and transformation system.
3.2. Effect of Rose Variety and Agrobacterium rhizogenes Strain on the Induction of Hairy Roots
Considering the genotypic differences among rose varieties and the differences in fluorescence-positive rates of different Agrobacterium strains, we used compound leaves as explant materials to screen rose varieties and A. rhizogenes strains for transformation. As shown in Table 2, after infection, “Sichun”, “Suehime”, “Hiogi”, and “Purple branch” failed to form roots, and the incision sites showed obvious browning. In addition, the rooting rate of “Old Blush” was extremely low, and no transformed hairy roots were observed. Furthermore, “Grevillei” had a low rooting rate, and the hairy roots that formed were extremely short, making them unsuitable for use to establish a transformation system (Figure 3). Notably, “Pink mooscoe” and “Samantha” had high regeneration frequencies, with multiple roots at the same incision site. Among the roots regenerated in “Pink mooscoe” and “Samantha”, 31.9% and 33.1% exhibited fluorescence, respectively (Figure 3, Table 2). In addition, explants infected by A. rhizogenes strains MSU440 and Ar Qual exhibited high rooting and fluorescence-positive rates compared with explants infected by Ar 1193 (Table 2). Therefore “Pink mooscoe” and “Samantha” were chosen as suitable candidate varieties, and MSU440 and Ar Qual were chosen as the A. rhizogenes strains for further study.

Table 2.
Hairy root induction status of different Rosa cultivars and Agrobacterium strains.

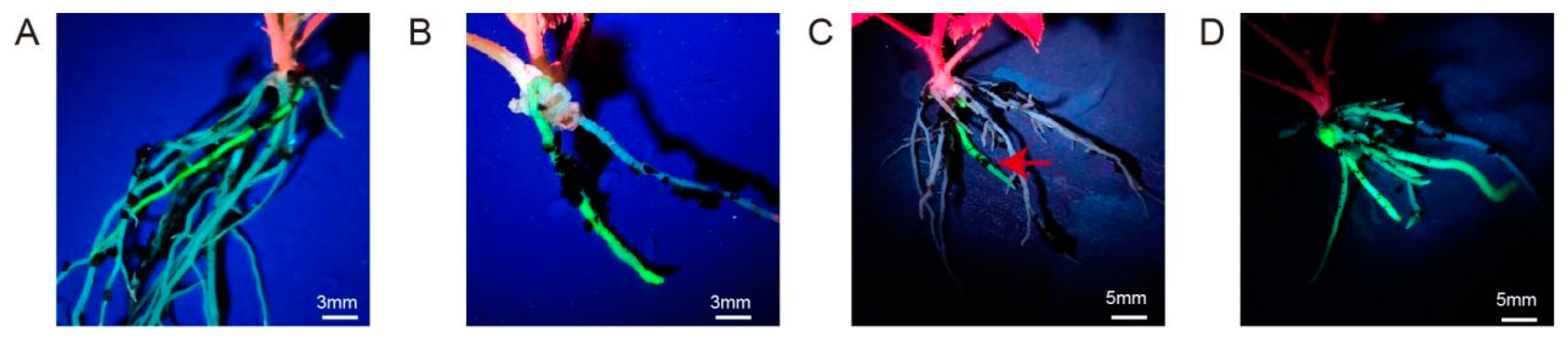
Figure 3.
Transgenic hairy root induction in different cultivars. (A) Transgenic hairy roots of “Grevillei”; (B) transgenic hairy roots of “Samantha”; (C) transgenic hairy roots of “Pink mooscoe”. All photographs were taken under UV light. Red arrow, root.
3.3. Effect of Different Types of Medium on the Rooting Rate of Transgenic Hairy Roots
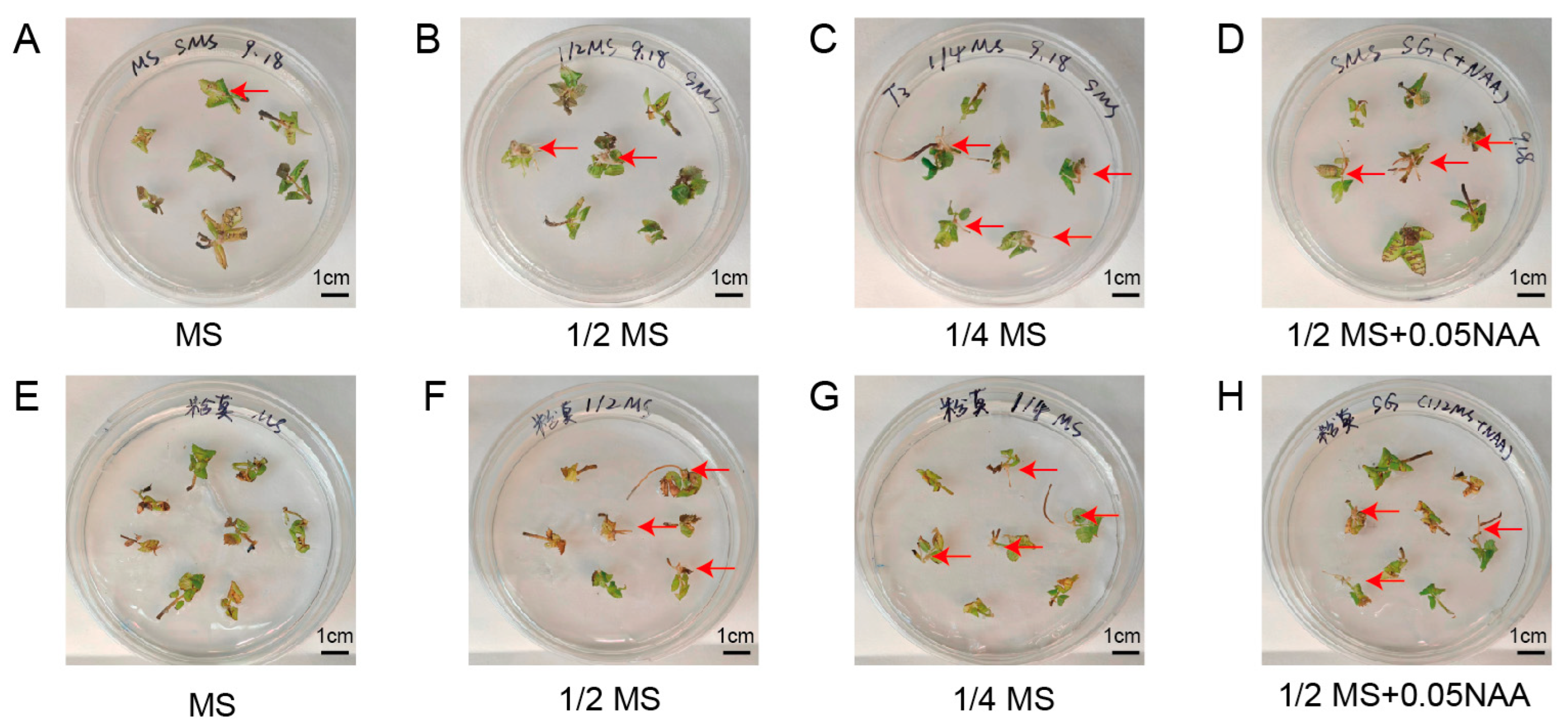
Because different culture media have different effects on regeneration, we tested four types of medium to identify the best culture medium for the induction of hairy roots in “Pink mooscoe” and “Samantha”. Among the media examined, the induction efficiency of “Pink mooscoe” and “Samantha” was significantly higher in 1/4 MS medium than in other treatments, reaching 30.8% and 49.4%, respectively (Figure 4, Table 3). The fluorescence-positive rates were also relatively high, reaching 20.9% and 24.8%, respectively. The addition of NAA was beneficial for inducing rooting in “Samantha”, increasing from 23.8% to 34.9%, but no significant effect of NAA on rooting was observed in “Pink mooscoe” (Table 3). In summary, we selected 1/4 MS medium for the induction and transformation of hairy roots of “Pink mooscoe” and “Samantha”.
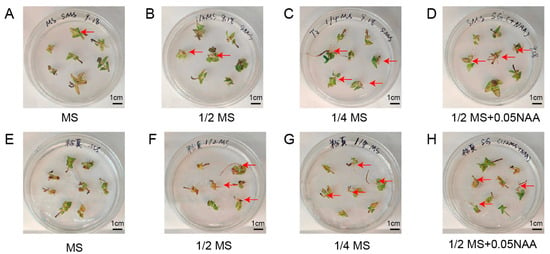
Figure 4.
Growth status of hairy roots in different types of medium. (A–D) Growth status of representative hairy roots of “Samantha” in four types of medium: MS, 1/2 MS, 1/4 MS, and 1/2 MS + 0.05 mg/L NAA, respectively; (E–H) Growth status of representative hairy roots of “Pink mooscoe” in four types of medium: MS, 1/2 MS, 1/4 MS, and 1/2 MS + 0.05 mg/L NAA, respectively.

Table 3.
Effects of different types of medium on the rooting rate of transgenic hairy roots.
3.4. Effect of Pre-Cultivation Time and Co-Cultivation Time on the Rooting Rate of Transgenic Hairy Roots
To identify suitable pre-cultivation and co-cultivation times for the induction of transgenic hairy roots, we analyzed the regeneration of hairy roots under different treatment times. After 2 days of pre-cultivation, the hairy root rooting rate of “Samantha” explants was significantly higher than after 0 or 1 day of pre-cultivation, reaching 46.9%; the rooting rates were lower without pre-cultivation and after 1 day of pre-cultivation, at 28.4% and 29.6%, respectively (Figure S3, Table S2). There were no significant differences in the fluorescence-positive rates of hairy roots among the three treatments. However, the rooting rate and fluorescence-positive rate of “Pink mooscoe” explants were significantly higher after 2 days of pre-cultivation than were those for explants without pre-cultivation. There was no significant difference between the results under pre-cultivation for 1 day vs. 2 days, with rooting rates of 30.9% and 31.3% and fluorescence-positive rates of 25.3% and 28.4%, respectively (Figure S3, Table S2). Therefore, we selected pre-cultivation for 2 days as the best treatment method.
To examine the effect of co-cultivation time on hairy root induction, we selected four different treatments in order to select the optimal co-cultivation time. As shown in Figure S4, the rooting rate and transformation efficiency of “Pink mooscoe” increased with increasing co-cultivation time. In addition, the growth of Agrobacterium was successfully inhibited by treatment with Timentin after 4 days of co-cultivation (Figure S4, Table S3). In contrast, “Samantha” showed the best induction and fluorescence-positive rates after 3 days of co-cultivation. After 4 days of co-cultivation, Timentin was unable to effectively inhibit Agrobacterium growth, resulting in death of the explant and a decrease in rooting rate (Figure S4, Table S3). Therefore, the optimal co-cultivation times for “Pink mooscoe” and “Samantha” were 4 days and 3 days, respectively.
3.5. Effect of Infection Times and Concentrations of Agrobacterium on the Rooting Rate of Transgenic Hairy Roots
The infection time and concentration of Agrobacterium are also important factors affecting the rooting rate. As shown in Table S4, the rooting rates were highest when the infection time was 30 min, with rates of 61.8% in “Pink mooscoe” and 94.1% in “Samantha”; both rates were significantly higher than for the other treatments. The fluorescence-positive rate of hairy roots also reached the highest values of 42.1% and 57.6%, respectively. In addition, the rooting rate and fluorescence-positive rate were highest when the OD value of the Agrobacterium culture was adjusted to 1.2 (Table S5).
3.6. Effect of AS and Carbon Source on the Rooting Rate of Transgenic Hairy Roots
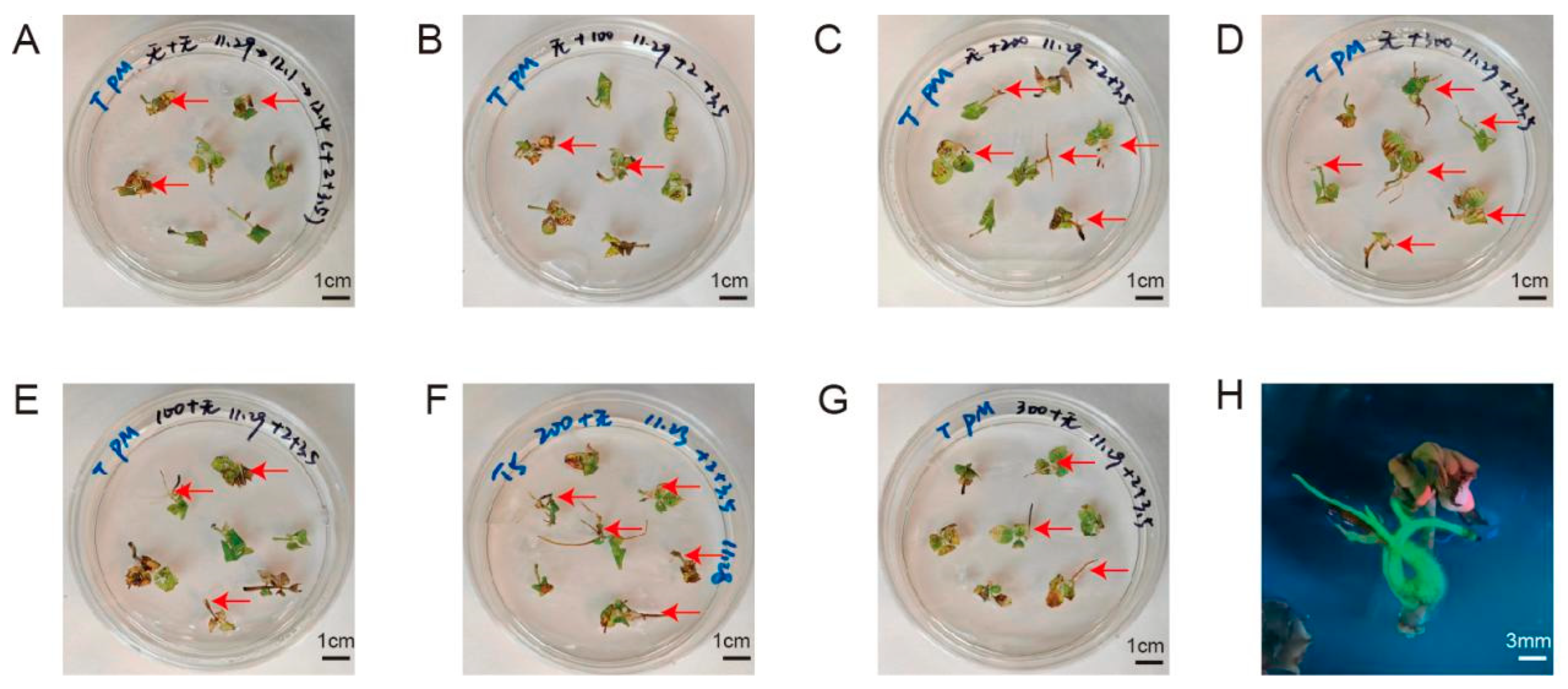
AS is a phenolic substance that can improve the transformation efficiency of plants. As shown in Figure 5 and Figure S5, AS had beneficial effects on the rooting rate and fluorescence-positive rate of both rose cultivars. Both cultivars had the highest rooting rates and fluorescence-positive rates when 200 μM AS were added to the 1/2 MS infiltration buffer. The optimal concentration of AS in solid medium was 200 μM and 300 μM for “Pink mooscoe” and “Samantha”, respectively (Table S6).
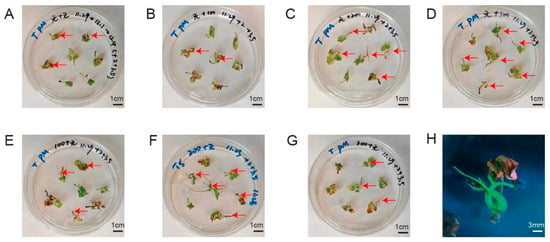
Figure 5.
Growth status of hairy roots from R. hybrida “Pink mooscoe” under different AS treatments. (A–G) Growth status of representative hairy roots of R. hybrida “Pink mooscoe” under seven combinations of treatment mode and AS concentration; the letters correspond to those in Table S8. Red arrow, root. (H) Transgenic hairy roots of R. hybrida “Pink mooscoe”, photographed under UV light.
To investigate the effects of different carbon sources on the rooting rate and fluorescence-positive rate of roses, we chose two carbon sources: sucrose and glucose. Glucose was a better carbon source, yielding a higher rooting rate and fluorescence-positive rate, as well as a higher average number of hairy roots (Figure 6, Table S7).
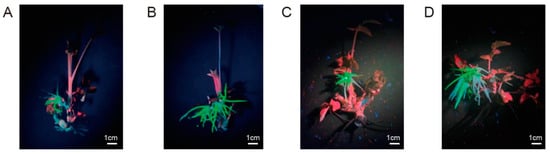
Figure 6.
Growth status of hairy roots provided with different carbon sources. (A,B) Transgenic hairy roots of “Pink mooscoe” provided with sucrose or glucose as the carbon source, respectively; (C,D) Transgenic hairy roots of “Samantha” provided with sucrose or glucose as the carbon source, respectively.
3.7. Effect of Solid Media on the Induction of Callus Tissue by Hairy Roots
Hairy roots can be induced to form adventitious buds in some plants, successfully achieving transgenic plant regeneration [39,40]. Therefore, we investigated the ability of the hairy roots to give rise to regenerated tissues. The regeneration of adventitious buds was quite difficult, but the use of the appropriate induction medium led to the production of stable, fluorescent callus tissue. Perhaps this tissue could ultimately be used for the indirect induction of adventitious buds (Figure 7 and Figure S6, Tables S8 and S9). Such transgenic callus tissues could also be used for biochemical experiments.
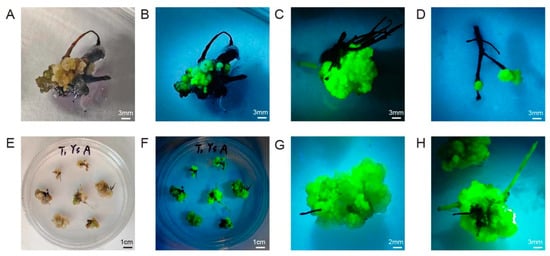
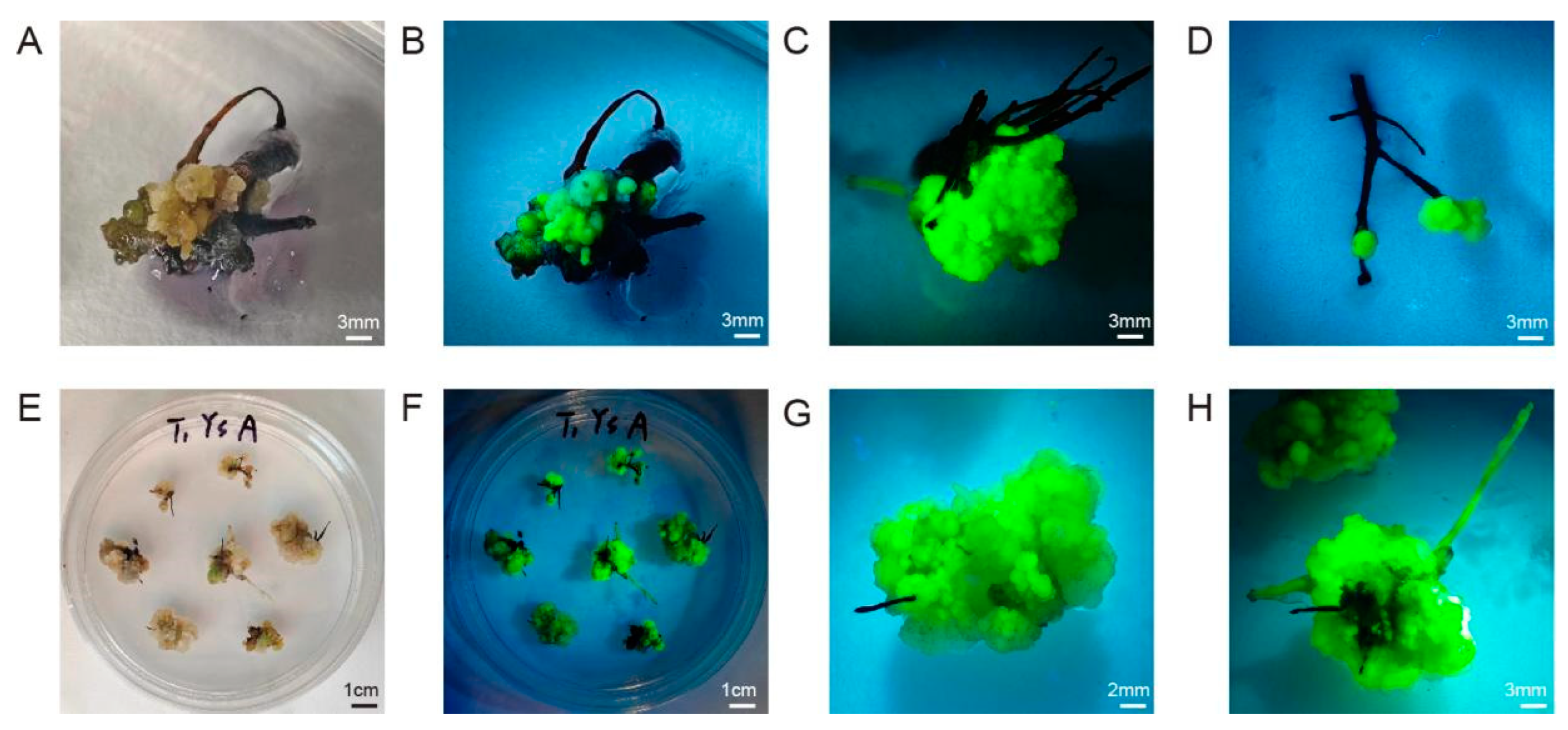
Figure 7.
Growth status of callus induced from hairy roots of R. hybrida “Samantha”. (A) Yellow granular callus; (B,C) transgenic callus induced from hairy roots; (D) swollen callus at the tips of roots; (E) continuously proliferating callus; (F,G) callus displaying strong green fluorescence; (H) new roots regrown from the callus.
3.8. Establishment of a Hairy Root Transformation System in Rose Under Non-Aseptic Conditions
The process of genetic transformation via tissue culture under aseptic conditions is relatively complex and cumbersome. Therefore, we also explored methods for inducing hairy root formation under non-aseptic conditions. The rooting rates of three methods (dip, immersion, vacuum) were more than 50%. However, the fluorescence-positive rates of three methods were almost 0% (only “Pink Mooscoe” and “Samantha” using the dip method were 3.6% and 6.1%) (Table S10). In addition, the fluorescence-positive rate of leaf cuttings and stems was 0% after infection and cutting (Figures S7 and S8). In addition, the hypocotyls of seedlings showed a 100% death rate after infection, making these explants also unsuitable for transformation (Figure S8, Table S11). However, in vitro-grown rose seedlings gave rise to roots with obvious fluorescent signals (Figure 8). Furthermore, JA treatment and irrigation with bacterial solution significantly improved the transformation efficiency (Figure 8 and Figure S9). The final optimized protocol of spraying the plants with 100 mg L−1 JA and irrigating with bacterial solution once every five days improved the transformation efficiency of R. hybrida “Samantha” to 82.8% (Table S12). Therefore, the use of “Samantha” seedlings as materials with JA treatment and irrigation with bacterial solution is the most simple and high-throughput technique under non-aseptic conditions, representing the best transformation system for hairy root regeneration in rose.
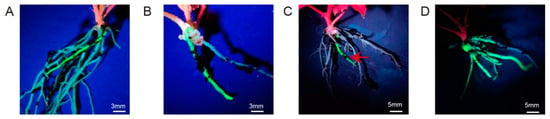
Figure 8.
Roots induced from in vitro-grown rose seedlings under non-aseptic conditions. (A) Transgenic hairy roots of R. hybrida “Pink mooscoe”; (B) transgenic hairy roots of R. hybrida “Samantha”; (C) transgenic hairy roots of “Samantha” without bacterial solution irrigation; (D) transgenic hairy roots of “Samantha” treated with bacterial solution irrigation.
4. Discussion
Owing to increasing living standards, ornamental plants, which serve as primary elements for landscaping and home gardening, have garnered significant attention because of their aesthetic and psychological benefits. Over the past two decades, the availability of extensive floral genomic resources, such as genome sequences, has facilitated advancements in genetic engineering and molecular breeding aimed at improving ornamental traits and plant resistance [17].
Current A. tumefaciens-mediated genetic transformation procedures for rose are not optimal for functional studies of rose genes because they are time-consuming, laborious, genotype-dependent, and hindered by low transformation frequencies [15,41]. Therefore, using A. rhizogenes to produce transformed hairy roots represents a novel approach to functional analysis of rose genes. The genetic background of roses is complex, and there are generally genotypic differences between varieties. The specific strain of A. rhizogenes employed is another critical factor for hairy root regeneration. Therefore, the root-promoting ability of A. rhizogenes depends on the combination of bacterial strains and plant genotypes [42,43]. Our results indicate that the combination of A. rhizogenes strain MSU440 or Ar Qual and “Pink mooscoe” or “Samantha” is excellent for hairy root regeneration in rose. In addition, there were significant differences in transformation efficiency for different explant types and infection methods.
The salt concentration in the culture medium can affect the absorption of nutrients by plants. When the salt concentration is low, the nitrogen concentration in the plant decreases to a level suitable for rooting [44]. In the current study, both “Pink mooscoe” and “Samantha” achieved the highest hairy root rooting rates on 1/4 MS medium. Factors including Agrobacterium concentration, infection time, and co-cultivation time affect the delivery of T-DNA and its integration into the plant genome [45,46]. In the present study, the co-transformation efficiencies of leaf and stem explants were highest after 2 or 3 days of co-cultivation. When the co-cultivation period is extended to 4 or 5 days, A. rhizogenes may overgrow, leading to cellular damage in the explant, consequently resulting in a low transformation efficiency [27]. Previous studies have demonstrated the requirement for the vir regulon of the Ti plasmid for the transfer of oncogenes from Agrobacterium tumefaciens to plant cells; the addition of AS to stimulate vir gene expression during the infection process is indispensable for this process [47,48]. In this study, the addition of AS promoted the rooting and transformation of two rose cultivars, especially at a relatively high concentration of 200–300 μmol/L.
A simple cut–dip–budding (CDB) delivery system was recently established that enables genetic modification of plants without the need for tissue culture. This method has enormous application potential [49,50,51,52,53]. Here, we established a regeneration system that does not rely on tissue culture by screening genotypes and identifying appropriate infection methods. JA treatment and irrigation with bacterial solution significantly increased the ability to regenerate hairy roots, which is similar to other recent findings [54,55]. However, it is still difficult to generate adventitious buds from hairy roots directly or indirectly in rose, which requires further exploration.
5. Conclusions
Here, we established a rapid, simple, highly efficient A. rhizogenes-mediated hairy root transformation system for rose under both aseptic and non-aseptic conditions. Our hairy root regeneration protocol does not require a tissue culture step, is high-throughput, and showed the highest regeneration efficiency among the techniques tested. This simple, rapid method could be used to analyze gene function in rose, with potential applications for improving ornamental properties, aroma production, disease resistance, and other traits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11010049/s1, Figure S1: The plasmid map of the vector used in hairy roots induction; Figure S2: Browning rates and contamination rates of compound leaves treated with different infection methods; Figure S3: Growth status of hairy roots pre-cultivated for different amounts of time; Figure S4: Growth status of hairy roots co-cultivated for different amounts of time; Figure S5: Growth status of hairy roots from R. hybrida “Samantha” under different acetosyringone (AS) treatments; Figure S6: Growth status of callus induced from hairy roots of R. hybrida “Pink mooscoe”; Figure S7: Rooted leaf cuttings of different rose cultivars; Figure S8: Rooting status of stem cuttings and the steps of hypocotyl infection in R. multiflora var. Spineless Thunb; Figure S9: Effect of jasmonic acid (JA) on the transformation efficiency of two rose cultivars under non-aseptic conditions; Table S1: Effects of different explants on rooting rate and fluorescence-positive rate; Table S2: Effects of different pre-cultivation time on the induction rate of transgenic hairy roots; Table S3: Effects of different co-cultivation time on the induction rate of transgenic hairy roots; Table S4: Effects of different infection time on the induction rate of transgenic hairy roots; Table S5: Effects of different concentration of Agrobacterium on the induction rate of transgenic hairy roots; Table S6: Effects of AS on the induction rate of transgenic hairy roots; Table S7: Effects of different carbon source on the induction rate of transgenic hairy roots; Table S8: The callus growth status induced by hairy roots of R. hybrida “Samantha” in different culture media; Table S9: The callus growth status induced by hairy roots of R. hybrida “Pink mooscoe” in different culture media; Table S10: Effect of different rose cultivars and infection methods on the rooting rate and transformation efficiency of tissue cultured seedlings; Table S11: Effect of different explants under non-aseptic condition on the rooting rate and transformation efficiency of tissue cultured seedlings; Table S12: Effect of frequency of bacterial solution irrigation on the transformation efficiency in two rose cultivars under non sterile conditions.
Author Contributions
Conceptualization, J.L. (Jun Lu) and C.W.; data curation, Y.G., G.Y., R.Z., J.S. and K.W.; funding acquisition, J.L. (Jun Lu), J.S., J.L. (Jinyi Liu) and C.W.; investigation, Y.H. and C.F.; methodology, J.L. (Jun Lu), J.L. (Jinyi Liu) and C.W.; writing—original draft, J.L. (Jun Lu) and Y.G.; writing—review and editing, J.L. (Jun Lu) and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the NSFC (32102418, 32302594, 32372744, and 32172615), the Natural Science Foundation of Jiangsu Province (BK20221008), the China Postdoctoral Science Foundation (2023T160325), the Jiangsu Funding Program for Excellent Postdoctoral Talent (2022ZB344), the “JBGS” Project of Seed Industry Revitalization in Jiangsu Province (JBGS (2021)020), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, as well as the high-performance computing platform of the Bioinformatics Center, Nanjing Agricultural University, and the open funds of the National Key Laboratory for Germplasm Innovation & Utilization of Horticultural Crops (Horti-KF-2023-17).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. We sincerely hope that readers can further develop a rapid and efficient system for adventitious shoot regeneration based on our research.
Acknowledgments
This work was supported by the high-performance computing platform of the Bioinformatics Center, Nanjing Agricultural University. We also thank Yuehua Ma (Central Laboratory of College of Horticulture, Nanjing Agricultural University) for assistance in using the stereo fluorescence microscope (M165FC, Leica, Germany).
Conflicts of Interest
Author Kun Wang was employed by the company Jinpu Landscape Architecture Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Bendahmane, M.; Dubois, A.; Raymond, O.; Le-Bris, M. Genetics and genomics of flower initiation and development in roses. J. Exp. Bot. 2013, 64, 847–857. [Google Scholar] [CrossRef]
- Kurokura, T.; Mimida, N.; Battey, N.H.; Hytonen, T. The regulation of seasonal flowering in the Rosaceae. J. Exp. Bot. 2013, 64, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.D.; Chen, H.; Byrne, D.; Liu, W.; Ranney, T.G. Cytogenetics, ploidy, and genome sizes of rose (Rosa spp.) cultivars and breeding lines. Ornam. Plant Res. 2023, 3, 15. [Google Scholar] [CrossRef]
- Reynders-Aloisi, S.; Bollereau, P. Characterisation of genetic diversity in genus Rosa by randomly amplified polymorphic DNA. Acta Hortic. 1996, 424, 253–259. [Google Scholar] [CrossRef]
- Gudin, S. Rose breeding technologies. Acta Hortic. 2001, 547, 23–26. [Google Scholar] [CrossRef]
- Vamosi, J.C.; Dickinson, T.A. Polyploidy and diversification: A phylogenetic investigation in Rosaceae. Int. J. Plant Sci. 2006, 167, 349–358. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, C.Y.; Liu, Y.; Gao, Y.R.; Lu, J.Y.; Aiwaili, P.; Fei, Z.J.; Jiang, C.Z.; Hong, B.; Ma, C.; et al. Auxin Regulates Sucrose Transport to Repress Petal Abscission in Rose (Rosa hybrida). Plant Cell 2020, 32, 3485–3499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Z.C.; Feng, M.; Chen, J.W.; Qin, M.Z.; Wang, W.R.; Bao, Y.; Xu, Q.; Ye, Y.; Ma, C.; et al. The circadian-controlled PIF8-BBX28 module regulates petal senescence in rose flowers by governing mitochondrial ROS homeostasis at night. Plant Cell 2021, 33, 2716–2735. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, V.M.; Zuzek, K.; Hokanson, S.C. Resistance of 12 rose genotypes to 14 isolates of Diplocarpon rosae Wolf (rose blackspot) collected from eastern North America. Plant Breed. 2007, 126, 83–88. [Google Scholar] [CrossRef]
- Ren, H.R.; Bai, M.J.; Sun, J.J.; Liu, J.Y.; Ren, M.; Dong, Y.W.; Wang, N.; Ning, G.G.; Wang, C.Q. RcMYB84 and RcMYB123 mediate jasmonate-induced defense responses against Botrytis cinerea in rose (Rosa chinensis). Plant J. 2020, 103, 1839–1849. [Google Scholar] [CrossRef]
- Tanaka, Y.; Katsumoto, Y.; Brugliera, F.; Mason, J. Genetic engineering in foriculture. Plant Cell Tissue Organ Cult. 2005, 80, 1–24. [Google Scholar] [CrossRef]
- Kishi-Kaboshi, M.; Aida, R.; Sasaki, K. Genome engineering in ornamental plants: Current status and future prospects. Plant Physiol. Biochem. 2018, 131, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.L.; Dohin, N.; Pornin, D.; Rolland, M. Overview and detectability of the genetic modifications in ornamental plants. Hortic. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Firoozabady, E.; Moy, Y.; Courtney-Gutterson, N.; Robinson, K. Regeneration of transgenic rose (Rosa hybrida) plants from embryogenic tissue. Nat. Biotechnol. 1994, 12, 609–613. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Y.; Jiang, H.; Bao, Y.; Ning, G.; Zhao, L.; Zhou, X.; Zhou, H.; Gao, J.; Ma, N. Agrobacterium tumefaciens-mediated transformation of modern rose (Rosa hybrida) using leaf-derived embryogenic callus. Hortic. Plant J. 2021, 7, 359–366. [Google Scholar] [CrossRef]
- Tang, J.; Ye, J.X.; Liu, P.X.; Wang, S.Q.; Chen, F.D.; Song, A.P. Ornamental plant gene editing: Past, present and future. Ornam. Plant Res. 2023, 3, 6. [Google Scholar] [CrossRef]
- Vergne, P.; Maene, M.; Gabant, G.; Chauvet, A.; Debener, T.; Bendahmane, M. Somatic embryogenesis and transformation of the diploid Rosa chinensis cv Old Blush. Plant Cell Tissue Organ Cult. 2010, 100, 73–81. [Google Scholar] [CrossRef]
- Chen, J.R.; Chen, Y.B.; Ziemianska, M.; Liu, R.; Deng, Z.N.; Niedzwiecka-Filipiak, I.; Li, Y.L.; Jiao, J.X.; Xiong, X.Y. Co-expression of MtDREB1C and RcXET enhances stress tolerance of transgenic China rose (Rosa chinensis Jacq.). J. Plant Growth Regul. 2016, 35, 586–599. [Google Scholar] [CrossRef]
- Wang, C.P.; Li, Y.; Wang, N.; Yu, Q.; Li, Y.H.; Gao, J.P.; Zhou, X.F.; Ma, N. An efficient CRISPR/Cas9 platform for targeted genome editing in rose (Rosa hybrida). J. Integr. Plant Biol. 2023, 65, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pei, H.X.; Zhang, S.; Chen, J.W.; Chen, W.; Yang, R.Y.; Meng, Y.L.; You, J.; Gao, J.P.; Ma, N. TRV-GFP: A modified Tobacco rattle virus vector for efficient and visualizable analysis of gene function. J. Exp. Bot. 2014, 65, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Shi, S.C.; Ma, N.; Cao, X.Q.; Zhang, H.; Qiu, X.Q.; Wang, Q.G.; Jian, H.Y.; Zhou, N.N.; Zhang, Z.; et al. Graft-accelerated virus-induced gene silencing facilitates functional genomics in rose flowers. J. Integr. Plant Biol. 2018, 60, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, J.J.; Jiang, A.Q.; Bai, M.J.; Fan, C.G.; Liu, J.Y.; Ning, G.G.; Wang, C.Q. Alternate expression of CONSTANS-LIKE 4 in short days and CONSTANS in long days facilitates day-neutral response in Rosa chinensis. J. Exp. Bot. 2020, 71, 4057–4068. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Liu, H.C.; Wang, W.N.; Fan, C.G.; Yuan, G.Z.; Zhou, R.; Lu, J.; Liu, J.Y.; Wang, C.Q. RcOST1L phosphorylates RcPIF4 for proteasomal degradation to promote flowering in rose. New Phytol. 2024, 243, 1387–1405. [Google Scholar] [CrossRef] [PubMed]
- Chilton, M.D.; Tepfer, D.A.; Petit, A.; David, C.; Casse-Delbart, F.; Tempé, J. Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature 1982, 295, 432–434. [Google Scholar] [CrossRef]
- Collier, R.; Fuchs, B.; Walter, N.; Lutke, W.K.; Taylor, C.G. Ex vitro composite plants: An inexpensive, rapid method for root biology. Plant J. 2005, 43, 449–457. [Google Scholar] [CrossRef]
- Liu, S.A.; Su, L.C.; Liu, S.A.; Zeng, X.J.; Zheng, D.M.; Hong, L.; Li, L. Agrobacterium rhizogenes-mediated transformation of Arachis hypogaea: An efficient tool for functional study of genes. Biotechnol. Biotechnol. Equip. 2016, 30, 869–878. [Google Scholar] [CrossRef]
- Gomes, C.; Dupas, A.; Pagano, A.; Grima-Pettenati, J.; Paiva, J.A.P. Hairy root transformation: A useful tool to explore gene function and expression in Salix spp. recalcitrant to transformation. Front. Plant Sci. 2019, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Veena, V.; Taylor, C.G. Agrobacterium rhizogenes: Recent developments and promising applications. In Vitro Cell Dev. Biol. Plant. 2007, 43, 383–403. [Google Scholar] [CrossRef]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in grapevine and suggests additional targets in the pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Lai, E.H.; Zhao, L.; Cai, Y.M.; Ogutu, C.; Cherono, S.; Han, Y.P.; Zheng, B.B. Development of a fast and efficient root transgenic system for functional genomics and genetic engineering in peach. Sci. Rep. 2020, 10, 2836. [Google Scholar] [CrossRef]
- Fan, Y.L.; Xu, F.L.; Zhou, H.Z.; Liu, X.X.; Yang, X.Y.; Weng, K.X.; Sun, X.L.; Lyu, S.H. A fast, simple, high efficient and one-step generation of composite cucumber plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. Plant Cell Tissue Organ Cult. 2020, 141, 207–216. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zhang, X.H.; Zhong, L.J.; Wang, X.Y.; Jin, L.S.; Lyu, S.H. One-step generation of composite soybean plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. BMC Plant Biol. 2020, 20, 208. [Google Scholar] [CrossRef]
- Yu, Y.C.; Xuan, Y.; Bian, X.F.; Zhang, L.; Pan, Z.Y.; Kou, M.; Cao, Q.H.; Tang, Z.H.; Li, Q.; Ma, D.F.; et al. Overexpression of phosphatidylserine synthase IbPSS1 affords cellular Na+ homeostasis and salt tolerance by activating plasma membrane Na+/H+ antiport activity in sweet potato roots. Hortic. Res. 2020, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Rüter, P.; Wehrenberg, F.; Bartels, J.; Debener, T.; Winkelmann, T. Optimization of Rhizobium rhizogenes-mediated transformation for a diversity set of rose genotypes. Acta Hortic. 2023, 1383, 225–233. [Google Scholar] [CrossRef]
- Rüter, P.; Debener, T.; Winkelmann, T. Unraveling the genetic basis of Rhizobium rhizogenes-mediated transformation and hairy root formation in rose using a genome-wide association study. Plant Cell Rep. 2024, 43, 300. [Google Scholar] [CrossRef]
- Yuan, G.L.; Lu, H.W.; Tang, D.; Hassan, M.M.; Li, Y.; Chen, J.G.; Tuskan, G.A.; Yang, X.H. Expanding the application of a UV-visible reporter for transient gene expression and stable transformation in plants. Hortic. Res. 2021, 8, 234. [Google Scholar] [CrossRef]
- Holsters, M.; de Waele, D.; Depicker, A.; Messens, E.; van Montagu, M.; Schell, J. Transfection and transformation of Agrobacterium tumefaciens. Mol. Gen. Genet. 1978, 163, 181–187. [Google Scholar] [CrossRef]
- Liu, L.; Qu, J.H.; Wang, C.Y.; Liu, M.; Zhang, C.M.; Zhang, X.Y.; Cheng, G.; Wu, C.G.; Yang, G.D.; Huang, J.G.; et al. An efficient genetic transformation system mediated by Rhizobium rhizogenes in fruit trees based on the transgenic hairy root to shoot conversion. Plant Biotechnol. J. 2024, 22, 2093–2103. [Google Scholar] [CrossRef]
- Yi, X.F.; Wang, C.C.; Yuan, X.Q.; Zhang, M.; Zhang, C.W.; Qin, T.J.; Wang, H.Y.; Xu, L.; Liu, L.W.; Wang, Y. Exploring an economic and highly efficient genetic transformation and genome-editing system for radish through developmental regulators and visible reporter. Plant J. 2024, 120, 1682–1692. [Google Scholar] [CrossRef]
- Dohm, A.; Ludwig, C.; Nehring, K.; Debener, T. Somatic embryogenesis in roses. Acta Hortic. 2001, 547, 341–347. [Google Scholar] [CrossRef]
- Ying, W.; Wen, G.C.; Xu, W.Y.; Liu, H.X.; Ding, W.A.; Zheng, L.Q.; He, Y.; Yuan, H.W.; Yan, D.L.; Cui, F.Q.; et al. Agrobacterium rhizogenes: Paving the road to research and breeding for woody plants. Front. Plant Sci. 2023, 14, 1196561. [Google Scholar] [CrossRef] [PubMed]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019, 37, 107405. [Google Scholar] [CrossRef]
- Predieri, S.; Drizzi, G.; Gatti, E. Effect of nitrogen supply on rooting of pear (Pyrus communis L.) cv. Conference microcuttings. Adv. Hortic. Sci. 1999, 13, 68–70. [Google Scholar]
- Ziemienowicz, A. Agrobacterium-mediated plant transformation: Factors, applications and recent advances. Biocatal. Agric. Biotechnol. 2014, 3, 95–102. [Google Scholar] [CrossRef]
- Zhao, J.T.; Li, Z.J.T.; Cui, J.; Henny, R.J.; Gray, D.J.; Xie, J.H.; Chen, J.J. Efficient somatic embryogenesis and Agrobacterium-mediated transformation of pothos (Epipremnum aureum) ‘Jade’. Plant Cell Tissue Organ Cult. 2013, 114, 237–247. [Google Scholar]
- Lai, E.M.; Kado, C.I. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 1998, 180, 2711–2717. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium virulence gene induction. Methods Mol. Biol. 2006, 343, 77–84. [Google Scholar]
- Cao, X.S.; Xie, H.T.; Song, M.L.; Lu, J.H.; Ma, P.; Huang, B.Y.; Wang, M.G.; Tian, Y.F.; Chen, F.; Peng, J.; et al. Cut–dip–budding delivery system enables genetic modifications in plants without tissue culture. Innovation 2023, 4, 100345. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.S.; Xie, H.T.; Song, M.L.; Zhao, L.H.; Liu, H.L.; Li, G.F.; Zhu, J.K. Simple method for transformation and gene editing in medicinal plants. J. Integr. Plant Biol. 2024, 66, 17–19. [Google Scholar] [CrossRef]
- Ma, H.J.; Liu, N.G.; Sun, X.P.; Zhu, M.L.; Mao, T.F.; Huang, S.Y.; Meng, X.Y.; Li, H.F.; Wang, M.; Liang, H.L. Establishment of an efficient transformation system and its application in regulatory mechanism analysis of biological macromolecules in tea plants. Int. J. Biol. Macromol. 2023, 244, 125372. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zheng, Y.S.; Zhou, Q.; Li, Y.; Liu, T.K.; Hou, X.L. Fast, simple, efficient Agrobacterium rhizogenes-mediated transformation system to non-heading Chinese cabbage with transgenic roots. Hortic. Plant J. 2024, 10, 450–460. [Google Scholar] [CrossRef]
- Lu, J.H.; Li, S.S.; Deng, S.; Wang, M.G.; Wu, Y.H.; Li, M.; Dong, J.S.; Lu, S.H.; Su, C.L.; Li, G.F.; et al. A method of genetic transformation and gene editing of succulents without tissue culture. Plant Biotechnol. J. 2024, 22, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Tian, Y.R.; Li, A.J.; Xia, X.L.; Lin, W.L. Jasmonic acid signaling and its research progress in woody plants. Sci. Sin. Vitae 2019, 50, 215–226. [Google Scholar]
- Han, X.; Kui, M.Y.; He, K.R.; Yang, M.L.; Du, J.C.; Jiang, Y.J.; Hu, Y.R. Jasmonate-regulated root growth inhibition and root hair elongation. J. Exp. Bot. 2022, 74, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).