Abstract
Chrysanthemum × morifolium Ramat. is a globally renowned ornamental flower. It includes numerous varieties, most of which are typical short-day (SD) plants, and the flowering characteristics of different chrysanthemum varieties in response to the photoperiod vary greatly. In this study, seven representative chrysanthemum varieties were selected for a comparative analysis of flowering traits under long-day conditions (16 h/8 h day/night) and short-day conditions (12 h/12 h day/night). It was found that three varieties (‘A44’, ‘C60’, and ‘183’) belonged to obligatory short-day varieties and four varieties (‘A20’, ‘C1’, ‘C27’, and ‘C31’) belonged to facultative short-day varieties. The short-day conditions not only induced earlier flowering but also improved flowering quality in the facultative SD varieties. Different chrysanthemum varieties required different light conditions to complete the vegetative stage and reach the floral competent state. Seven chrysanthemum varieties, ‘A44’, ‘C60’, ‘183’, ‘A20’, ‘C1’, ‘C27’, and ‘C31’, reached a floral competent state in the L20, L20, L22, L22, L18, L20, and L24 periods, respectively, and were most sensitive to SD induction at this time. The expression patterns of key floral genes in the photoperiod pathway were analyzed and it was found that CmCRY1, CmCRY2, CmGI1, CmGI2, and CmCO were mainly expressed in leaves. Then, comparing the expression levels of these genes under LD and SD conditions, the expression of CmGI1, CmGI2, CmCO, and CmFTL were not significantly induced in the obligatory SD varieties, while the expression of them in the facultative SD varieties were induced by SD conditions. This may be the reason why the facultative varieties could respond to SD conditions more quickly to complete the floral transition. In addition, SD induction under different photoperiodic conditions and growth states resulted in differences in the phenotype of flowering. This result provides guidance for the artificial regulation of chrysanthemum flowering and improvement of ornamental quality, as well as clues for analyzing the flowering mechanism of chrysanthemums under different photoperiod conditions.
1. Introduction
Flowering is a critical developmental phase in which plants transition from vegetative growth to reproductive growth, and it involves complex regulatory mechanisms. It is influenced by environmental factors such as photoperiod, vernalization, and ambient temperature, and endogenous signals such as sugar and hormones [1,2,3]. Plants precisely regulate flowering based on one or multiple environmental and endogenous signals to ensure reproductive success. Photoperiod is an important signal that controls plant flowering [4,5,6]. In 1920, Garner and Allard discovered and demonstrated that some species flower in response to changes in day length and described this phenomenon as “photoperiodism” [7,8]. Plants can be classified into short-day plants (SDP), long-day plants (LDP), and day-neutral plants (DNP) based on their response to photoperiod. For SDP, flowering occurs when the night length is longer than a critical minimum, whereas LDP flower when the day becomes longer than some crucial length [4]. In addition, plants that are responsive to day length may be further subdivided into obligatory (or qualitative) types, where a particular day length is essential for flowering, and facultative (or quantitative) types, where a particular day length accelerates but is not essential for flowering [9].
In recent years, with the continuous development of sequencing technology and genetic engineering, floral-related genes have been isolated and characterized in a variety of plants, and the regulatory pathways of flowering have been clarified [1]. Currently, five major pathways of floral transition have been identified in Arabidopsis thaliana: the photoperiod pathway, autonomous pathway, gibberellin pathway, age pathway, and ambient temperature pathway. The photoperiod pathway is one of the most widely studied pathway, which regulates plants’ flowering by transmitting the flowering signals received by the leaves to the shoot apical meristem (SAM), leading to the occurrence of flowering [10,11]. The photoreceptor first recognizes the external light signal and transmits the light signal to the circadian clock. The circadian clock generally comprises three different modules: the input pathway, central oscillator that consists of the canonical clock gene, and output pathway [12]. After that, the light signal continues to be transmitted downstream through the central oscillator; then, the expression of the flowering gene FLOWERING LOCUS T (FT) is induced by the transcriptional activator CONSTANS (CO) which integrating the light signal and the circadian clock signal [13,14]. Currently identified photoreceptors mainly include phytochromes (PHYs), cryptochromes (CRYs), and UV-B receptors (UVB-resistance locus 8, UVR8), which receive and recognize external light signals of different wavelengths [15,16,17]. GIGANTEA (GI), CO, and FT, as the key genes of this pathway, play a crucial role in signal integration in regulating plant flowering through the photoperiod pathway. Among them, FT is one of the important genes regulating flower formation in plants and encodes florigenin. It is a key gene for sensing photoperiod and an important genetic factor in determining whether or not to enter reproductive growth [18,19,20].
Chrysanthemum, as one of the world’s famous flowers and one of the four major cut flowers, has a long history of cultivation and extremely high ornamental value. Chrysanthemum, especially most of the autumn flowering chrysanthemum varieties, are typically SD plants, and the flowering characteristics of them in response to SD induction greatly limit their application and promotion. When a plant enters adulthood from the juvenile stage, its shoot apical meristem possesses the ability to sense the floral signal stimulus and then initiate flower bud differentiation, and this state is called the floral competent state [21]. Some studies have shown that plants have different sensitivities to flower formation induced by SD in different developmental stages. It is most sensitive to photoperiod when it reaches the floral competent state. At present, it has been widely recognized that the number of leaves instead of the growth time is used as a marker of plants’ developmental stage [22]. Moreover, it has been shown that chrysanthemum will not bloom unless a minimum number of leaves is reached [23]. Therefore, it is important to clarify the floral competent state of chrysanthemums to regulate the flowering period of chrysanthemums and reduce energy loss. At the same time, the genetic regulation mechanism of the different flowering times of different chrysanthemum varieties has not been fully analyzed, and it is especially important to find the key regulatory genes of different flowering time varieties for the molecular breeding of chrysanthemum flowering time improvement. In addition to influencing the floral transition, photoperiod, as a necessary condition for photosynthesis, also affects vegetative growth and physiological characteristics. Research has shown that there are differences in the flowering characteristics of different chrysanthemum varieties in response to light induction. ‘Hongmian’ chrysanthemum varieties achieved the shortest time to flower and the best flower morphology under 10 h light/14 h dark conditions [24]; cut ‘C029’ chrysanthemum varieties treated with a 10 h light/14 h dark photoperiod accelerated the process of flower bud differentiation, bloomed earliest, and had an excellent quality [25]. Although using photoperiod regulation measures to change the flowering time is a safe and simple means, these measures often blindly use shading and supplementary lighting due to a lack of understanding of the flowering trait differences among different chrysanthemum varieties, ultimately leading to a series of problems such as increased production energy consumption and decreased flowering quality. Therefore, exploring chrysanthemum flowering characteristics and the flowering quality of different chrysanthemum varieties in response to photoperiod are of great significance in guiding the production of chrysanthemums to artificially and accurately regulate flowering time and improve flowering quality.
In this study, seven self-fertilized chrysanthemum varieties with relatively stable genetic traits were used as study materials. We compared the flowering characteristics and quality of the seven chrysanthemum varieties under different photoperiod conditions and analyzed the expression patterns of key floral genes in the photoperiod pathway. The aim was to explore the flowering characteristics of different chrysanthemums in response to photoperiod, laying the foundation for further analysis of the flowering mechanism of chrysanthemums and providing theoretical guidance for flowering period control in chrysanthemum cultivation.
2. Materials and Methods
2.1. Plant Materials and Treatment
The seven chrysanthemum varieties self-bred by Beijing Forestry University (‘Dongli Hongzhuang’ (‘A44’), ‘Dongli Xiaotaiyang’ (‘C60’), ‘Dongli Lianghuang’ (‘183’), ‘Dongli Jiaofen’ (‘A20’), ‘Dongli Qiuhong’ (‘C1’), ‘Dongli Fanxing’ (‘C27’), and ‘Dongli Hongshou’ (‘C31’)) were utilized as plant materials. Chrysanthemums of the same status were selected and put into 10 × 9 cm plastic pots when the tissue-cultured seedlings had grown five to six leaves and had just taken root. The plants were planted in red trays with one plant per pot, and the growing medium was a 1:1 mixture of peat and vermiculite. After that, they were transferred to an artificial climatic chamber for an extended number of days. They were transferred into a short-day artificial climatic environment and SD induction was conducted consistently when they displayed a specific quantity of fully grown leaves (Table 1). The temperature was 24 ± 2 °C, and the relative humidity was about 60%. The SD was 12 h of light/12 h of dark (12 h L/12 h D), while the LD was 16 h light/8 h dark (16 h L/8 h D). An incandescent lamp with an average light intensity of 3000 lx served as the light source. Every three to four days, one liter of water was added to the tray, depending on the plants’ water deficit.

Table 1.
Different varieties with different number of leaves represent different nutrient growth states.
The growth characteristics of different chrysanthemum varieties vary greatly. We used the leaf number as an indication of growth and development status and uniformly used the number of leaves (Leaf, L) of plants grown under LD conditions for 1 month as the basis for starting to enter the treatment in SD conditions. Each variety had 5 treatments under SD conditions and a control (CK) under LD conditions, with 3 replicates of each treatment and 5 individual plants per replicate (Table 1). Fifteen plants were replicated for each treatment, and one treatment was uniformly placed in a red tray of 530 mm × 390 mm × 43 mm. A growth and developmental state in which the time between bud appearance and flowering was the shortest and the subsequent time between bud appearance and flowering was essentially unchanged, and an increasing leaf number was used as the floral competent state.
When the flower buds at the top of chrysanthemums grew to a visible state (about 1 mm), each organ of the plant was taken: upper leaves (UL), middle leaves (ML), bottom leaves (BL), stems (S), flowers (F), and roots (R). Leaves were taken as samples and grown under LD conditions for one month and SD induction for 7 days. After material harvesting, they were immediately frozen with liquid nitrogen and stored at −80 °C for subsequent analysis.
2.2. Observations of Flowering Time and Other Phenotypic Traits
The time of bud appearance and time of flowering (from transplant to budding and flowering) were recorded when the buds appeared visible to the naked eye at the top of chrysanthemums (about 1 mm) and when the ray florets were all unfolded. The plant height and crown width at the beginning of SD induction, as well as the plant height, crown width, number of leaves, number of capitula per plant, diameter of ray florets, and diameter of disk florets in different vegetative growth status treatments were recorded, and the rate of flowering was calculated based on the proportion of flowering plants under each treatment to the total number of plants in each treatment.
2.3. RT-qPCR and Gene Heat Map
Total RNA was extracted from the samples using the Plant RNA Rapid Extraction Kit (HUAYUEYANG Biotechnology, Beijing, China). First-strand cDNA was generated by using M-MLV reverse transcriptase (Promega, Madison, WI, USA) and anchored with the Oligo AP primer. Gene expression was measured by RT-qPCR by real-time quantitative reverse transcription-PCR (RT-qPCR). RT-qPCR amplification reactions were performed using SYBR Green (Roche, Basel, Switzerland) with a LightCycler 480 system (Roche, Basel, Switzerland) with three replicates. The CmPP2A (Protein phosphatases Type2 A) gene was used as an internal reference gene for the RT-qPCR. The RT-qPCR primers are shown in Table S1. Relative expression levels were calculated using the 2−ΔΔCT method [26], and the data are presented as the mean ± S.D.
Based on the expression results obtained by the RT-qPCR, gene heat maps were constructed using TBtools v2.142 software with the obtained data results [27].
2.4. Statistics and Analysis of Data
Experimental data were analyzed using Excel 2010 and SPSS 20.0; graphs were processed and plotted using Origin 9.0 software. The calculation of the mean was performed using Excel, and the t-test, least significant difference (LSD) method, and Duncan’s new multiple range test were performed using SPSS.
3. Results
3.1. Flowering Types of Different Chrysanthemum Varieties in Response to Photoperiod
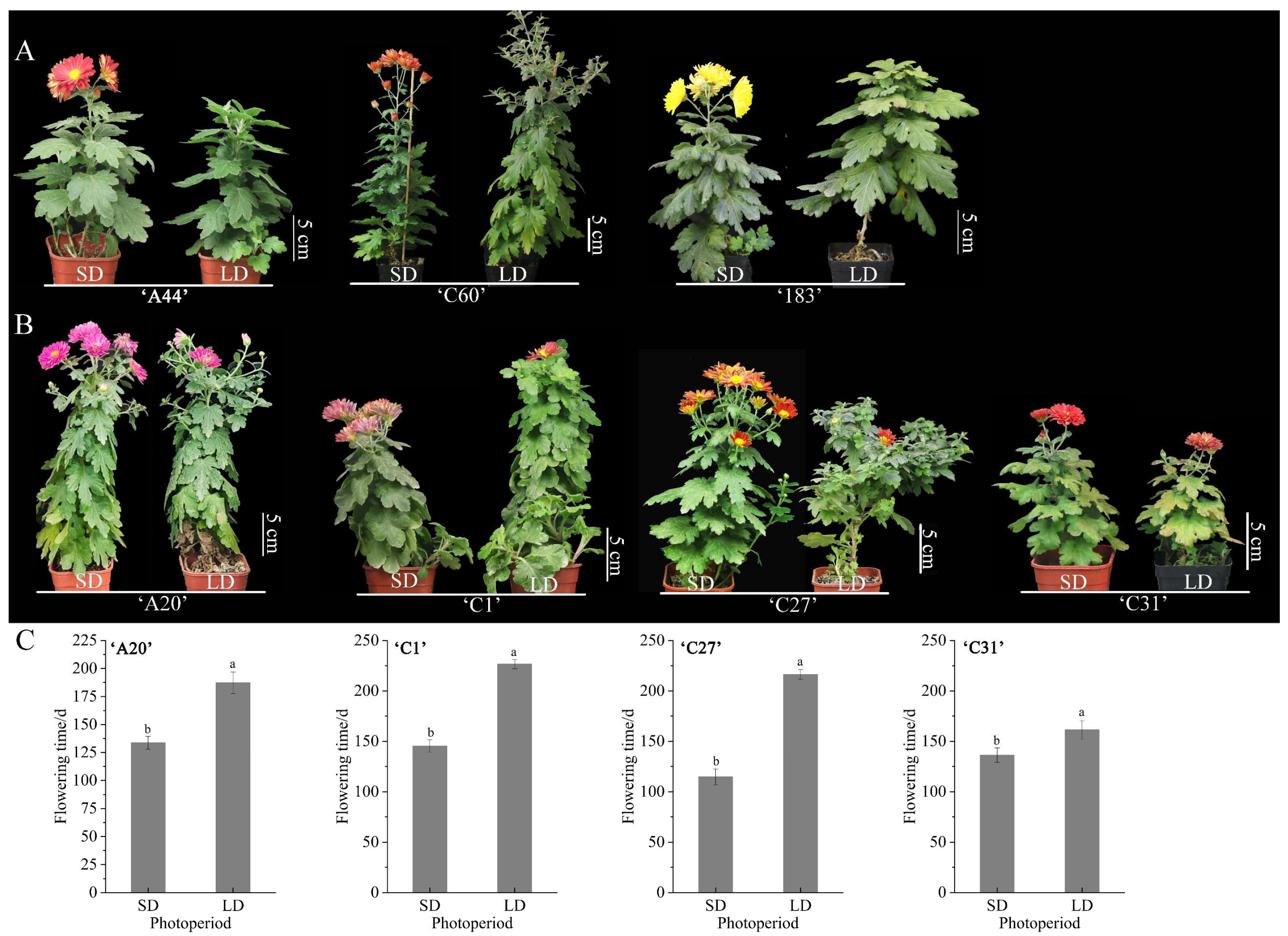
The flowering time of the seven chrysanthemum varieties were compared under treatments with LD and SD conditions. It was found that three chrysanthemum varieties, ‘A44’, ‘C60’, and ‘183’, could only flower under SD conditions and could not flower normally under LD conditions, which were typical obligatory short-day chrysanthemums, of which ‘C60’ could not open normally even though some flower buds appeared under LD conditions (Figure 1A). Four chrysanthemum varieties, ‘A20’, ‘C1’, ‘C27’, and ‘C31’, bloomed normally under both SD and LD conditions, so they were typical facultative SD chrysanthemums. Comparing the flowering time under SD and LD conditions, it was found that different varieties of facultative SD chrysanthemums flowered earlier under SD-induced conditions compared to LD conditions, with a minimum of about 20 d and a maximum of about 102 d ahead of schedule (Figure 1B,C). This suggests that obligatory SD chrysanthemums had a stringent SD requirement, while facultative short-day chrysanthemums did not have a stringent SD requirement, but short-day conditions could significantly promote the advancement of their flowering time.
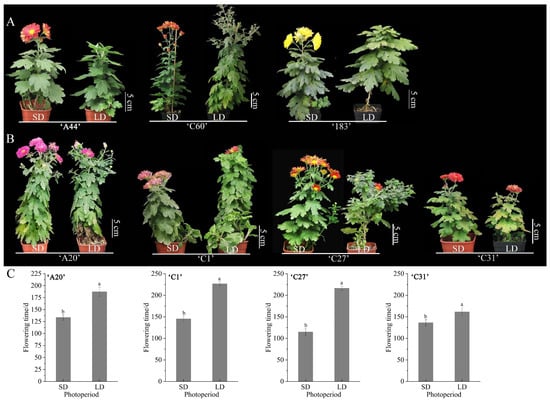
Figure 1.
The flowering types of seven chrysanthemum varieties under different photoperiods. (A) The flowering phenotypes of obligatory SD chrysanthemums ‘A44’, ‘C60’, and ‘183’. (B) The flowering phenotypes of facultative SD chrysanthemums ‘A20’, ‘C1’, ‘C27’, and ‘C31’. (C) The flowering time of facultative SD chrysanthemums ‘A20’, ‘C1’, ‘C27’, and ‘C31’ under SD and LD conditions, respectively. The flowering time was calculated based on the time it took from potting until the first flower was fully open. Error bars are the standard deviation (S.D.) of at least three biological replicates. Different letters on each bar are significant differences at a 5% level of probability (p < 0.05) according to the t-test.
3.2. Differences in the Floral Competent State of Different Chrysanthemum Varieties
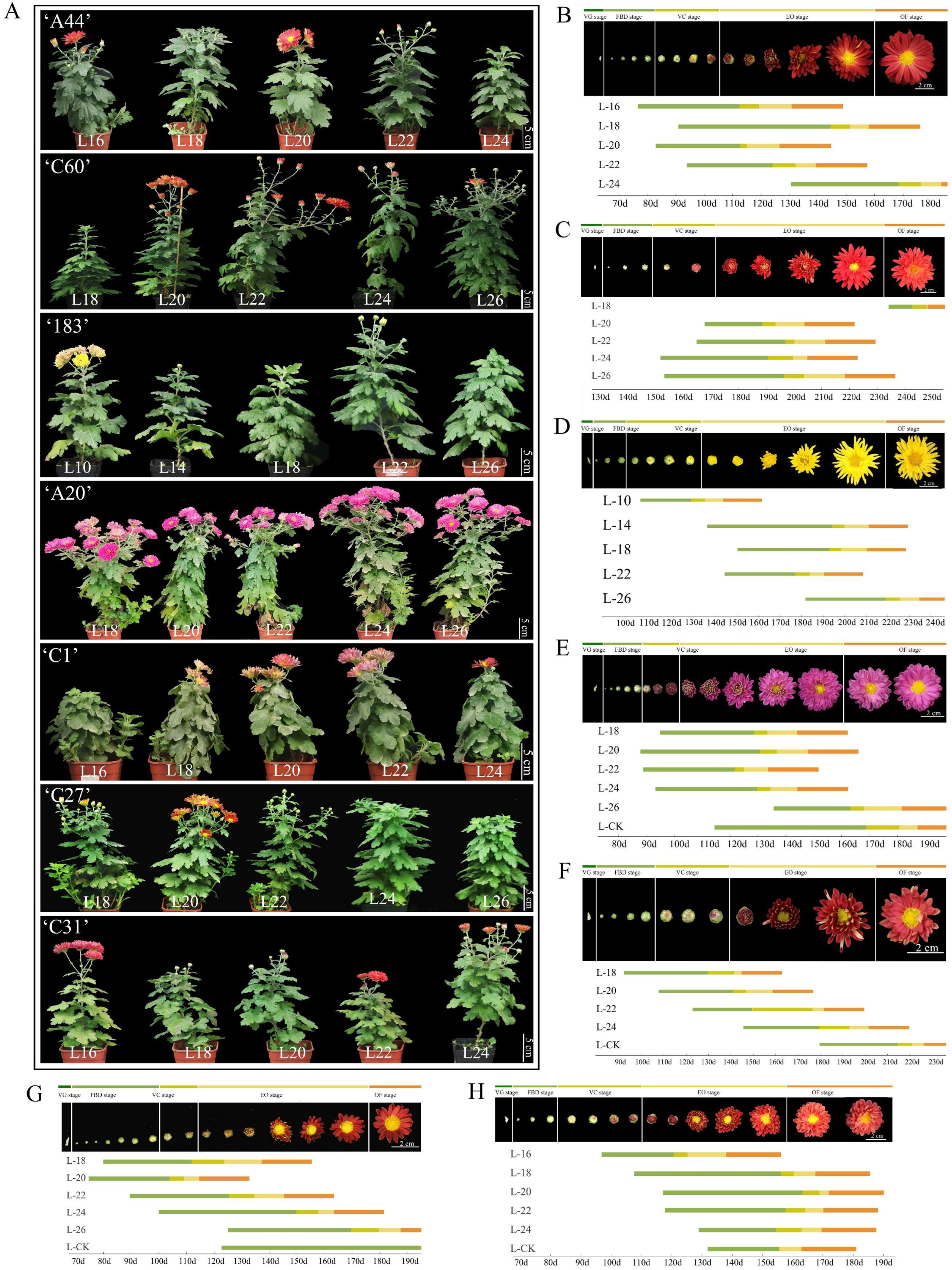
There are many varieties of chrysanthemums and the flowering characteristics of different varieties vary greatly. They also have different stages of completing vegetative growth and reaching the floral competent state. Seven chrysanthemum varieties were investigated for the stage of reaching the floral competent state using the number of intact leaves on the main stem as a criterion (Figure S1). The results showed that under the same light conditions and different growth and development states, the time from bud to flower for the same variety of chrysanthemum was basically the same, and an early or late time for the bud appearing basically determined an early or late time for the flower appearing. Among obligatory SD chrysanthemums, ‘A44’ and ‘C60’ flowered the earliest when the number of leaves reached 20 (L20), and the time required from SD induction to bud initiation and flowering was the shortest, and the time required for bud initiation and flowering no longer changed significantly when the number of leaves reached 20 and above, suggesting that ‘A44’ and ‘C60’ had already completed vegetative growth and reached the floral competent state at L20, while ‘183’ at reached it at L22 (Figure 2A–D and Figure S2). In facultative SD chrysanthemums, ‘A20’ reached the floral competent state when it reached L22, and ‘C1’ did so at L18; ‘C27’ and ‘C31’ were most sensitive to SD induction with leaves at L20 and L24, respectively, and took the shortest time to reach bud appearance and flowering (Figure 2A,E–H and Figure S2). In summary, the seven chrysanthemum varieties, ‘A44’, ‘C60’, ‘183’, ‘A20’, ‘C1’, ‘C27’, and ‘C31’, reached the floral competent state in the L20, L20, L22, L22, L18, L20, and L24 periods, respectively.
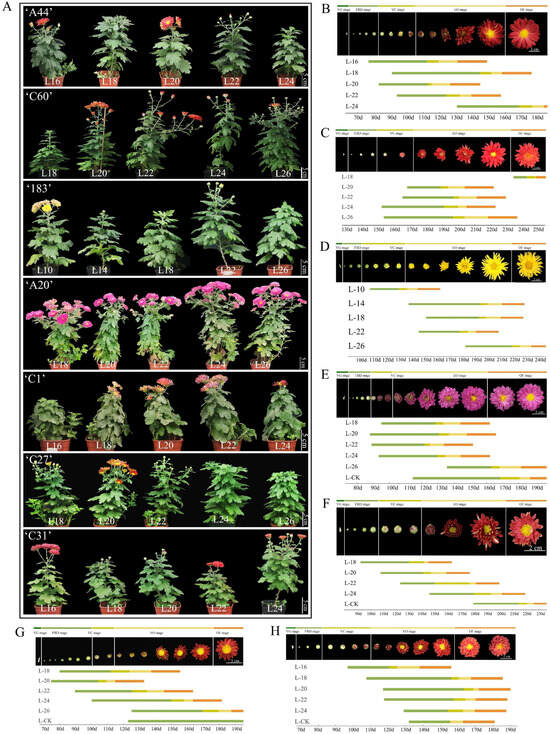
Figure 2.
An exploration of the floral competent state of different chrysanthemum varieties. (A) The flowering phenotypes of seven chrysanthemum varieties after SD induction in different growth states. (B–H) ‘A44’, ‘C60’, ‘183’, ‘A20’, ‘C1’, ‘C27’, and ‘C31’ in different stages of vegetative growth for the demonstration of SD-induced flowering time. VG, FBD, VC, EO, and OF stages represent the vegetative growth stage, flower bud developmental stage, visible color stage, early opening stage, and open flower stage, respectively. Different colors are used to represent different developmental stages.
Comparing the time from SD induction to flowering after the seven varieties reached the floral competent state, it was found that the time required from SD induction to flowering in the three obligatory SD varieties was the same. Among the four facultative SD varieties, except for ‘C1’ which required the longest time for flowering, the varieties responded to SD induction and reached flowering in a shorter time than the obligatory SD varieties (Figure S3). It can be seen that different chrysanthemum varieties require different lengths of time to reach the floral competent state, and chrysanthemums are most sensitive to the induction of SD only after reaching the floral competent state. Chrysanthemum requires the shortest time from SD induction to flowering after reaching the flowering state, and there is also specificity in the time from SD induction to flowering among different varieties.
3.3. Analysis of Tissue-Specific Expression Patterns of Key Flowering Genes
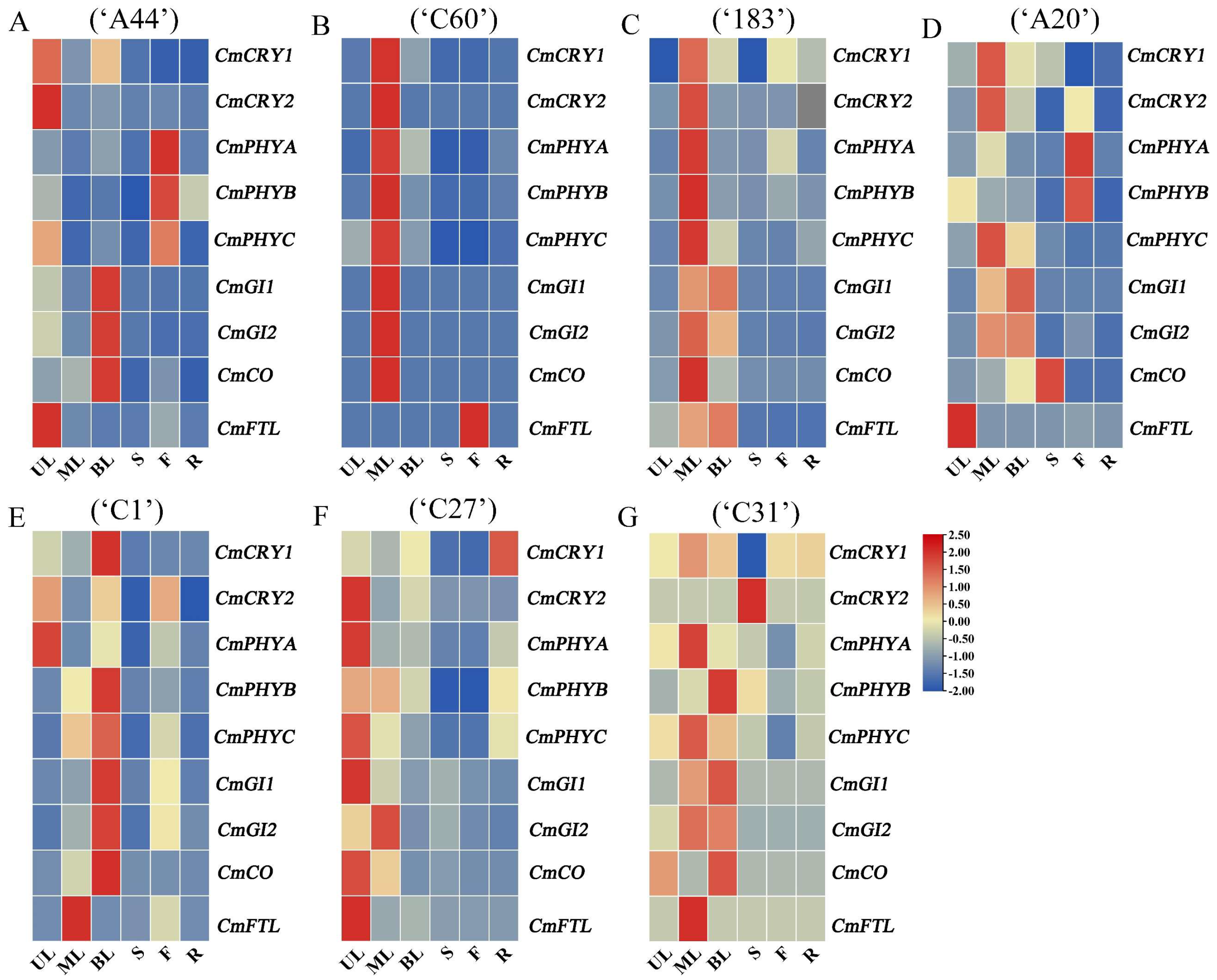
The photoreceptor genes CmCRY1, CmCRY2, CmPHYA, CmPHYB, and CmPHYC and the integrator genes CmGI1, CmGI2, CmCO, and CmFTL (FLOWERING LOCUS T-like) are all key members involved in the photoperiod regulation of flowering pathways [28,29]. In order to subsequently investigate the mechanism of action of flowering in response to external light signals in different chrysanthemum varieties, the tissue-specific expression patterns of some flowering genes in the photoperiodic pathway were analyzed firstly. The results showed that CmCRY1, CmCRY2, CmGI1, CmGI2, and CmCO in most chrysanthemum varieties were mainly expressed in the leaves (Figure 3 and Figure S4). CmPHYA, CmPHYB, and CmPHYC in ‘C60’, ‘183’, ‘C1’, ‘C27’, and ‘C31’ were mainly expressed in the leaves, while CmPHYs in ‘A44’ and ‘A20’ were mainly expressed in the flower (Figure 3B and Figure S5). In addition, except for CmFTL in ‘C60’, which was mainly expressed in the flower (Figure 3B and Figure S6), CmFTL in other chrysanthemums was mainly expressed in the leaves. Overall, the major floral genes in chrysanthemums were predominantly expressed in the leaves, with some differences in the leaf site of expression in different chrysanthemums, which is consistent with the pathway of chrysanthemums that completes the floral transition by sensing light signals through leaves and then transmitting the floral signal to the SAM. And, this is also effectively in agreement with the expression pattern of genes in the photoperiod pathway in other species [30,31,32].
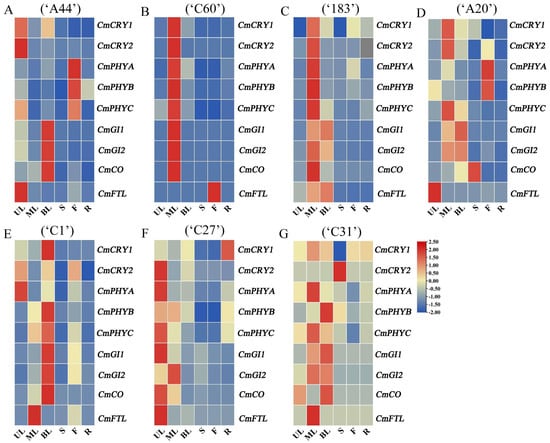
Figure 3.
Analysis of tissue-specific expression pattern of key floral genes in different chrysanthemums. (A−G) Tissue-specific expression pattern of key floral genes in ‘A44’, ‘C60’, ‘183’, ‘A20’, ‘C1’, ‘C27’, and ‘C31’. UL, ML, BL, S, F, and R, respectively, represent upper leaves, middle leaves, bottom leaves, stems, flowers, and roots.
3.4. Expression Pattern of Key Floral Genes in Response to Photoperiod
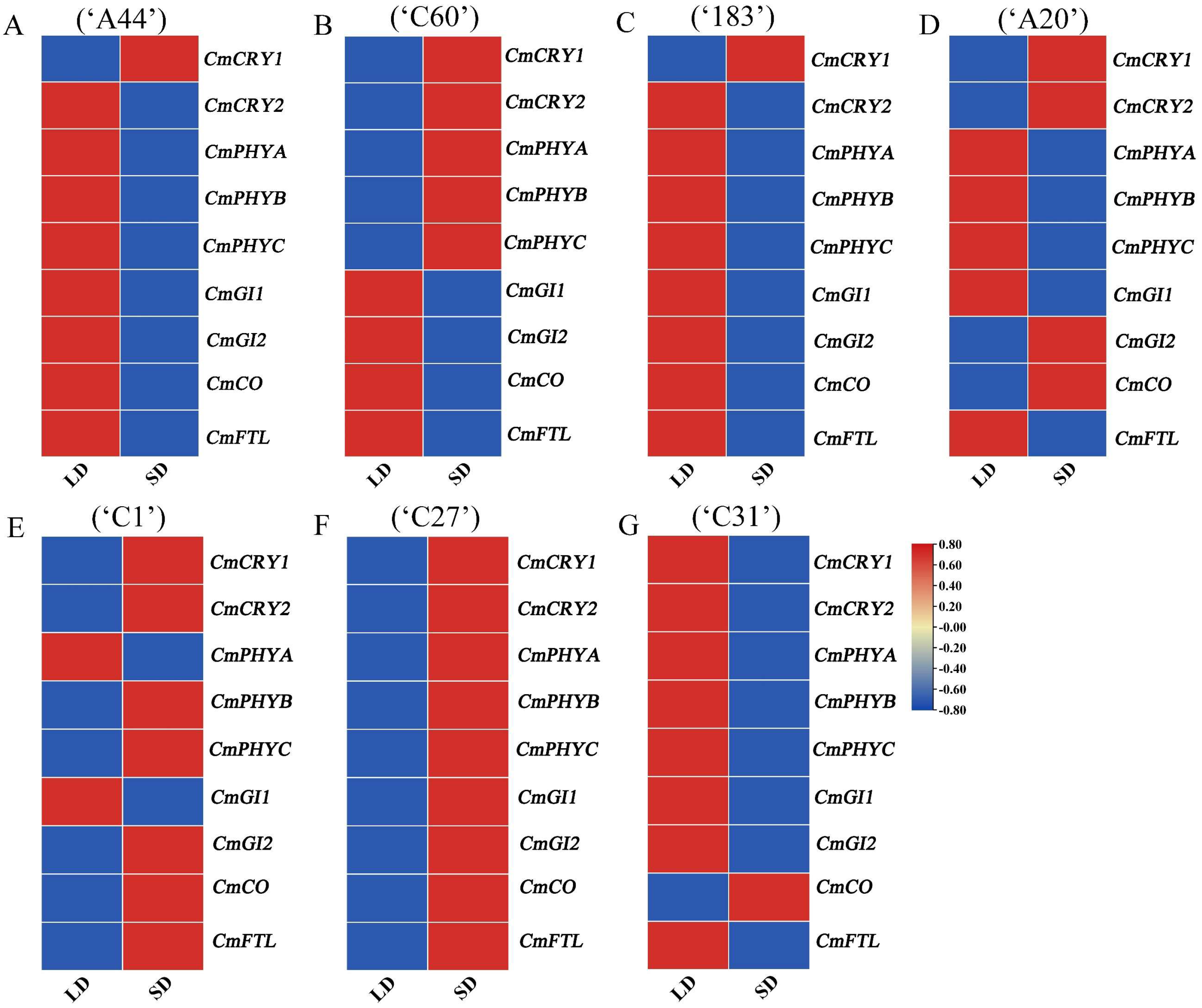
Based on the results that different flowering genes were mainly expressed in leaves, the expression patterns of the above key flowering genes in response to photoperiod were further analyzed using qRT-PCR. Under SD conditions, the expression of CmGI1, CmGI2, CmCO, and CmFTL in obligatory SD chrysanthemums (‘A44’, ‘C60’, ‘183’) were not significantly induced, while the expression of the photoreceptor genes CmCRYs and CmPHYs in the photoperiod pathway of ‘C60’ was significantly induced (Figure 4A–C and Figure S7). Compared to the other photoreceptor genes, the expression of CmCRY1 in all three varieties was significantly up-regulated at the beginning of SD induction, indicating that CmCRY1 can rapidly receive external SD signals in obligatory short-day chrysanthemums.
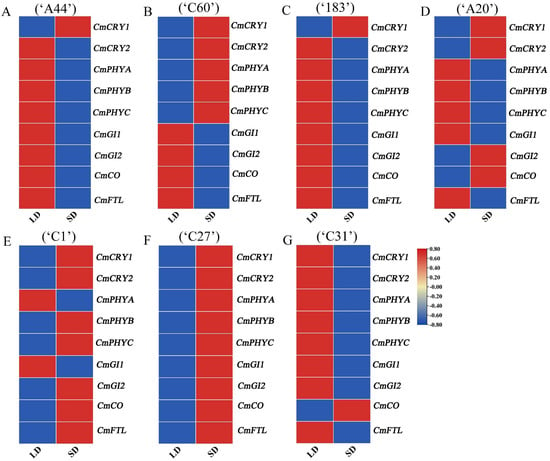
Figure 4.
Expression pattern analysis of key floral genes in response to photoperiod in different chrysanthemums. (A–G) Expression pattern of key floral genes in response to photoperiod in ‘A44’, ‘C60’, ‘183’, ‘A20’, ‘C1’, ‘C27’, and ‘C31’.
In addition to the expression of the photoreceptor genes induced, the expression of integrated genes was also induced after 7 days of SD induction in facultative SD chrysanthemums (‘A20’, ‘C1’, ‘C27’, ‘C31’) (Figure 4D–G and Figure S7). Among them, the expression of CmCRY1, CmCRY2, CmGI2, and CmCO were induced by the SD in ‘A20’, and the expression of CmCRY1, CmCRY2, CmPHYB, CmPHYC, CmGI2, CmCO, and CmFTL in ‘C1’ were induced by the SD. All photoreceptors and integrative genes were induced by the SD in ‘C27’, which may also be the reason for the earliest flowering time of ‘C27’ under SD conditions. Combined with the phenotype that most facultative SD chrysanthemums (‘A20’, ‘C27’, and ‘C31’) required less time to enter SD induction and bloom, it indicates that facultative SD chrysanthemums could not only flower under LD but also responded more quickly to SD conditions compared to obligatory SD chrysanthemums (Figure S3). Among them, the photoreceptor genes CmCRY1 and CmCRY2 were induced by the SD in all three facultative SD chrysanthemums, and CmCO showed upregulation in the early stage of SD induction only in ‘C31’ (Figure 4G and Figure S7). Combined with the phenotype of ‘C31’ which could bloom under both LD and SD conditions (Figure 1B,C), it is speculated that there may be other flowering pathways that jointly regulate its early flowering, and the expression patterns of known or candidate genes of other flower-forming pathways in this variety can be explored to see if there is any influence of other flower-forming pathways.
Based on the above results, this study suggests that compared to obligatory SD chrysanthemums, facultative SD chrysanthemums can respond more quickly to SD induction, leading to the expression of some downstream integration genes, except for the photoreceptor genes. But, the induced integrator genes are not entirely the same. This indicates that obligatory SD chrysanthemums have a relatively fixed mechanism for responding to photoperiod, strictly relying on short-day signals. In contrast, facultative SD chrysanthemums may possess a more flexible photoperiod perception mechanism that allows them to adjust their growth and flowering under various light conditions. Their quicker response to SD conditions, along with the faster induction of downstream integration genes, may also contribute to the earlier flowering of some facultative SD chrysanthemums compared to obligatory SD chrysanthemums. Additionally, there may be different regulatory mechanisms among various facultative SD varieties. Furthermore, some facultative SD varieties exhibit an earlier induction of integration genes but do not flower earlier. As indicated by Figure 2, ‘C1’ had a longer floral development stage compared to other cultivars, suggesting that the timing of flower bud differentiation and development varied among cultivars, which was also a reason for the differences in flowering time.
3.5. Different Flowering Qualities of Chrysanthemums INDUCED to Flower in Different Vegetative Stages Under Different Photoperiods
In addition to affecting flowering time, past studies have also shown that photoperiod affects the flowering quality of chrysanthemums, so this study looked at the flowering quality of chrysanthemums under different treatments. Firstly, the plant height and the number of leaves of the seven chrysanthemum varieties were analyzed. These varieties continued to accumulate vegetative growth until the final peak was reached after entering SD conditions. Among them, the plant height and number of leaves of ‘C31’ during flowering under LD and SD conditions were relatively small, indicating that ‘C31’ could quickly reach the floral competent state and sense the light signal to promote early flowering (Table 2, Figure S8). Compared to the crown width before SD induction, the crown width of chrysanthemum increased to a certain extent under flowering. However, there were differences in the increase in crown width among different varieties under different treatments. ‘A20’, ‘C1’, and ‘C27’ reached their maximum crown width under LD conditions, indicating that some facultative SD chrysanthemums may exhibit excessive vegetative growth under LD conditions (Table 2, Figure S9).

Table 2.
Statistics of ornamental quality of chrysanthemums under different photoperiodic conditions and different vegetative growth states.
Further comparison of the flowering traits differed somewhat in the number of capitula per plant, diameter of ray florets, and diameter of disk florets among the seven chrysanthemum varieties, regardless of the different vegetative growth states induced by SD conditions (Table 2). Although the SD induced flowering earlier, it resulted in a decrease in the number of capitula per plant and diameter of ray florets in ‘A44’. ‘C60’, ‘183’, and ‘C31’, which entered SD conditions before reaching the floral competent state, not only delayed flowering but also significantly reduced the number of capitula per plant. ‘C1’ even failed to bloom normally due to premature entry into SD conditions (Figure 1 and Figure S2, Table 2). While ‘A20’, ‘C27’, and ‘C31’ significantly increased the number of capitula per plant and diameter of ray florets and disk florets under SD conditions, LD conditions inhibited these plants’ capitulum development. The rate of flowering could reach 100% regardless of the different states for SD induction in some varieties (‘A44’, ‘183’, ‘A20’, ‘C1’, ‘C27’, ‘C31’), while the obligatory SD chrysanthemum ‘C60’ could only achieve a flowering rate of 100% after reaching the floral competent state and entering SD induction. In the facultative SD chrysanthemums ‘A20’ and ‘C1’, the rate of flowering was 100% under LD conditions. Those of ‘C27’ and ‘C31’ under LD conditions were only 18.33% and 60%, respectively; even though there were many visible flower buds, most of them could not bloom normally (Table 2, Figure S10). Combined with the results in Figure 2, some facultative SD chrysanthemums varieties had prolonged bud development stages under LD conditions compared to SD conditions, suggesting that photoperiod may also influence the process of chrysanthemum flower development to some extent. In addition, by calculating the correlation coefficients between the different response variables, it was found that there were some differences between varieties, and the effects of different nutritional states for flower formation induction were different for different varieties (Figure S11).
In summary, the induction of flowering under different photoperiodic conditions and different stages of vegetative growth not only affect flowering time but also affect other flowering traits. The induction of flowering under unsuitable conditions causes chrysanthemum to fail to bloom normally and even seriously reduces the quality of flowering.
4. Discussion
4.1. Chrysanthemums Exhibit Diverse Types of Flowering in Response to Photoperiodic Stimuli
Photoperiod is one of the key environmental factors that determine plant flowering. During the long evolutionary process, chrysanthemums have developed different types of photoperiod-responsive flowering, including SD-dependent autumn chrysanthemum, which blooms in autumn, and summer–autumn chrysanthemum, which blooms in summer to autumn and is less sensitive to photoperiod. The former of these is an obligatory SD chrysanthemum and the latter is a facultative SD chrysanthemum [33,34]. In this study, by comparing the flowering characteristics of different chrysanthemums in response to photoperiod, we found that ‘A44’, ‘C60’, and ‘183’ were sensitive to SD conditions and were classified as obligatory SD chrysanthemum, and ‘A20’, ‘C1’, ‘C27’, and ‘C31’ were not sensitive to SD conditions and were classified as facultative SD chrysanthemum. This suggest that different chrysanthemum varieties respond to photoperiod with various flowering types, which is consistent with the previous classification of chrysanthemum types. Therefore, research on the flowering types of chrysanthemums responding to photoperiod can facilitate breeders to adopt different breeding methods for different types of chrysanthemum varieties according to their response characteristics to photoperiod, as well as selecting and breeding chrysanthemum varieties insensitive to photoperiod, which is conducive to lowering the cost of chrysanthemums’ annual production.
4.2. Different Chrysanthemum Varieties Vary Widely in Reaching the Floral Competent State
The genetic background of chrysanthemum is very complex, and there are some differences in the floral transition characteristics of different varieties in response to light induction. The determination of the floral competent state is beneficial to shorten the time of floral transition and precisely regulate the industrialized production of chrysanthemum. Some studies have shown that plants have different sensitivities to flower formation induced by SD in different developmental stages. In this study, the number of leaves was used as a marker to determine the floral competent state of chrysanthemums, and the stage of the floral competent state was clarified for seven varieties, which differed from each other. It also differed significantly from the previously clarified species of Chrysanthemum lavandulifolium [35], Chrysanthemum vestitum [36], and cut-flower chrysanthemum ‘Reagan’ [37] and had a floral competent state in L14, suggesting that there are significant differences in floral characteristics in response to light induced among different species of chrysanthemum and among different varieties of chrysanthemum. In summary, the SD induction of chrysanthemum at the time when they reach the floral competent state can advance the flowering period.
4.3. The Mechanism of the Response to Photoperiod Flowering Varies Among Different Chrysanthemum Varieties
Through this study, it was found that facultative SD chrysanthemums could bloom in both LD and SD conditions and bloom earlier in SD than LD conditions, and obligatory SD chrysanthemums could bloom only in SD conditions. Facultative SD chrysanthemums were able to respond to SD conditions more quickly to complete the floral transition to flowering than obligatory SD chrysanthemums that required a longer period of SD conditions for completion of the floral transition (Figure 1 and Figure 2). Previous studies have also shown that obligatory SD chrysanthemum must be continuously maintained under SD conditions to flower. This requirement for SD repetition seems to be related to the induction of flowering-promoting signals in leaves [38]. For obligatory SD chrysanthemums, photoperiod is the most critical factor affecting their flowering, and sufficient SD conditions are required to induce the floral transition. However, facultative SD chrysanthemums are less sensitive to photoperiod, and there may be other factors that cooperate with SD regulation to regulate its flowering. This suggests that there are different characteristics of flowering in response to photoperiod in chrysanthemum.
Photoreceptor genes and floral integrated genes are important nodal genes in the photoperiod pathway, and they serve as important players in the response to and integration of external light signals, which are essential components for the completion of the floral transition through the photoperiod pathway in SD plants [28,29]. Comparing the expression of photoreceptor and integrated genes in different species, it was found that they differed greatly in both obligatory and facultative SD chrysanthemums under SD conditions (Figure 4). Among them, CmCRY1 was significantly induced by SD conditions in six varieties, indicating that this gene may be a key gene in response to external SD signals both in obligatory and facultative SD chrysanthemums. In the past, it was also found that ClCRY1a and ClCRY1b could affect chrysanthemum flowering by regulating the expression of the downstream integrative genes GI, CO, and FT in the study of C. lavandulifolium, a closely related wild species of chrysanthemum [30]. Especially in ‘C27’, except for CmCRY1, other photoreceptor genes and integrator genes can respond to SD signals, which suggest that the ‘C27’ variety is the most sensitive to SD induction combined with the fact that it can rapidly respond to SD conditions and it flowers the earliest. And, the photoperiod pathway plays an important role in the ‘C27’ variety’s floral transition (Figure 1 and Figure 4F). Although ‘C31’ is a facultative SD chrysanthemum, the photoreceptor genes did not show obvious up-regulated expression under SD conditions, and there was a significant difference in the expression of floral transition genes in other varieties. Therefore, it was hypothesized that other floral transition pathways might exist in the ‘C31’ variety (Figure 1 and Figure 4G). In chrysanthemum, in addition to the photoperiod pathway, the vernalization pathway [34], the age pathway [39], and the gibberellin pathway [40] have also been found to be involved in the regulation of flowering, and the joint involvement of multiple pathways is also a question worth investigating.
In this study, some of the downstream integrative genes of facultative SD chrysanthemums were induced to be expressed more rapidly in response to SD conditions than those of obligatory SD chrysanthemums, but the integrative genes that were induced to be expressed were not exactly the same. This suggests that the sensitivity of downstream integrated genes to photoperiod may influence the sensitivity of chrysanthemum varieties to photoperiod to some extent. Previous studies have also found that loss of the function of ghd7 (a homolog of the CONSTANS gene in A. thaliana) in rice reduces photoperiodic sensitivity [41], and mutations in the FTL1 gene in tomato also affect the induction of flowering under short-day conditions and alter sensitivity to photoperiod [42]. This suggests that mutations in a number of integrated genes in the photoperiod pathway have the potential to affect plant sensitivity to photoperiods. Whether such a situation also exists in different facultative SD chrysanthemums, or whether there are other photoperiod-insensitive flowering genes and different upstream regulatory genes, remains to be investigated. In conclusion, the factors contributing to the reduced sensitivity to photoperiod in different facultative SD chrysanthemums may be diverse. All these conclusions lay a foundation for further analysis of the complex flowering mechanism in chrysanthemum.
In addition, this study also found that different photoperiodic conditions and different stages of vegetative growth for flower induction not only affected the early and late flowering of chrysanthemums but also regulated ornamental traits of chrysanthemums. Previous studies have also found that the flowering-related genes, such as AtFT not only promote flowering but also participate in lateral shoot development [43]; MdFT1 and MdFT2 in apple may affect the development of leaves and fruits [44], so whether the differential expression of flowering-related genes affects other ornamental traits of chrysanthemums needs to be explored in subsequent studies.
In order to achieve the annual supply of chrysanthemum production, the flower time control technology of extending light time combined with shading treatment is usually adopted, which has problems such as being time-consuming, being labor-intensive, generating high-energy consumption, and making it difficult to control the quality, resulting in high production costs and affecting economic benefits. More and more molecular mechanisms of chrysanthemum flowering have been revealed, which not only lay the foundation to utilize molecular breeding to improve chrysanthemum flowering time but also provide a new theoretical basis for the related research of simplified flower regulation technology in production.
5. Conclusions
The seven chrysanthemum varieties involved in this study had different flowering characteristics and could be classified into obligatory SD chrysanthemums and facultative SD chrysanthemums based on their response to photoperiod. The floral competent state of different chrysanthemums differed greatly, and the timely induction of the floral competent state could shorten the SD induction time and advance the flowering time. The key genes of the photoperiod pathway were mainly expressed in leaves, and the expression patterns of them in response to photoperiod were different between obligatory SD chrysanthemums and facultative SD chrysanthemums. Facultative SD chrysanthemums could respond quickly to SD and the expression of the floral genes could be up-regulated by SD conditions. However, obligatory SD chrysanthemums were not only sensitive to the induction of SD conditions but also required a longer SD induction to up-regulate the expression of floral genes and ultimately flowering. The reasons for the decreased photoperiod sensitivity may vary among different facultative SD chrysanthemums. Additionally, in facultative SD chrysanthemums, there may be other pathways, in addition to the photoperiod pathway, that dominantly influence flowering. In addition to influencing the flowering time of different varieties, different photoperiodic conditions also had great influence on their ornamental traits. Although facultative SD chrysanthemums could bloom under LD conditions, their ornamental quality was impaired under the LD environment. This study not only lays the foundation for further analyzing the complex flowering mechanism in chrysanthemum but also has important significance for artificially regulating the flower time and improving the flowering quality of chrysanthemum production.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11010005/s1, Figure S1: Phenotypes of seven chrysanthemum varieties subjected to floral competent state probes in different growth stages; Figure S2: Time taken to enter SD induction and budding and flowering in different growth stages of seven chrysanthemum varieties; Figure S3: Time required to enter SD induction and flowering after reaching floral competent state in seven varieties; Figure S4: Tissue-specific expression patterns of CmCRY1 and CmCRY2 in seven chrysanthemum varieties; Figure S5: Tissue-specific expression patterns of PHYs in different chrysanthemum varieties; Figure S6: Tissue-specific expression patterns of integrative genes in different chrysanthemum varieties; Figure S7: Expression patterns of key flowering genes in different chrysanthemum varieties in response to different photoperiods; Figure S8: Plant height of chrysanthemums in different vegetative growth states before entering SD induction and at flowering; Figure S9: Crown width of chrysanthemums in different growth states at time of entry into SD induction and at time of flowering; Figure S10: Flowering phenotypes of C27 under LD conditions; Figure S11: Heat map of correlation coefficients between different response variable relationships; Table S1: Primer list.
Author Contributions
Conceptualization, Q.Z. and S.D.; methodology, Q.Z., X.L. and J.L.; software, Q.Z.; validation, Q.Z., X.L. and S.C.; formal analysis, Q.Z. and X.L.; investigation, Q.Z.; resources, X.L. and S.C.; data curation, Q.Z., X.L., J.W. and Y.L.; writing—original draft preparation, Q.Z. and X.L.; writing—review and editing, Q.Z., X.L., J.L., J.W., Y.L. and S.D.; visualization, Q.Z. and X.L.; supervision, S.D.; project administration, S.D.; funding acquisition, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32371948) and Fujian Provincial Department of Science and Technology (No. 2022S0004).
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
We are particularly indebted to Beijing Dadongliu Nursery for providing test sites, and we also thank Yushan Ji and Hao Liu for their guidance and help in plant materials’ cultivation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Perrella, G.; Vellutini, E.; Zioutopoulou, A.; Patitaki, E.; Headland, L.R.; Kaiserli, E. Let it bloom: Cross-talk between light and flowering signaling in Arabidopsis. Physiol. Plant 2020, 169, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Richter, R. Genetic and molecular basis of floral induction in Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 2490–2504. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef]
- Brambilla, V.; Gomez-Ariza, J.; Cerise, M.; Fornara, F. The importance of being on time: Regulatory networks controlling photoperiodic flowering in Cereals. Front. Plant Sci. 2017, 8, 665. [Google Scholar] [CrossRef]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod control of plant growth: Flowering time genes beyond flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Garner, W.W.; Allard, H.A. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. Mon. Weather Rev. 1920, 48, 415. [Google Scholar] [CrossRef]
- Higuchi, Y.; Narumi, T.; Oda, A.; Nakano, Y.; Sumitomo, K.; Fukai, S.; Hisamatsu, T. The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in Chrysanthemums. Proc. Natl. Acad. Sci. USA 2013, 110, 17137–17142. [Google Scholar] [CrossRef]
- Thomas, B.; Vince-Prue, D. Photoperiodism in Plants, 2nd ed.; Academic Press: Salt Lake City, UT, USA, 1997; pp. 3–4. [Google Scholar]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Blümel, M.; Dally, N.; Jung, C. Flowering time regulation in crops—What did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015, 32, 121–129. [Google Scholar] [CrossRef]
- Venkat, A.; Muneer, S. Role of circadian rhythms in major plant metabolic and signaling pathways. Front Plant Sci. 2022, 13, 836244. [Google Scholar] [CrossRef] [PubMed]
- Komeda, Y. Genetic regulation of time to flower in Arabidopsis thaliana. Annu. Rev. Plant Biol. 2004, 55, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Fujiwara, S.; Suh, S.S.; Kim, J.; Kim, Y.; Han, L.; David, K.; Putterill, J.; Nam, H.G.; Somers, D.E. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 2007, 449, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Lin, C. Photoreceptors and regulation of flowering time. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef]
- Endo, M.; Araki, T.; Nagatani, A. Tissue-specific regulation of flowering by photoreceptors. Cell Mol. Life Sci. 2016, 73, 829–839. [Google Scholar] [CrossRef]
- Zhu, Y.; Klasfeld, S.; Wagner, D. Molecular regulation of plant developmental transitions and plant architecture via PEPB family proteins: An update on mechanism of action. J. Exp. Bot. 2021, 72, 2301–2311. [Google Scholar] [CrossRef]
- Yang, M.; Lin, W.; Xu, Y.; Xie, B.; Yu, B.; Chen, L.; Huang, W. Flowering-time regulation by the circadian clock: From Arabidopsis to crops. Crop J. 2023, 12, 17–27. [Google Scholar] [CrossRef]
- Wang, X.; Hao, Y.; Altaf, M.A.; Shu, H.; Cheng, S.; Wang, Z.; Zhu, G. Evolution and dynamic transcriptome of key genes of photoperiodic flowering pathway in Water Spinach (Ipomoea aquatica). Int. J. Mol. Sci. 2024, 25, 1420. [Google Scholar] [CrossRef]
- Second Botanical Nomenclature Review Committee. Chinese Terms in Botany, 2nd ed.; Science Press: Beijing, China, 2019; pp. 250–254. [Google Scholar]
- Adams, S.R.; Pearson, S.; Hadley, P.; Patefield, W.M. The effects of temperature and light integral on the phases of photoperiod sensitivity in Petunia × hybrida. Ann. Bot. 1999, 83, 263–269. [Google Scholar] [CrossRef]
- Grigoras, C.D.; Toma, F. Photoperiodism, an important element for the growth and flowering of Chrysanthemums. Sci. Pap. Ser. B Hortic. 2021, 65, 215–224. [Google Scholar]
- Lu, S.; Yang, Z.; Yang, L.; Zhang, Y.; Zheng, H. Effects of different photoperiods on the growth and development process and endogenous hormones of Chrysanthemum. Acta Agric. Boreali Sin. (In Chinese). 2021, 36, 106–115. [Google Scholar]
- Mao, H.; Gu, Z.; Zhu, P. Effects of different photoperiods on floral bud differentiation and flowering of Chrysanthemum ‘C029’. Acta Bot. Boreali Occident. Sin. 2010, 30, 2074–2080. (In Chinese) [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, L.J.; Sun, J.; Ren, L.P.; Zhou, M.; Han, X.Y.; Ding, L.; Zhang, F.; Guan, Z.Y.; Fang, W.M.; Chen, S.M.; et al. CmBBX8 accelerates flowering by targeting CmFTL1 directly in summer Chrysanthemum. Plant Biotechnol. J. 2020, 18, 1562–1572. [Google Scholar] [CrossRef]
- Wang, L.J.; Cheng, H.; Wang, Q.; Si, C.N.; Yang, Y.M.; Yu, Y.; Zhou, L.J.; Ding, L.; Song, A.P.; Xu, D.Q.; et al. CmRCD1 represses flowering by directly interacting with CmBBX8 in summer Chrysanthemum. Hortic. Res. 2021, 8, 79. [Google Scholar] [CrossRef]
- Kotoda, N.; Hayashi, H.; Suzuki, M.; Igarashi, M.; Hatsuyama, Y.; Kidou, S.; Igasaki, T.; Nishiguchi, M.; Yano, K.; Shimizu, T.; et al. Molecular characterization of FLOWERING LOCUS T-like genes of apple (Malus × domestica Borkh.). Plant Cell Physiol. 2010, 51, 561–575. [Google Scholar] [CrossRef]
- Yang, L.W.; Fu, J.X.; Qi, S.; Hong, Y.; Huang, H.; Dai, S.L. Molecular cloning and function analysis of ClCRY1a and ClCRY1b, two genes in Chrysanthemum lavandulifolium that play vital roles in promoting floral transition. Gene 2017, 617, 32–43. [Google Scholar] [CrossRef]
- Yang, L.W.; Wen, X.H.; Fu, J.X.; Dai, S.L. ClCRY2 facilitates floral transition in Chrysanthemum lavandulifolium by affecting the transcription of circadian clock-related genes under short-day photoperiods. Hortic. Res. 2018, 5, 58. [Google Scholar] [CrossRef]
- Kawata, J. The phasic development of Chysanthemum as a basis for the regulation of vegetative growth and flowerring in Japan. Acta Hortic. 1987, 197, 115–124. [Google Scholar] [CrossRef]
- Lyu, J.; Aiwaili, P.; Gu, Z.Y.; Xu, Y.J.; Zhang, Y.H.; Wang, Z.L.; Huang, H.F.; Zeng, R.H.; Ma, C.; Gao, J.P.; et al. Chrysanthemum MAF2 regulates flowering by repressing gibberellin biosynthesis in response to low temperature. Plant J. 2022, 112, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.X. Molecular Mechanism of Floral Transition Induced by Short-Day in Chrysanthemum lavandulifolium. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2014. [Google Scholar]
- Zhang, Q.L.; Li, J.Z.; Deng, C.Y.; Chen, J.Q.; Han, W.J.; Yang, X.Z.; Wang, Z.M.; Dai, S.L. The mechanisms of optimal nitrogen conditions to accelerate flowering of Chrysanthemum vestitum under short day based on transcriptome analysis. J. Plant Physiol. 2023, 285, 153982. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, Z.L.; Dai, S.L. Light induction on flowering characteristics of cut Chrysanthemum ‘Reagan’. J. Beijing For. Univ. 2015, 37, 133–138. (In Chinese) [Google Scholar]
- Nakano, Y.; Higuchi, Y.; Sumitomo, K.; Hisamatsu, T. Flowering retardation by high temperature in Chrysanthemums: Involvement of FLOWERING LOCUS T-like 3 gene repression. J. Exp. Bot. 2013, 64, 909–920. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, C.; Xu, Y.J.; Wang, T.L.; Chen, Y.Y.; Lü, J.; Zhang, L.L.; Jiang, C.Z.; Hong, B.; Gao, J.P. Control of Chrysanthemum flowering through integration with an aging pathway. Nat. Commun. 2017, 8, 829. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.W.; Aiwaili, P.; Zhang, H.; Xu, Y.J.; Gu, Z.Y.; Gao, J.P.; Hong, B. PHOTOLYASE/BLUE LIGHT RECEPTOR2 regulates Chrysanthemum flowering by compensating for gibberellin perception. Plant Physiol. 2023, 193, 2848–2864. [Google Scholar] [CrossRef]
- Gao, H.; Jin, M.N.; Zheng, X.M.; Chen, J.; Yuan, D.Y.; Xin, Y.Y.; Wang, M.Q.; Huang, D.Y.; Zhang, Z.; Zhou, K.N.; et al. Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 16337–16342. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.B.; Wang, X.T.; Sun, S.; Liu, Z.Q.; Wang, K.T.; Wan, H.J.; Zhou, G.Z.; Li, R.; Yu, H.; et al. Variations in both FTL1 and SP5G, two tomato FT Paralogs, control day-neutral flowering. Mol. Plant. 2020, 13, 939–942. [Google Scholar] [CrossRef]
- Hiraoka, K.; Yamaguchi, A.; Abe, M.; Araki, T. The florigen genes FT and TSF modulate lateral shoot outgrowth in Arabidopsis thaliana. Plant Cell Physiol. 2013, 54, 352–368. [Google Scholar] [CrossRef]
- Mimida, N.; Kidou, S.; Iwanami, H.; Moriya, S.; Abe, K.; Voogd, C.; Varkonyi-Gasic, E.; Kotoda, N. Apple FLOWERING LOCUS T proteins interact with transcription factors implicated in cell growth and organ development. Tree Physiol. 2011, 31, 555–566. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).