A SMALL AUXIN UP-REGULATED RNA Gene Isolated from Watermelon (ClSAUR1) Positively Modulates the Chilling Stress Response in Tobacco via Multiple Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
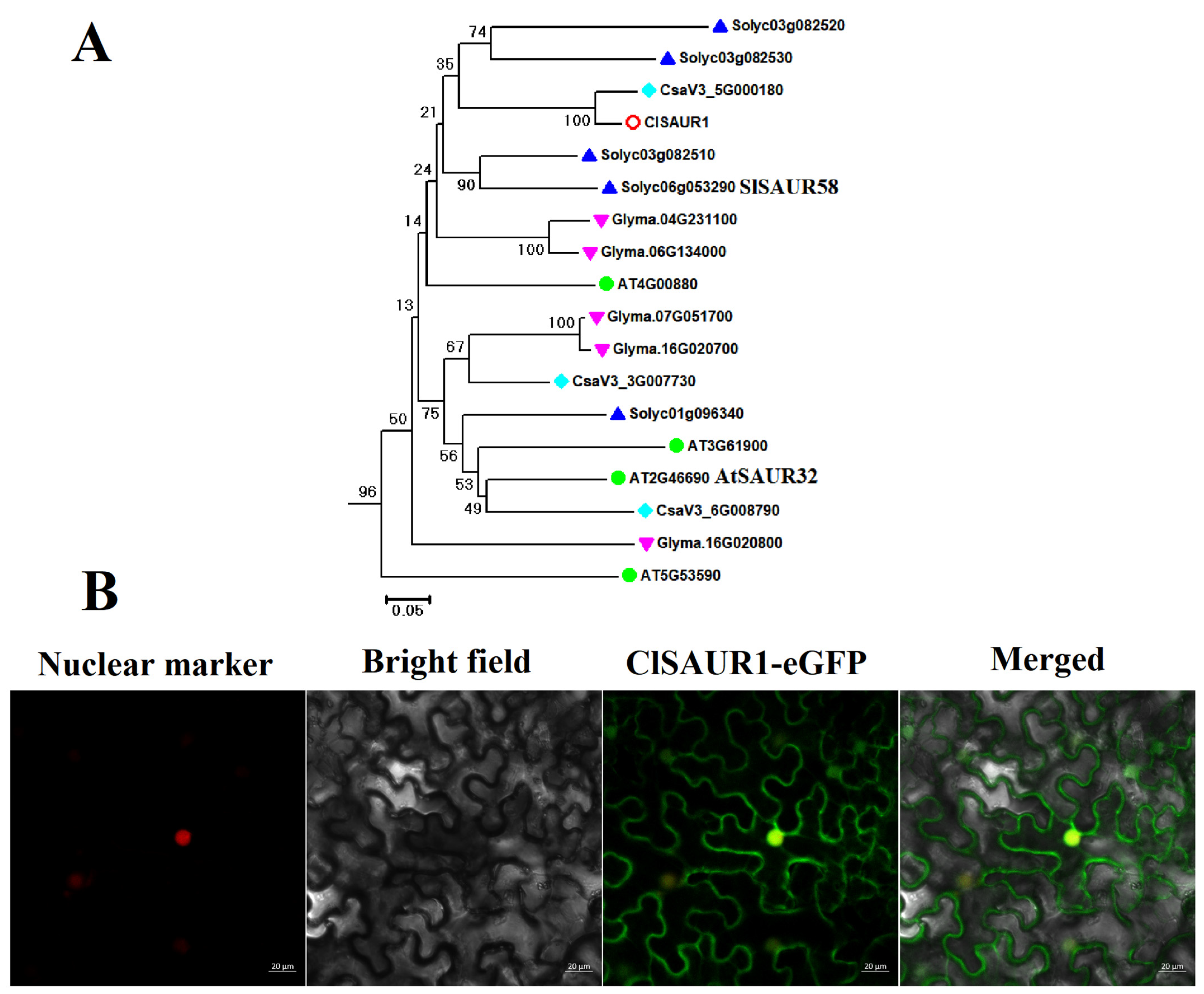
2.2. Identification and Bioinformatics Analysis of ClSAUR1
2.3. Analysis of Subcellular Localization
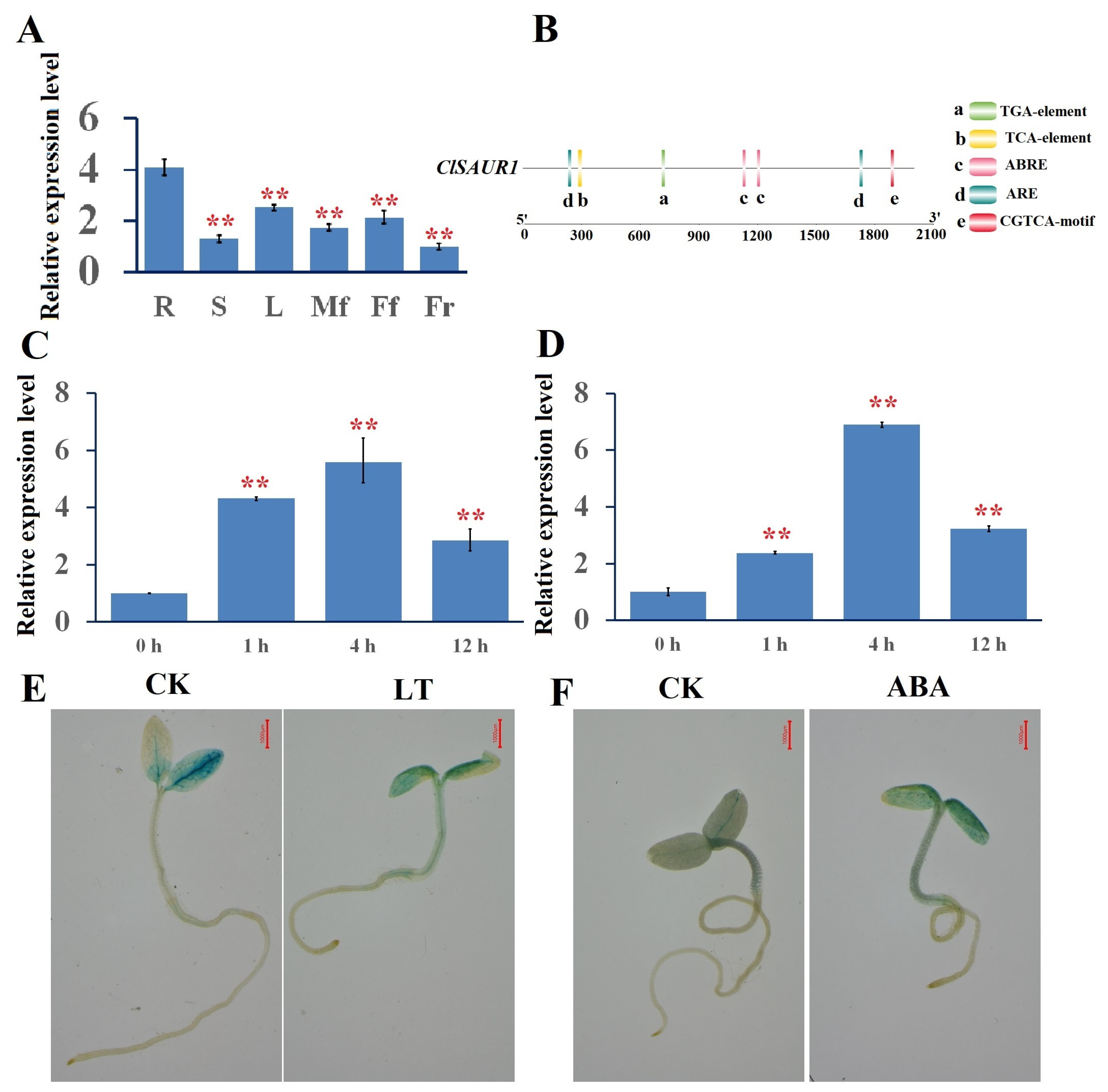
2.4. GUS Staining Promoter Assay
2.5. Plasmid Construction and Generation of Tobacco Transgenic Plants
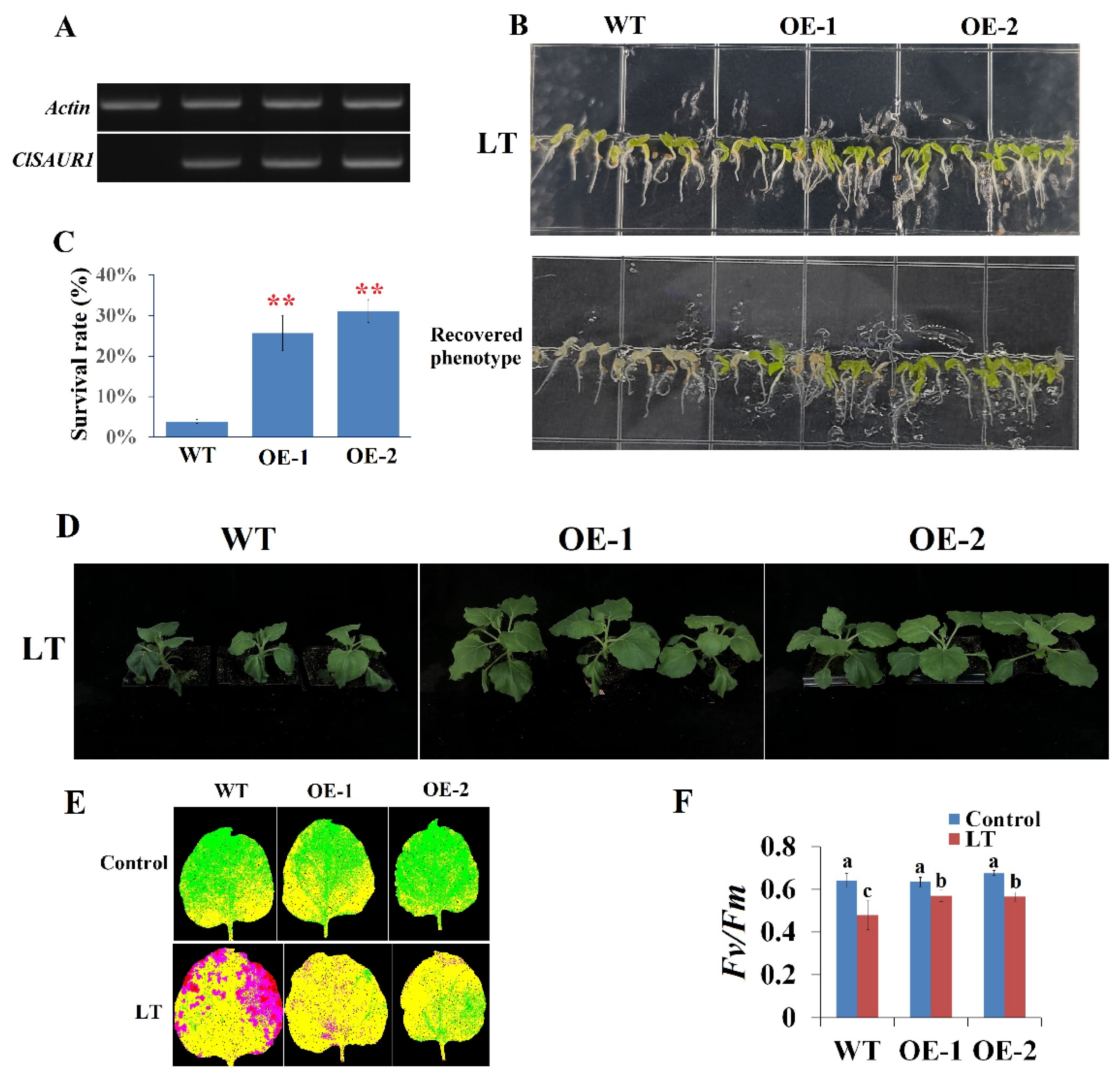
2.6. Chilling Tolerance in Transgenic Tobacco Lines
2.7. Determination of Physiological Indices
2.8. Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Isolation and Basic Informatics Analysis of ClSAUR1
3.2. Response Patterns of ClSAUR1 to ABA and Chilling Stress in Tobacco Plants
3.3. Overexpression of ClSAUR1 Enhanced Tobacco’s Chilling Stress Tolerance
3.4. Alterations in Physiological and Biochemical Indices of Tobacco Overexpressing ClSAUR1 in Response to Chilling Stress
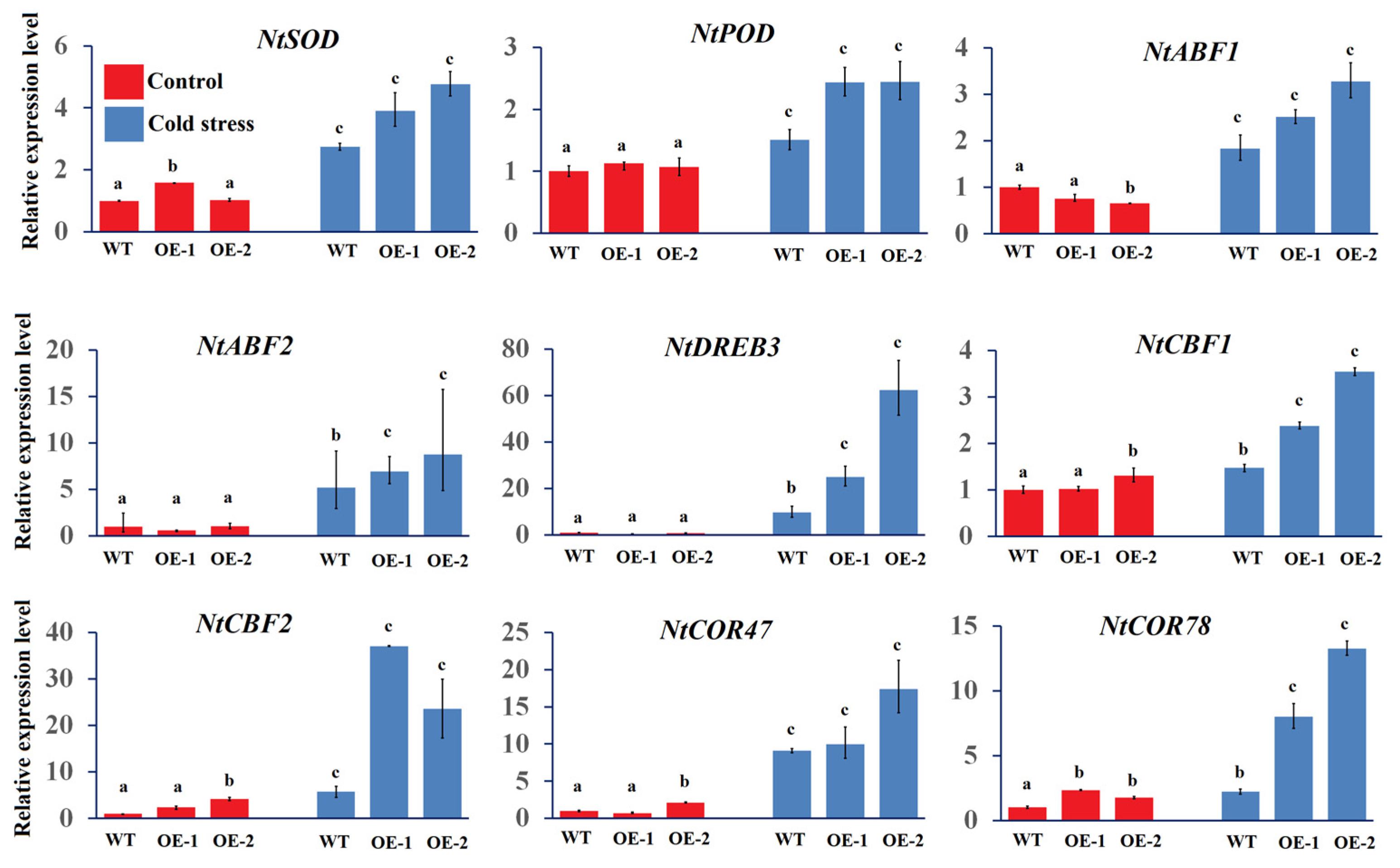
3.5. Up-Regulation of Cold Stress-Related Genes in ClSAUR1-Overexpressing Lines Under Conditions of Low-Temperature Stress
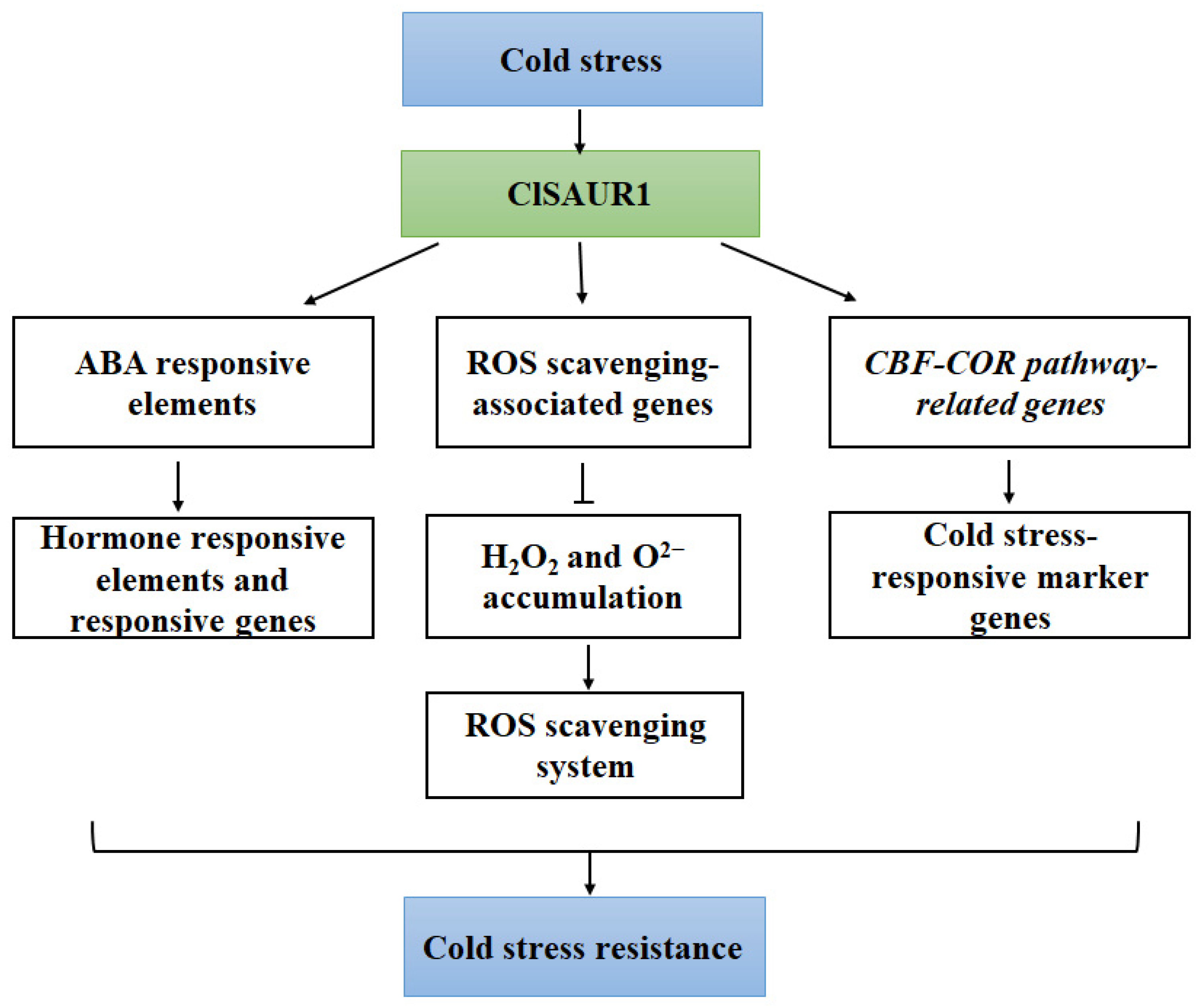
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, H.; Gray, W.M. SAUR Proteins as Effectors of Hormonal and Environmental Signals in Plant Growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Franklin, K.A.; Lee, S.H.; Patel, D.; Kumar, S.V.; Spartz, A.K.; Gu, C.; Ye, S.; Yu, P.; Breen, G.; Cohen, J.D.; et al. PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. USA 2011, 108, 20231–20235. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Lor, V.S.; Ren, H.; Olszewski, N.E.; Miller, N.D.; Wu, G.; Spalding, E.P.; Gray, W.M. Constitutive Expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in Tomato Confers Auxin-Independent Hypocotyl Elongation. Plant Physiol. 2016, 173, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, R.K.; Kobayashi, Y.; Enomoto, T.; Miyachi, T.; Sakuma, M.; Fujita, M.; Ogata, T.; Fujita, Y.; Iuchi, S.; Kobayashi, M.; et al. STOP1-regulated SMALL AUXIN UP RNA55 (SAUR55) is involved in proton/malate co-secretion for Al tolerance in Arabidopsis. Plant Direct 2023, 8, e557. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR Inhibition of PP2C-D Phosphatases Activates Plasma Membrane H+-ATPases to Promote Cell Expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Han, N.; Zhu, M.; Bian, H.; Wang, J. The SAUR41 subfamily of SMALL AUXIN UP RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis SMALL AUXIN UP RNA32 Protein Regulates ABA-Mediated Responses to Drought Stress. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Chen, F.; Wang, R.-J.; Wu, C.-J.; Lin, M.; Yan, H.-W.; Xiang, Y. SAUR8, a small auxin-up RNA gene in poplar, confers drought tolerance to transgenic Arabidopsis plants. Gene 2022, 837, 146692. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, L.; Chi, J.; Li, R.; Han, Y.; Cui, F.; Peng, Z.; Wan, S.; Li, G. Genome-wide identification and expression of SAUR gene family in peanut (Arachis hypogaea L.) and functional identification of AhSAUR3 in drought tolerance. BMC Plant Biol. 2022, 22, 178. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Jiang, M.; Tian, H.; Bin Khalid, M.H.; Wang, Y.; Yu, H. The Small Auxin-Up RNA 50 (SAUR50) Gene from Ammopiptanthus nanus Negatively Regulates Drought Tolerance. Plants 2024, 13, 2512. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, C.-B.; Sun, X.-J.; Hu, Z.; Fan, S.-J.; Jiang, Q.-Y.; Zhang, H. TaSAUR78 enhances multiple abiotic stress tolerance by regulating the interacting gene TaVDAC1. J. Integr. Agric. 2019, 18, 2682–2690. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhai, H.; Wang, F.; Yang, N.; Wang, B.; He, S.; Liu, Q. Cloning and characterization of a carbohydrate metabolism-associated gene IbSnRK1 from sweetpotato. Sci. Hortic. 2013, 158, 22–32. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Yao, Y.; Zhang, H.; Wang, Y.; Gao, J.; Fan, M. Overexpression of watermelon m6A methyltransferase ClMTB enhances drought tolerance in tobacco by mitigating oxidative stress and photosynthesis inhibition and modulating stress-responsive gene expression. Plant Physiol. Biochem. 2021, 168, 340–352. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Huang, D.; Dong, Q.; Li, P.; Ma, F. High-efficient utilization and uptake of N contribute to higher NUE of ‘Qinguan’ apple under drought and N-deficient conditions compared with ‘Honeycrisp’. Tree Physiol. 2019, 39, 1880–1895. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, Y.H.; Ji, C.Y.; Park, S.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Stable internal reference genes for the normalization of real-time PCR in different sweet potato cultivars subjected to abiotic stress conditions. PLoS ONE 2012, 7, e51502–e51518. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, Y.; Yan, Y.; Yu, X.; Ali, M.; Pan, C.; Lu, G. Cytokinin-inducible response regulator SlRR6 controls plant height through gibberellin and auxin pathways in tomato. J. Exp. Bot. 2023, 74, 4471–4488. [Google Scholar] [CrossRef]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When Bad Guys Become Good Ones: The Key Role of Reactive Oxygen Species and Nitric Oxide in the Plant Responses to Abiotic Stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. Cell Mol. Biol. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Zolla, L.; Rinalducci, S. Low temperature tolerance in plants: Changes at the protein level. Phytochemistry 2015, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Lipid peroxidation—DNA damage by malondialdehyde. Mutat. Res. Mol. Mech. Mutagen. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Kodaira, K.S.; Qin, F.; Tran, L.S.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol. 2011, 157, 742–756. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Sun, T.; Wang, X.; Zhu, C.; Ai, Y.; Gu, H. The precise regulation of different COR genes by individual CBF transcription factors in Arabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 118–133. [Google Scholar] [CrossRef]
- Park, S.; Lee, C.-M.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Ma, G.; Shen, J.; Xu, X.; Shou, W.; Xuan, Z.; He, Y. A SMALL AUXIN UP-REGULATED RNA Gene Isolated from Watermelon (ClSAUR1) Positively Modulates the Chilling Stress Response in Tobacco via Multiple Signaling Pathways. Horticulturae 2025, 11, 52. https://doi.org/10.3390/horticulturae11010052
Wang D, Ma G, Shen J, Xu X, Shou W, Xuan Z, He Y. A SMALL AUXIN UP-REGULATED RNA Gene Isolated from Watermelon (ClSAUR1) Positively Modulates the Chilling Stress Response in Tobacco via Multiple Signaling Pathways. Horticulturae. 2025; 11(1):52. https://doi.org/10.3390/horticulturae11010052
Chicago/Turabian StyleWang, Duo, Gangli Ma, Jia Shen, Xinyang Xu, Weisong Shou, Zhengying Xuan, and Yanjun He. 2025. "A SMALL AUXIN UP-REGULATED RNA Gene Isolated from Watermelon (ClSAUR1) Positively Modulates the Chilling Stress Response in Tobacco via Multiple Signaling Pathways" Horticulturae 11, no. 1: 52. https://doi.org/10.3390/horticulturae11010052
APA StyleWang, D., Ma, G., Shen, J., Xu, X., Shou, W., Xuan, Z., & He, Y. (2025). A SMALL AUXIN UP-REGULATED RNA Gene Isolated from Watermelon (ClSAUR1) Positively Modulates the Chilling Stress Response in Tobacco via Multiple Signaling Pathways. Horticulturae, 11(1), 52. https://doi.org/10.3390/horticulturae11010052





