Balancing Yield and Sustainability: A Comparative Analysis of Supplemental Lighting in Commercial-Scale Cucumber Cultivation
Abstract
1. Introduction
2. Results
2.1. Morphological Measurements
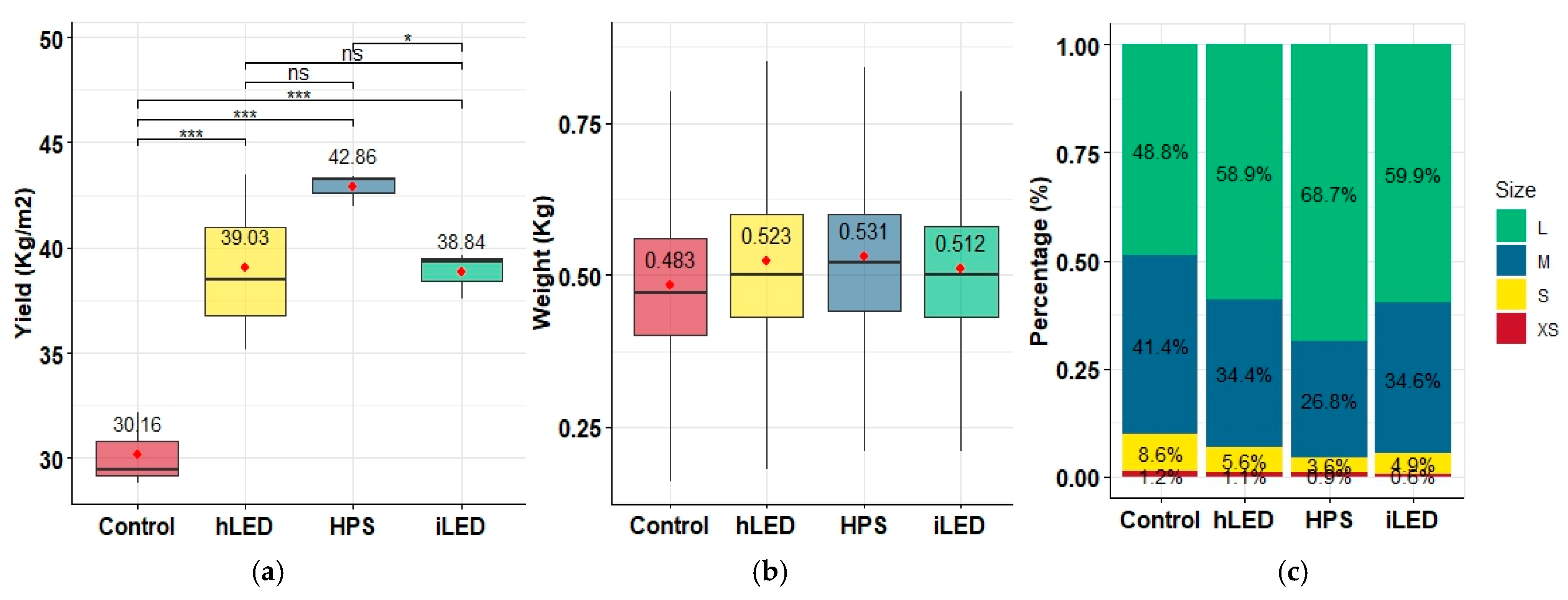
2.2. Plant Yield and Fruit Quality
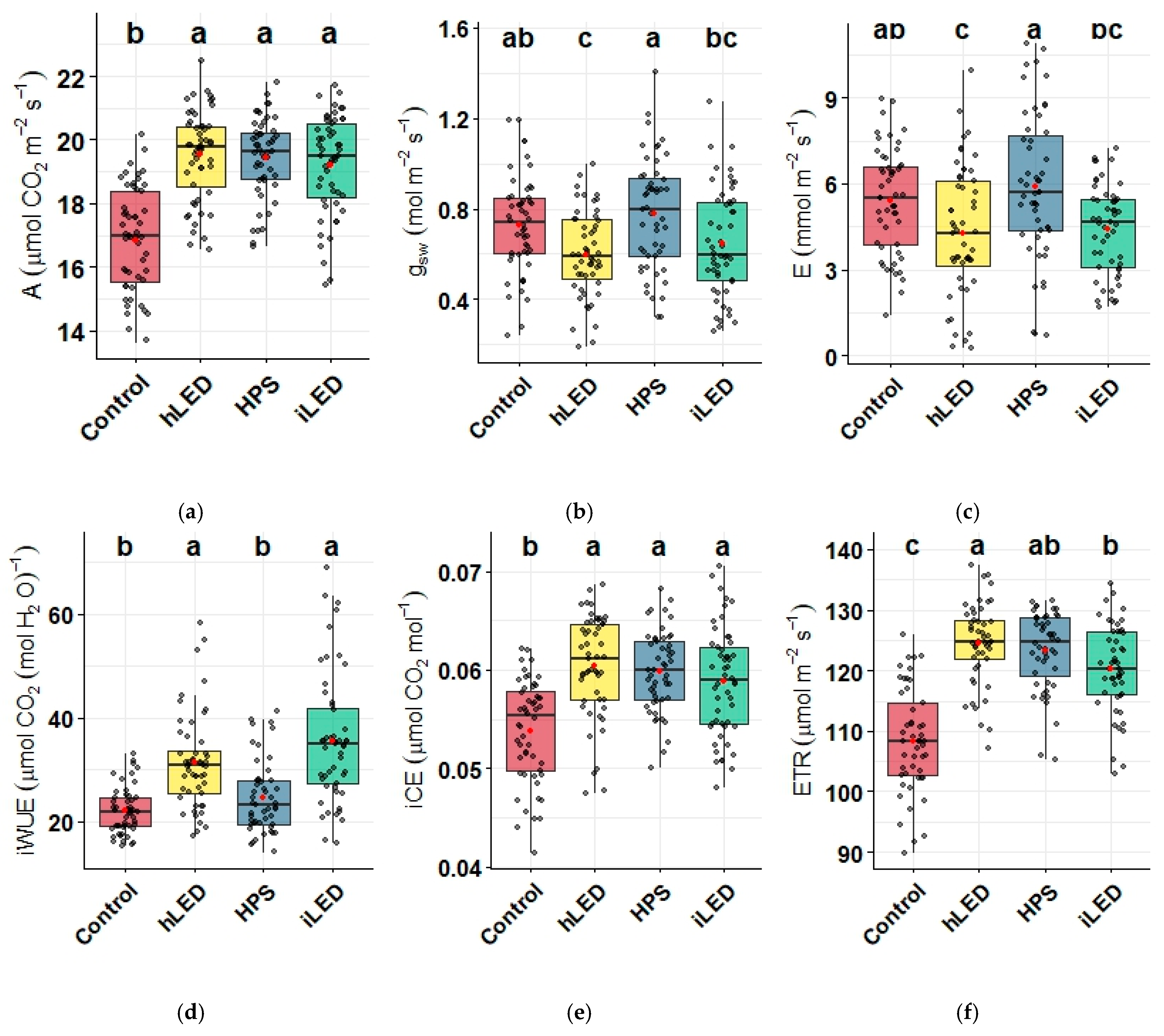
2.3. Photosynthetic Parameters
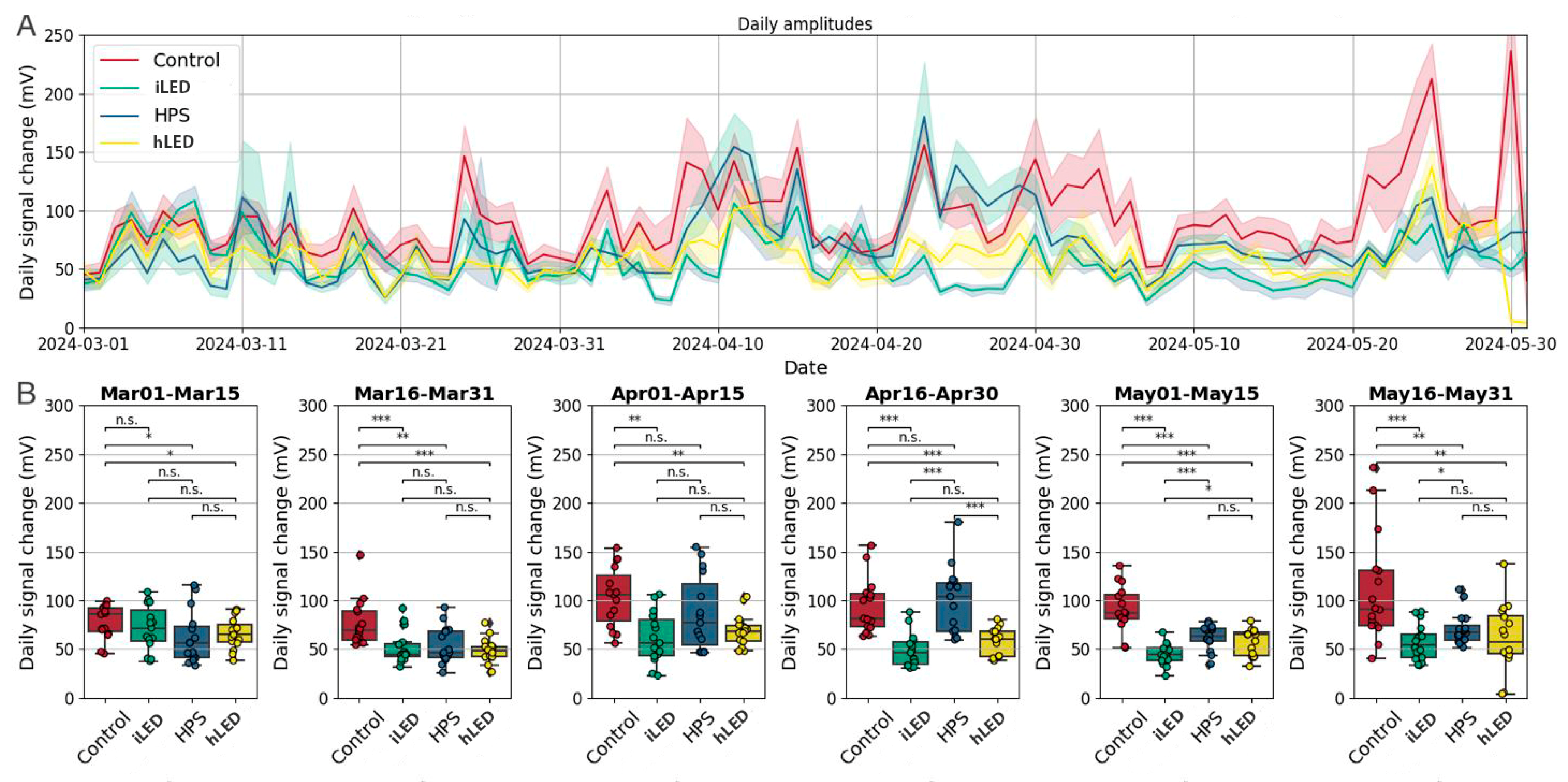
2.4. Plant Stress Response
2.5. Economic and Environmental Sustainability
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Greenhouse Conditions
4.2. Experimental Design
4.3. Morphological Measurments
4.4. Plant Yield and Fruit Quality Analysis
4.5. Photosynthetic Measurments
4.6. Electrophysiology Measurements
4.7. Energy Use Efficiency and Carbon Emissions
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roser, M.; Ritchie, H. Two Centuries of Rapid Global Population Growth Will Come to an End. Available online: https://ourworldindata.org/world-population-growth-past-future (accessed on 6 January 2025).
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture-Systems at Breaking Point: Synthesis Report; Agriculture Organization of T.U.N.: Rome, Italy, 2021. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, A.; Newman, L.; Graham, T.; Fraser, E.D.G. Exploring the Landscape of Controlled Environment Agriculture Research: A Systematic Scoping Review of Trends and Topics. Agric. Syst. 2023, 209, 103673. [Google Scholar] [CrossRef]
- Neo, D.C.J.; Ong, M.M.X.; Lee, Y.Y.; Teo, E.J.; Ong, Q.; Tanoto, H.; Xu, J.; Ong, K.S.; Suresh, V. Shaping and Tuning Lighting Conditions in Controlled Environment Agriculture: A Review. ACS Agric. Sci. Technol. 2022, 2, 3–16. [Google Scholar] [CrossRef]
- Appolloni, E.; Paucek, I.; Pennisi, G.; Stringari, G.; Gabarrell Durany, X.; Orsini, F.; Gianquinto, G. Supplemental LED Lighting Improves Fruit Growth and Yield of Tomato Grown under the Sub-Optimal Lighting Condition of a Building Integrated Rooftop Greenhouse (i-RTG). Horticulturae 2022, 8, 771. [Google Scholar] [CrossRef]
- Eylands, N.J.; Mattson, N.S. Influence of Far-Red Intensity during the Seedling Stage on Biomass Production and Photomorphogenic Characteristics in Leafy Greens under Sole-Source Lighting. Horticulturae 2023, 9, 1100. [Google Scholar] [CrossRef]
- Hamedalla, A.M.; Ali, M.M.; Ali, W.M.; Ahmed, M.A.A.; Kaseb, M.O.; Kalaji, H.M.; Gajc-Wolska, J.; Yousef, A.F. Increasing the Performance of Cucumber (Cucumis sativus L.) Seedlings by LED Illumination. Sci. Rep. 2022, 12, 852. [Google Scholar] [CrossRef]
- Tarakanov, I.G.; Tovstyko, D.A.; Lomakin, M.P.; Shmakov, A.S.; Sleptsov, N.N.; Shmarev, A.N.; Litvinskiy, V.A.; Ivlev, A.A. Effects of Light Spectral Quality on Photosynthetic Activity, Biomass Production, and Carbon Isotope Fractionation in Lettuce, Lactuca sativa L., Plants. Plants 2022, 11, 441. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Sim, H.S.; Kim, J.H.; Son, K.H. Growth, Fruit Yield, and Bioactive Compounds of Cherry Tomato in Response to Specific White-Based Full-Spectrum Supplemental LED Lighting. Horticulturae 2022, 8, 319. [Google Scholar] [CrossRef]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for Photons, Physiology and Food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, J.; Liu, M.; Li, Y.; Zheng, J. Effects of Different LED Spectra on the Antioxidant Capacity and Nitrogen Metabolism of Chinese Cabbage (Brassica rapa L. Ssp. Pekinensis). Plants 2024, 13, 2958. [Google Scholar] [CrossRef] [PubMed]
- Katzin, D.; Marcelis, L.F.M.; van Mourik, S. Energy Savings in Greenhouses by Transition from High-Pressure Sodium to LED Lighting. Appl. Energy 2021, 281, 116019. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for Energy Efficient Greenhouse Lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light Emitting Diode (LED) Lights for the Improvement of Plant Performance and Production: A Comprehensive Review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Economic Analysis of Greenhouse Lighting: Light Emitting Diodes vs. High Intensity Discharge Fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Olewnicki, D.; Mirgos, M.; Gajc-Wolska, J. Comparison of Selected Costs in Greenhouse Cucumber Production with LED and HPS. Agronomy 2020, 10, 1342. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and Control of Horticultural Plant Traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Spall, C.E.; Lopez, R.G. Supplemental Lighting Quality Influences Time to Flower and Finished Quality of Three Long-Day Specialty Cut Flowers. Horticulturae 2023, 9, 73. [Google Scholar] [CrossRef]
- Bugbee, B. Toward an Optimal Spectral Quality for Plant Growth and Development: The Importance of Radiation Capture. Acta Hortic. 2016, 1134, 1134. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Statistics Canada. Table 32-10-0456-01 Production and Value of Greenhouse Fruits and Vegetables. Geography: Alberta. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210045601 (accessed on 8 June 2024).
- Average Electricity Price for Farm Use (Excluding Service or Delivery Charge) by the End of May 2024 per 100 kwh. Available online: https://www.agric.gov.ab.ca/app21/farminputprices (accessed on 8 June 2024).
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of Red and Blue Light on Cucumber Seedlings Grown in a Plant Factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Wu, B.S.; Hitti, Y.; MacPherson, S.; Orsat, V.; Lefsrud, M.G. Comparison and Perspective of Conventional and LED Lighting for Photobiology and Industry Applications. Environ. Exp. Bot. 2020, 171, 103953. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Cheng, R.; Kaiser, E.; Yang, Q.; Li, T. Acclimating Cucumber Plants to Blue Supplemental Light Promotes Growth in Full Sunlight. Front. Plant Sci. 2021, 12, 782465. [Google Scholar] [CrossRef]
- Dutta Gupta, S. Light Emitting Diodes for Agriculture: Smart Lighting; Springer Nature: Singapore, 2017. [Google Scholar] [CrossRef]
- Brini, F.; Mseddi, K.; Brestic, M.; Landi, M. Hormone-Mediated Plant Responses to Light Quality and Quantity. Environ. Exp. Bot. 2022, 202, 105026. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Carbon Assimilation in Crops at High Temperatures. Plant Cell Environ. 2019, 42, 2750–2758. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves Are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Zhang, N.; van Westreenen, A.; He, L.; Evers, J.B.; Anten, N.P.R.; Marcelis, L.F.M. Light from below Matters: Quantifying the Consequences of Responses to Far-Red Light Reflected Upwards for Plant Performance in Heterogeneous Canopies. Plant Cell Environ. 2021, 44, 102–113. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, Z.; Marcelis, L.F.M.; Heuvelink, E.; Yang, Q.; Kaiser, E. Green Light Is Similarly Effective in Promoting Plant Biomass as Red/Blue Light: A Meta-Analysis. J. Exp. Bot. 2024, 75, 5655–5666. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Q.; Zheng, Y.; Xu, Y.; Liu, B.; Li, Q. Effects of Red and Blue Light on the Growth, Photosynthesis, and Subsequent Growth under Fluctuating Light of Cucumber Seedlings. Plants 2024, 13, 1668. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light Added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis Sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Tian, S.; Liang, Z.; Li, S.; Li, Y.; Wei, M.; Zhang, D. Effect of Supplemental Lighting on Water Transport, Photosynthetic Carbon Gain and Water Use Efficiency in Greenhouse Tomato. Sci. Hortic. 2019, 256, 108630. [Google Scholar] [CrossRef]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the Blue Light Photoreceptor Cryptochrome 2 in Tomato Affects Vegetative Development, Flowering Time, and Fruit Antioxidant Content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef]
- Pech, R.; Volná, A.; Špunda, V.; Nezval, J. Blue Light as an Important Factor Increasing Plant Tolerance to Acute Photooxidative Stress. Environ. Exp. Bot. 2024, 226, 105923. [Google Scholar] [CrossRef]
- Borbély, P.; Gasperl, A.; Pálmai, T.; Ahres, M.; Asghar, M.A.; Galiba, G.; Müller, M.; Kocsy, G. Light Intensity-and Spectrum-Dependent Redox Regulation of Plant Metabolism. Antioxidants 2022, 11, 1311. [Google Scholar] [CrossRef]
- Jiménez-Viveros, Y.; Núñez-Palenius, H.G.; Fierros-Romero, G.; Valiente-Banuet, J.I. Modification of Light Characteristics Affect the Phytochemical Profile of Peppers. Horticulturae 2023, 9, 72. [Google Scholar] [CrossRef]
- Yavari, N.; Tripathi, R.; Wu, B.S.; MacPherson, S.; Singh, J.; Lefsrud, M. The Effect of Light Quality on Plant Physiology, Photosynthetic, and Stress Response in Arabidopsis Thaliana Leaves. PLoS ONE 2021, 16, e0247380. [Google Scholar] [CrossRef]
- Nicholsa, M. LEDs (Light Emitting Diodes) in Horticulture. Acta Hortic. 2017, 1176, 23–24. [Google Scholar] [CrossRef]
- Iddio, E.; Wang, L.; Thomas, Y.; McMorrow, G.; Denzer, A. Energy Efficient Operation and Modeling for Greenhouses: A Literature Review. Renew. Sustain. Energy Rev. 2020, 117, 109480. [Google Scholar] [CrossRef]
- Available online: https://rijkzwaanusa.com/cucumber/VERDON-RZ-F1-24-150-prdCS10684-ctgCrops.cucumber (accessed on 8 June 2024).
- Campbell, G.S.; Norman, J.M. The Description and Measurement of Plant Canopy Structure. In Plant Canopies: Their Growth Form Function; Cambridge University Press: Cambridge, UK, 1990; pp. 1–19. [Google Scholar]
- CFIA, A.C.F.I. Grades and Requirements for Greenhouse Long Seedless Cucumbers. Available online: https://inspection.canada.ca/en/about-cfia/acts-and-regulations/list-acts-and-regulations/documents-incorporated-reference/canadian-grade-compendium-volume-2#s27c3 (accessed on 8 June 2024).
- Tran, D.; Dutoit, F.; Najdenovska, E.; Wallbridge, N.; Plummer, C.; Mazza, M.; Raileanu, L.E.; Camps, C. Electrophysiological Assessment of Plant Status Outside a Faraday Cage Using Supervised Machine Learning. Sci. Rep. 2019, 9, 17073. [Google Scholar] [CrossRef] [PubMed]
- A Consumption Intensity Indicator Is Derived to Reflect the GHG Emission Intensity of Electricity as It Is Delivered to the Consumer. Available online: https://www.canada.ca/en/environment-climate-change/services/climate-change/pricing-pollution-how-it-will-work/output-based-pricing-system/federal-greenhouse-gas-offset-system/emission-factors-reference-values.html (accessed on 8 June 2024).
- Available online: https://www.canada.ca/en/revenue-agency/services/forms-publications/publications/fcrates/fuel-charge-rates.html (accessed on 8 June 2024).
- The MathWorks Inc. MATLAB, Version: 9.13.0 (R2022b); The MathWorks Inc.: Natick, MA, USA, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
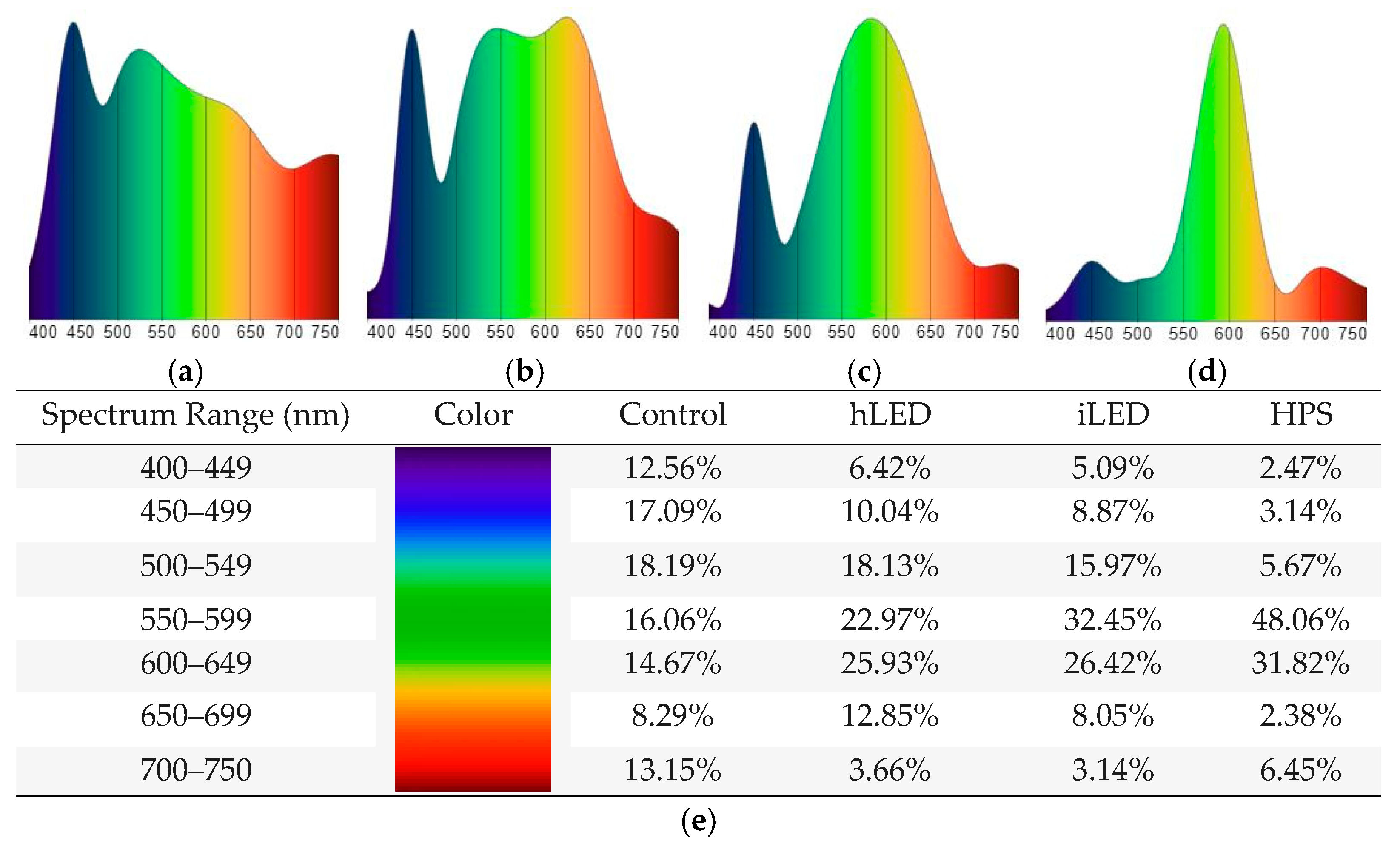
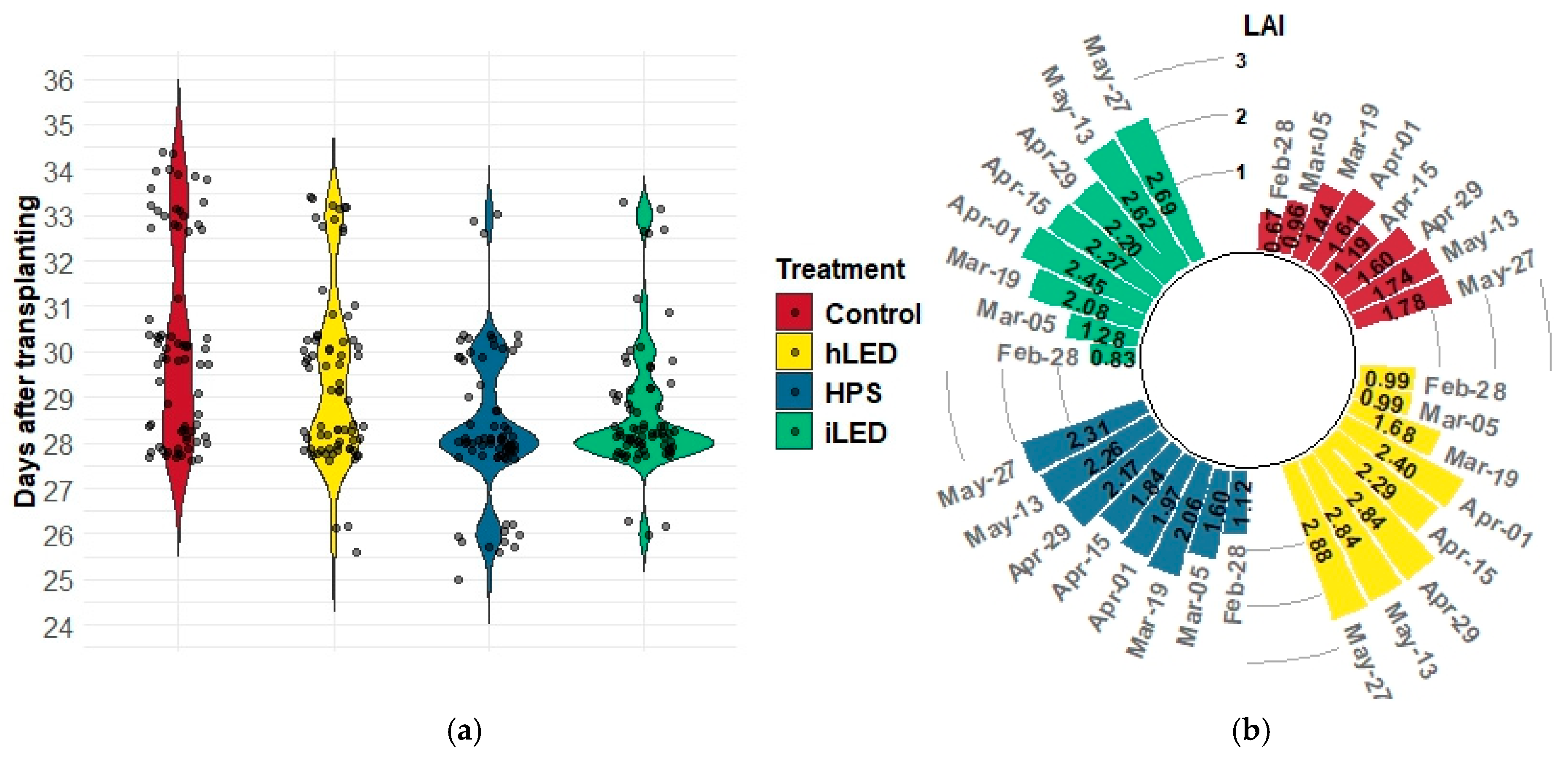

| Treatment | SE (cm) | SD (mm) | ID (cm) | LAI | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | Mean | Std | |
| Control | 881.21 a | 38.35 | 13.35 a | 1.10 | 9.99 a | 0.49 | 1.34 b | 0.61 |
| HPS | 871.67 a | 35.33 | 14.48 a | 1.14 | 9.54 b | 0.39 | 1.87 a | 0.62 |
| Industrial LED | 854.56 b | 43.38 | 14.26 b | 1.03 | 9.04 c | 0.57 | 1.95 a | 0.67 |
| Horticultural LED | 838.04 c | 37.64 | 13.67 b | 1.23 | 8.89 c | 0.45 | 2.10 a | 0.85 |
| Parameters | Light Type | ||
|---|---|---|---|
| iLED | hLED | HPS | |
| Number of lights (Light m−2) | 0.69 | 0.28 | 0.62 |
| Plant density (Plant m−2) | 1.65 | 1.65 | 1.65 |
| Total hours of light application (h) | 2193.08 | 2193.08 | 2193.08 |
| Energy consumption (kWh m−2) | 378.31 | 465.39 | 634.53 |
| Carbon Emission (CO2e m−2) | 204.29 | 251.31 | 342.65 |
| * Energy saving compared to HPS lighting (%) | 40.38 | 26.65 | - |
| Initial light cost (CAD m−2) | 124.2 | 252.0 | 164.3 |
| ** Initial cost compared to iLED lighting (%) | - | 102.91 | 32.29 |
| Company warranted lifespan (h) | 50,000 | 50,000 | 24,000 |
| ** Pollution cost compared to iLED (CAD m−2) | - | 3.06 | 8.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi, S.M.; Kurenda, A.; Karatepe, S.; Savidov, N.A. Balancing Yield and Sustainability: A Comparative Analysis of Supplemental Lighting in Commercial-Scale Cucumber Cultivation. Horticulturae 2025, 11, 79. https://doi.org/10.3390/horticulturae11010079
Hashemi SM, Kurenda A, Karatepe S, Savidov NA. Balancing Yield and Sustainability: A Comparative Analysis of Supplemental Lighting in Commercial-Scale Cucumber Cultivation. Horticulturae. 2025; 11(1):79. https://doi.org/10.3390/horticulturae11010079
Chicago/Turabian StyleHashemi, Seyed Mohammad, Andrzej Kurenda, Selin Karatepe, and Nick A. Savidov. 2025. "Balancing Yield and Sustainability: A Comparative Analysis of Supplemental Lighting in Commercial-Scale Cucumber Cultivation" Horticulturae 11, no. 1: 79. https://doi.org/10.3390/horticulturae11010079
APA StyleHashemi, S. M., Kurenda, A., Karatepe, S., & Savidov, N. A. (2025). Balancing Yield and Sustainability: A Comparative Analysis of Supplemental Lighting in Commercial-Scale Cucumber Cultivation. Horticulturae, 11(1), 79. https://doi.org/10.3390/horticulturae11010079






