Leveraging Multispectral and 3D Phenotyping to Determine Morpho-Physiological Changes in Peppers Under Increasing Drought Stress Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Details
2.2. PhenoHort Phenotyping Platform Layout
2.3. Data Analysis
3. Results
3.1. Greenhouse Climatic Conditions
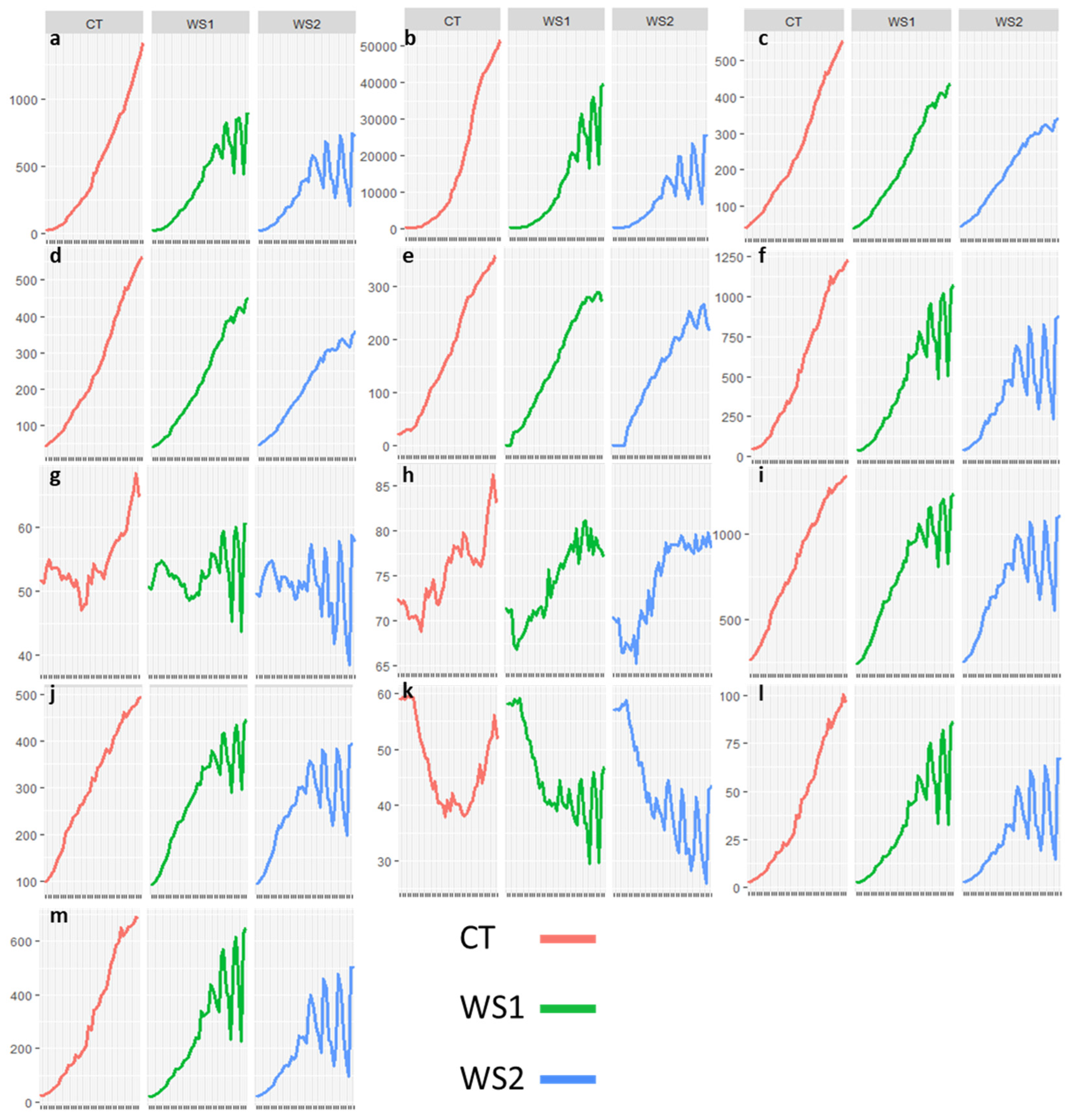
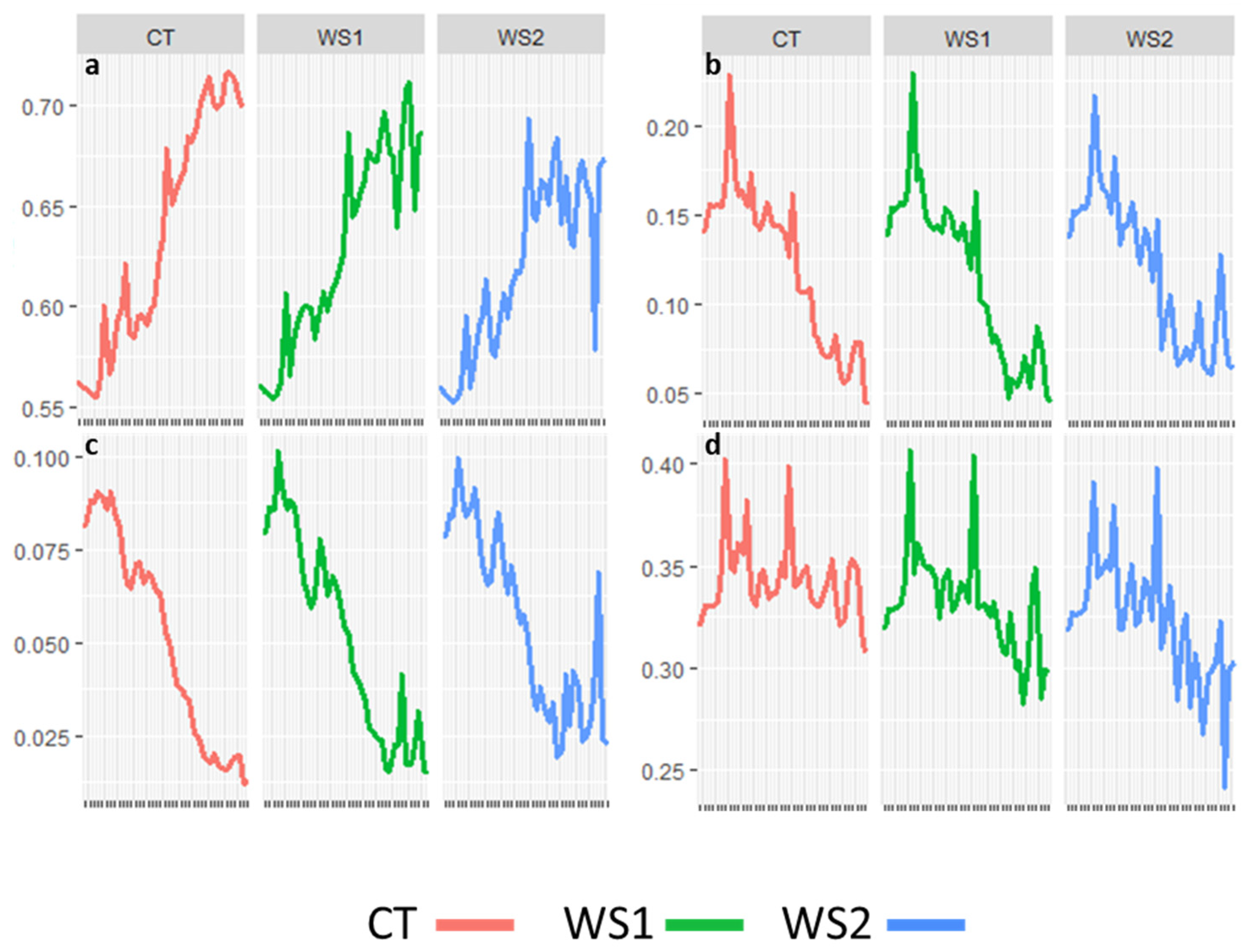
3.2. Phenotypic Variation Across Trials
3.3. Genotype by Trait Interaction and Genotypic Variation
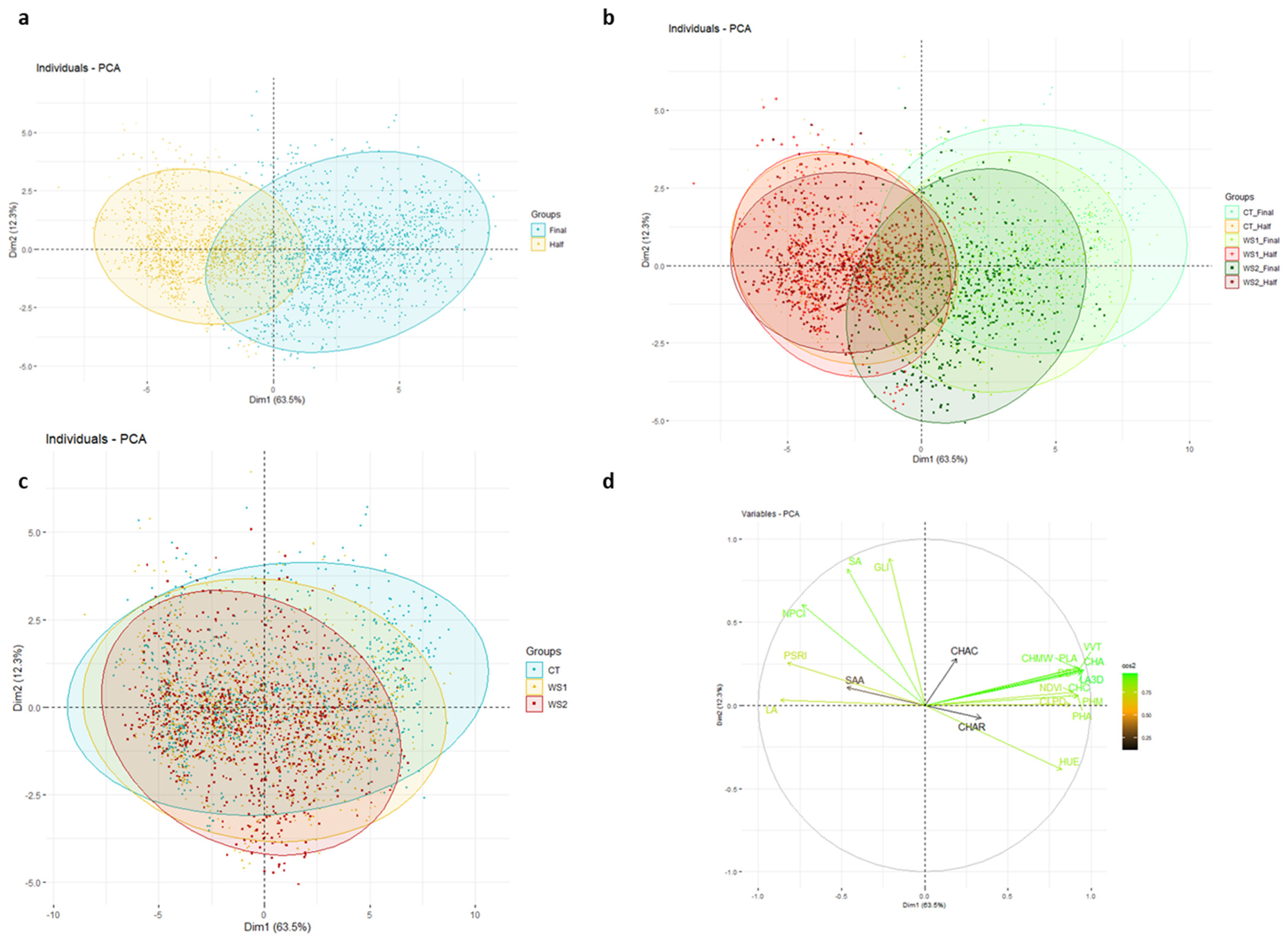
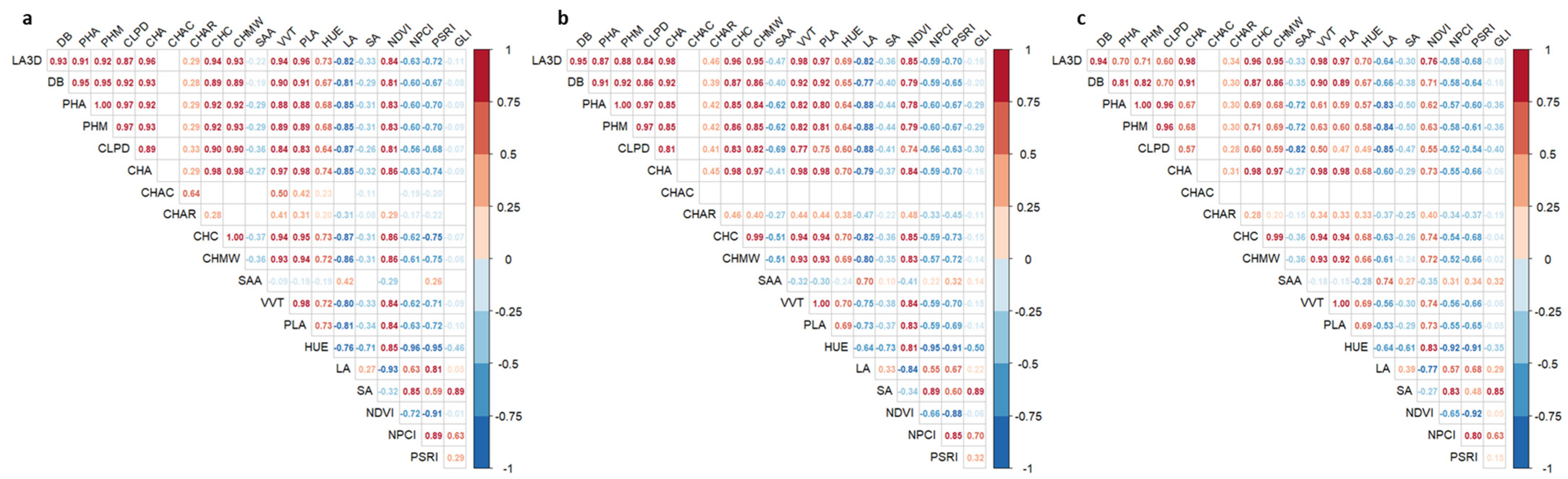
3.4. Multivariate Analysis
4. Discussion
4.1. Potentiality of PhenoHort for Morpho-Physiological Traits and Stress Indices Assessment
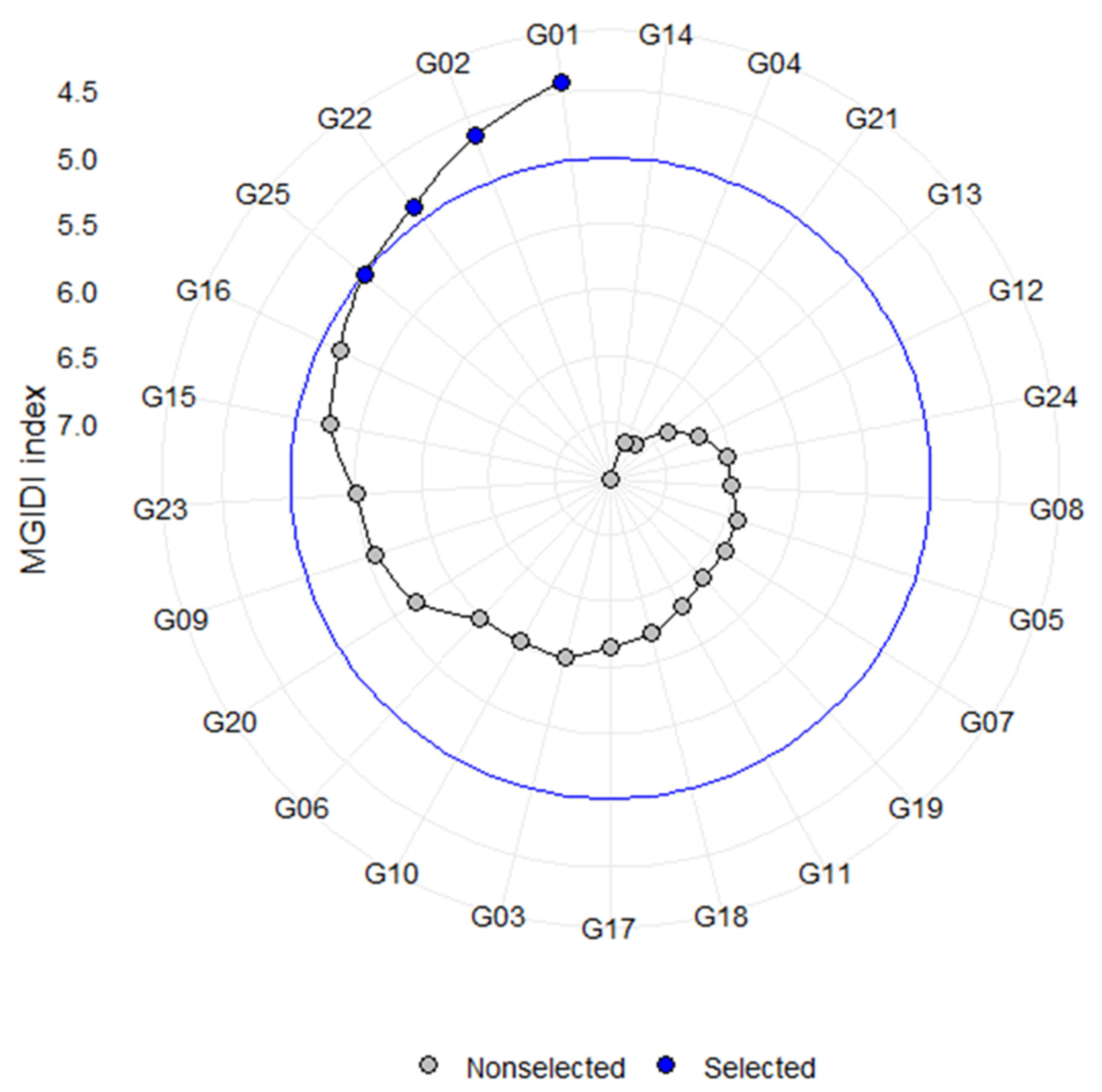
4.2. Identification of Best Accessions Resilient to Water Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. 2025. Available online: http://www.fao.org/faostat/en/#home (accessed on 27 September 2025).
- Tripodi, P.; Kumar, S. The Capsicum Crop: An introduction. In The Capsicum Genome; Ramchiary, N., Kole, C., Eds.; Springer International Publishing: Basel, Switzerland, 2019. [Google Scholar]
- Agyemang Duah, S.; Silva e Souza, C.; Nagy, Z.; Pék, Z.; Neményi, A.; Daood, H.G.; Vinogradov, S.; Helyes, L. Effect of Water Supply on Physiological Response and Phytonutrient Composition of Chili Peppers. Water 2021, 13, 1284. [Google Scholar] [CrossRef]
- Fallik, E.; Alkalai-Tuvia, S.; Chalupowicz, D.; Zaaroor-Presman, M.; Offenbach, R.; Cohen, S.; Tripler, E. How Water Quality and Quantity Affect Pepper Yield and Postharvest Quality. Horticulturae 2019, 5, 4. [Google Scholar] [CrossRef]
- Wang, L.; Ren, W. Drought in Agriculture and Climate-Smart Mitigation Strategies. Cell Rep. Sustain. 2025, 2, 100386. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Fourth Assessment Report: Climate Change 2007—Working Group II: Impacts, Adaptation and Vulnerability. Available online: https://archive.ipcc.ch/ipccreports/tar/vol4/english/114.htm (accessed on 25 September 2025).
- OECD-FAO (2023a). ‘OECD-FAO Agricultural Outlook 2023–2032’, Technical Report, OECD, and Food and Agriculture Organization of the United Nations). Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2023-2032_08801ab7-en (accessed on 30 October 2025).
- Brook, B.W.; Buettel, J.C.; Hong, S. Constrained scenarios for twenty-first century human population size based on the empirical coupling to economic growth. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Okur, B.; Örçen, N. Soil Salinization and Climate Change. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–350. ISBN 9780128180327. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Borràs, D.; Plazas, M.; Moglia, A.; Lanteri, S. The Influence of Acute Water Stresses on the Biochemical Composition of Bell Pepper (Capsicum annuum L.) Berries. J. Sci. Food Agric. 2021, 101, 4724–4734. [Google Scholar] [CrossRef] [PubMed]
- Ntanasi, T.; Karavidas, I.; Savvas, D.; Spyrou, G.P.; Giannothanasis, E.; Consentino, B.B.; Papasotiropoulos, V.; Sabatino, L.; Ntatsi, G. Physiological and Yield Responses of Pepper (Capsicum annuum L.) Genotypes to Drought Stress. Plants 2025, 14, 1934. [Google Scholar] [CrossRef]
- Thin, K.K.; Lee, S.; Lee, J.M. Genetic and morpho-physiological attributes of drought resistance in Capsicum accessions. Hort. Plant J. 2025, in press. [Google Scholar] [CrossRef]
- Ahmadi, S.Z.; Zahedi, B.; Ghorbanpour, M.; Mumivand, H. Comparative morpho-physiological and biochemical responses of Capsicum annuum L. plants to multi-walled carbon nanotubes, fullerene C60 and graphene nanoplatelets exposure under water deficit stress. BMC Plant Biol. 2024, 24, 116. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Martín-García, R.; Bažon Zidarić, I.; Pascual-Seva, N.; Pascual, B.; Padilla, Y.G.; Calatayud, Á.; López-Galarza, S. A Water Stress-Tolerant Pepper Rootstock Improves the Behavior of Pepper Plants under Deficit Irrigation through Root Biomass Distribution and Physiological Adaptation. Horticulturae 2023, 9, 362. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Atefi, A.; Ge, Y.; Pitla, S.; Schnable, J.C. Robotic Technologies for High-Throughput Plant Phenotyping: Contemporary Reviews and Future Perspectives. Front. Plant Sci. 2021, 12, 611940. [Google Scholar] [CrossRef]
- Bethge, H.L.; Weisheit, I.; Dortmund, M.S.; Landes, T.; Zabic, M.; Linde, M.; Debener, T.; Heinemann, D. Automated Image Registration of RGB, Hyperspectral and Chlorophyll Fluorescence Imaging Data. Plant Methods 2024, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, P.; Nicastro, N.; Pane, C. Digital applications and artificial intelligence in agriculture toward next-generation plant phenotyping. Crop Pasture Sci. 2022, 74, 597–614. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.; Guo, X.; Yang, W.; Zhao, C. High-Throughput Phenotyping: Breaking through the Bottleneck in Future Crop Breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Angidi, S.; Madankar, K.; Tehseen, M.M.; Bhatla, A. Advanced High-Throughput Phenotyping Techniques for Managing Abiotic Stress in Agricultural Crops—A Comprehensive Review. Crops 2025, 5, 8. [Google Scholar] [CrossRef]
- Xu, R.; Li, C. A Review of High-Throughput Field Phenotyping Systems: Focusing on Ground Robots. Plant Phenomics 2022, 2022, 9760269. [Google Scholar] [CrossRef] [PubMed]
- Phenospex. FieldScan: High-Throughput Field Phenotyping. Available online: https://phenospex.com/products/plant-phenotyping/fieldscan-high-throughput-field-phenotyping (accessed on 25 September 2025).
- Abebe, A.M.; Kim, Y.; Kim, J.; Kim, S.L.; Baek, J. Image-Based High-Throughput Phenotyping in Horticultural Crops. Plants 2023, 12, 2061. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.; Hong, S.; Kim, S.L.; Baek, J.-H.; Kim, K.-H. High-Throughput Phenotyping Methods for Breeding Drought-Tolerant Crops. Int. J. Mol. Sci. 2021, 22, 8266. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.; Chee, P.W.; Paterson, A.H.; Meng, C.; Zhang, J.; Adhikari, J. High Resolution 3D Terrestrial LiDAR for Cotton Plant Main Stalk and Node Detection. Comput. Electron. Agric. 2021, 187, 106276. [Google Scholar] [CrossRef]
- IPPN Infrastructure. Available online: https://www.plant-phenotyping.org/db_infrastructure#/tool/182/ (accessed on 27 September 2025).
- Tripodi, P.; Vincenzo, C.; Venezia, A.; Cocozza, A.; Pane, C. Precision Phenotyping of Wild Rocket (Diplotaxis tenuifolia) to Determine Morpho-Physiological Responses under Increasing Drought Stress Levels Using the PlantEye Multispectral 3D System. Horticulturae 2024, 10, 496. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 30 October 2025).
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extract and visualize the results of multivariate data analyses. RCRAN 2017, 76, 1–74. [Google Scholar]
- Factormine Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
- Wei, T.S.V. R Package ‘Corrplot’: Visualization of a Correlation Matrix; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Olivoto, T.; Nardino, M. MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics 2021, 37, 1383–1389. [Google Scholar] [CrossRef]
- Fu, Q.S.; Zhao, B.; Wang, Y.J.; Ren, S.; Guo, Y.D. Stomatal development and associated photosynthetic performance of capsicum in response to differential light availabilities. Photosynthetica 2010, 48, 189–198. [Google Scholar] [CrossRef]
- Egea-Gilabert, C.; Pagnotta, M.A.; Tripodi, P. Genotype × Environment Interactions in Crop Breeding. Agronomy 2021, 11, 1644. [Google Scholar] [CrossRef]
- Maphosa, L.; Thoday-Kennedy, E.; Vakani, J.; Phelan, A.; Badenhorst, P.; Slater, A.; Spangenberg, G.; Kant, S. Phenotyping wheat under salt stress conditions using a 3D laser scanner. Isr. J. Plant Sci. 2017, 64, 55–62. [Google Scholar] [CrossRef]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Front. Plant Sci. 2021, 12, 629441. [Google Scholar] [CrossRef]
- Cappello Fusaro, M.; Lucchetta, I.; Bona, S. Water Stress Effects on Biomass Allocation and Secondary Metabolism in CBD-Dominant Cannabis sativa L. Plants 2025, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Schafleitner, R.; Lin, C.-Y.; Lin, Y.-P.; Wu, T.-H.; Hung, C.-H.; Phooi, C.-L.; Chu, S.-H.; Jhong, Y.-C.; Hsiao, Y.-Y. The World Vegetable Center Okra (Abelmoschus esculentus) Core Collection as a Source for Flooding Stress Tolerance Traits for Breeding. Agriculture 2021, 11, 165. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Ramamoorthy, P.; Poudel, S.; Samiappan, S.; Wijewardane, N.; Reddy, K.R. Effects of drought and heat stresses during reproductive stage on pollen germination, yield, and leaf reflectance properties in maize (Zea mays L.). Plant Direct 2022, 6, e434. [Google Scholar] [CrossRef]
- Slaton, M.R.; Hunt, E.R.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef]
- Index Database. Available online: https://www.indexdatabase.de/ (accessed on 27 September 2025).
- Anderegg, J.; Yu, K.; Aasen, H.; Walter, A.; Liebisch, F.; Hund, A. Spectral Vegetation Indices to Track Senescence Dynamics in Diverse Wheat Germplasm. Front. Plant Sci. 2020, 10, 1749. [Google Scholar] [CrossRef]
- Govind, A.; Bhavanarayana, M.; Kumari, J.; Govind, A. Efficacy of Different Indices Derived from Spectral Reflectance of Wheat for Nitrogen Stress Detection. J. Plant Interact. 2005, 1, 93–105. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Value of using different vegetative indices to quantify agricultural crop chateristics at different growth stages under varying management practices. Remote Sens. 2010, 2, 562–578. [Google Scholar] [CrossRef]
- Shukla, S.; Felderhoff, T.J.; Saballos, A.; Vermerris, W. The relationship between plant height and sugar accumulation in the stems of sweet sorghum (Sorghum bicolor (L.) Moench). Field Crops Res. 2017, 203, 181–191. [Google Scholar] [CrossRef]
- McLeod, L.; Barchi, L.; Tumino, G.; Tripodi, P.; Salinier, J.; Gros, C.; Boyaci, H.F.; Ozalp, R.; Borovsky, Y.; Schafleitner, R.; et al. Multi-environment association study highlights candidate genes for robust agronomic quantitative trait loci in a novel worldwide Capsicum core collection. Plant J. 2023, 116, 1508–1528. [Google Scholar] [CrossRef]
- Miao, L.; Wang, X.; Yu, C.; Ye, C.; Yan, Y.; Wang, H. What factors control plant height? J. Integr. Agric. 2024, 23, 1803–1824. [Google Scholar] [CrossRef]





| Acronym | Trait | Measurement Unit |
|---|---|---|
| Morphological parameters | ||
| LA3D | Three-dimensional leaf area | mm2 |
| DB | Digital biomass | mm2 |
| PHA | Plant height averaged | mm |
| PHM | Plant height max | mm |
| CLPD | Canopy light penetration depth | mm |
| CHA | Convex hull area | mm2 |
| CHAC | Convex hull area coverage | % |
| CHAR | Convex hull aspect ratio | index |
| CHC | Convex hull circumference | mm |
| CHMW | Convex hull maximum width | mm |
| SAA | Surface angle average | A° |
| VVT | Voxel volume total | mm3 |
| PLA | Projected leaf area | mm2 |
| Color and multispectral | ||
| HUE | Hue average | ° |
| LA | Lightness average | % |
| SA | Saturation average | % |
| Vegetation indices | ||
| NDVI | Normalized differential vegetation index | index |
| NPCI | Normalized pigment chlorophyll ratio index | index |
| PSRI | Plant senescence reflection index | index |
| GLI | Green leaf index average | index |
| CT (dd 20) | WS1 (dd 20) | WS2 (dd 20) | CT (dd 40) | WS1 (dd 40) | WS2 (dd 40) | |
|---|---|---|---|---|---|---|
| LA3D (cm2) | 168.42 | 153.08 | 169.22 | 884.25 | 650.11 * | 495.34 * |
| (13.77–774.60) | (0.00–721.59) | (14.93–880.49) | (259.81–1861.65) | (194.77–1374.54) | (102.82–1099.29) | |
| DB (cm3) | 2826.79 | 2368.39 * | 2680.65 | 33218.78 | 23256.55 * | 14635.05 * |
| (36.478–21,555.87) | (0.00–19,974.47) | (45.754–18,800.05) | (6248.753–73,256.43) | (3032.527–66,919.20) | (2433.45–47,261.80) | |
| PHA (mm) | 119.42 | 106.86 * | 112.77 | 400.17 | 338.74 * | 287.27 * |
| (20.92–370.50) | (0.00–321.73) | (27.15–304.88) | (151.96–720.84) | (147.53–577.29) | (94.04–480.72) | |
| PHM (mm) | 123.08 | 110.51 * | 116.56 | 411.00 | 350.05 * | 297.77 * |
| (21.16–372.43) | (0.00–326.49) | (27.68–310.39) | (157.66–730.54) | (154.62–588.08) | (97.34–499.20) | |
| CLPD (mm) | 75.69 | 70.10 | 74.63 | 272.29 | 238.67 * | 213.26 * |
| (10.12–242.13) | (4.48–209.04) | (10.61–217.06) | (89.53–543.00) | (93.24–466.29) | (65.59–378.98) | |
| CHA (mm2) | 226.42 | 207.76 | 221.35 | 939.36 | 754.23 * | 571.35 * |
| (23.94–780.46) | (0.00–803.75) | (20.60–941.79) | (326.33–1570.95) | (222.49–1567.52) | (100.98–1315.57) | |
| CHAC (%) | 51.65 | 51.82 | 51.62 | 54.99 | 54.33 * | 50.68 * |
| (17.43–82.38) | (23.97–91.54) | (25.23–73.18) | (26.70–72.35) | (21.82–71.18) | (23.25–70.95) | |
| CHAR (index) | 72.07 | 70.01 * | 71.11 | 79.22 | 77.85 | 78.41 |
| (20.89–89.57) | (38.88–97.51) | (35.78–87.75) | (58.41–97.96) | (53.06–92.14) | (54.04–93.70) | |
| CHC (mm) | 550.41 | 521.31 * | 536.73 | 1151.30 | 1024.52 * | 870.54 * |
| (179.90–1063.62) | (0.00–1079.76) | (180.30–1179.04) | (719.58–1531.93) | (543.03–1528.68) | (389.05–1375.63) | |
| CHMW (mm) | 205.47 | 195.60 | 200.56 | 416.60 | 366.86 * | 310.90 * |
| (65.31–406.37) | (0.00–388.52) | (65.82–425.84) | (252.09–569.98) | (186.32–563.37) | (141.03–497.91) | |
| SAA (A°) | 49.88 | 49.78 | 48.46 * | 42.22 | 40.04 * | 36.19 * |
| (29.32–66.74) | (29.63–66.03) | (26.41–65.61) | (27.55–65.96) | (17.86–64.85) | (17.03–64.58) | |
| VVT (cm3) | 15.17 | 13.84 | 15.00 | 69.24 | 56.52* | 41.46 * |
| (1.68–54.89) | (0.00–56.71) | (1.71–69.49) | (20.46–120.66) | (13.04–128.06) | (6.66–110.87) | |
| PLA (cm2) | 113.77 | 103.93 | 112.39 | 518.65 | 421.55 * | 306.96 * |
| (12.03–409.77) | (0.00–425.19) | (12.38–537.27) | (150.25–916.95) | (73.91–987.18) | (37.17–859.85) | |
| HUE (°) | 107.53 | 107.80 | 107.59 | 115.14 | 114.87 | 113.79 * |
| (97.95–118.52) | (95.93–122.15) | (97.48–117.88) | (102.30–127.25) | (103.93–126.78) | (99.01–127.02) | |
| LA (%) | 9.47 | 9.40 | 9.43 | 6.28 | 6.51 * | 6.58 * |
| (6.48–12.67) | (7.10–12.45) | (6.46–12.64) | (4.29–11.46) | (4.48–9.21) | (4.23–9.63) | |
| SA (%) | 44.97 | 44.87 | 45.27 | 42.88 | 41.42 * | 41.16 * |
| (36.32–59.86) | (34.89–59.44) | (38.65–56.71) | (32.15–57.18) | (32.07–60.77) | (34.03–55.53) | |
| NDVI | 0.585 | 0.587 | 0.581 | 0.689 | 0.67 * | 0.65 * |
| (0.444–0.679) | (0.470–0.651) | (0.437–0.684) | (0.528–0.755) | (0.488–0.747) | (0.470–0.763) | |
| NPCI | 0.157 | 0.153 | 0.156 | 0.083 | 0.078 | 0.085 |
| (0.036–0.346) | (0.001–0.347) | (0.046–0.306) | (−0.031–0.287) | (−0.026–0.317) | (−0.029–0.251) | |
| PSRI | 0.075 | 0.075 | 0.079 | 0.025 | 0.027 | 0.034 * |
| (0.012–0.200) | (0.009–0.412) | (0.022–0.203) | (−0.013–0.123) | (−0.012–0.117) | (−0.016–0.168) | |
| GLI | 0.345 | 0.342 | 0.338 * | 0.340 | 0.320 * | 0.307 * |
| (0.275–0.449) | (0.240–0.436) | (0.261–0.443) | (0.242–0.444) | (0.228–0.474) | (0.194–0.442) |
| Trait | G (df = 24) | T (df = 2) | G × T (df = 48) | Error (df = 448) |
|---|---|---|---|---|
| TSS % | TSS % | TSS % | TSS % | |
| LA3D | 16.04 ** | 28.55 ** | 11.81 ** | 43.59 |
| DB | 13.29 ** | 51.02 ** | 12.50 ** | 23.19 |
| PHA | 31.23 ** | 50.61 ** | 12.28 ** | 5.88 |
| PHM | 30.02 ** | 50.75 ** | 12.37 ** | 6.86 |
| CLPD | 49.77 ** | 22.44 ** | 15.19 ** | 12.60 |
| CHA | 12.49 ** | 38.86 ** | 10.29 ** | 38.35 |
| CHAC | 31.99 ** | 3.43 ** | 12.41 ** | 52.17 |
| CHAR | 24.40 ** | 13.19 ** | 14.99 ** | 47.41 |
| CHC | 10.97 ** | 36.77 ** | 9.42 ** | 42.85 |
| CHMW | 11.73 ** | 39.08 ** | 8.84 ** | 40.35 |
| SAA | 32.03 ** | 15.02** | 6.01 NS | 46.94 |
| VVT | 15.89 ** | 29.86 ** | 11.06 ** | 43.19 |
| PLA | 16.39 ** | 28.56 ** | 10.76 ** | 44.29 |
| HUE | 58.27 ** | 7.76 * | 9.96 ** | 24.01 |
| LA | 40.32 ** | 11.57 ** | 20.18 ** | 27.93 |
| SA | 43.72 ** | 6.21 ** | 9.94 ** | 40.13 |
| NDVI | 19.98 ** | 23.30 ** | 16.10 ** | 40.63 |
| NPCI | 64.97 ** | 2.90 * | 9.11 ** | 23.02 |
| PSRI | 39.36 ** | 9.93 ** | 13.48 ** | 37.23 |
| GLI | 29.54 ** | 16.78 ** | 8.32 * | 45.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocozza, A.; Venezia, A.; Macellaro, R.; Di Cesare, C.; Milanesi, C.; Tripodi, P. Leveraging Multispectral and 3D Phenotyping to Determine Morpho-Physiological Changes in Peppers Under Increasing Drought Stress Levels. Horticulturae 2025, 11, 1318. https://doi.org/10.3390/horticulturae11111318
Cocozza A, Venezia A, Macellaro R, Di Cesare C, Milanesi C, Tripodi P. Leveraging Multispectral and 3D Phenotyping to Determine Morpho-Physiological Changes in Peppers Under Increasing Drought Stress Levels. Horticulturae. 2025; 11(11):1318. https://doi.org/10.3390/horticulturae11111318
Chicago/Turabian StyleCocozza, Annalisa, Accursio Venezia, Rosaria Macellaro, Carlo Di Cesare, Chiara Milanesi, and Pasquale Tripodi. 2025. "Leveraging Multispectral and 3D Phenotyping to Determine Morpho-Physiological Changes in Peppers Under Increasing Drought Stress Levels" Horticulturae 11, no. 11: 1318. https://doi.org/10.3390/horticulturae11111318
APA StyleCocozza, A., Venezia, A., Macellaro, R., Di Cesare, C., Milanesi, C., & Tripodi, P. (2025). Leveraging Multispectral and 3D Phenotyping to Determine Morpho-Physiological Changes in Peppers Under Increasing Drought Stress Levels. Horticulturae, 11(11), 1318. https://doi.org/10.3390/horticulturae11111318









