Storage Temperature Effect on Quality and Shelf-Life of Hericium erinaceus Mushroom
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom Sampling and Preparation
2.2. Respiration Measurements
2.3. Physico-Chemical Analysis Contenct
2.4. Color and Texture Analysis
2.5. Microbiology Analysis
2.6. Determination of Total Phenolic Content and Antioxidant Capacity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Respiration Measurement
3.2. Physicochemical Parameters Evaluation
3.3. Color and Texture
3.4. Microbiology Evaluation
3.5. Total Phenolic Content and Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karbowy-Thongbai, B.; Rapior, S.; Wittstein, K.; Hyde, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and Neuroprotective Effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Morales, P.; Fernández-Ruiz, V.; Ferreira, I.C.F.R. Chemical composition, antioxidant activity and bioaccessibility studies in phenolic extracts of two Hericium wild edible species. LWT-Food Sci. Technol. 2015, 63, 475–481. [Google Scholar] [CrossRef]
- Ba, D.M.; Gao, X.; Al-Shaar, L.; Muscat, J.E.; Chinchilli, V.M.; Beelman, R.B.; Richie, J.P. Mushroom intake and depression: A population-based study using data from the US National Health and Nutrition Examination Survey (NHANES), 2005–2016. J. Affect. Disord. 2021, 294, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, X.; Wang, Y.; Zhao, Y.; Wang, J. Polysaccharides from Hericium erinaceus and its immuno-modulatory effects on RAW 264.7 macrophages. Int. J. Biol. Macromol. 2024, 278, 134947. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, W.; Lee, D.-S.; Shim, S.H.; Kim, Y.-C.; Kim, Y.H. Hericirine, a novel anti-inflammatory alkaloid from Hericium erinaceum. Tetrahedron Lett. 2014, 55, 4086–4090. [Google Scholar] [CrossRef]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.E.; Reyes, J.E.; Rivera, C.S.; Oria, R.; Blanco, D. Microbiological quality and safety of fresh cultivated and wild mushrooms commercialized in Spain. Food Microbiol. 2011, 28, 1492–1498. [Google Scholar] [CrossRef]
- Qiu, W.; Huang, Y.; Zhao, C.; Lin, Z.; Lin, W.; Wang, Z. Microflora of fresh white button mushrooms (Agaricus bisporus) during cold storage revealed by high-throughput sequencing and MALDI-TOF mass spectrometry finger-printing. J. Sci. Food Agric. 2019, 99, 4498–4503. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Ramos, A.C.; Lidon, F.J.; Reboredo, F.H.; Gonçalves, E.M. Pre- and Postharvest Strategies for Pleurotus ostreatus Mushroom in a Circular Economy Approach. Foods 2024, 13, 1464. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Phillippoussis, A. Cultivated Mushrooms: Preservation and Processing. In Handbook of Vegetable Preservation and Processing; CRC Press: Boca Raton, FL, USA, 2015; pp. 516–547. [Google Scholar]
- Schill, S.; Stessl, B.; Meier, N.; Tichy, A.; Wagner, M.; Ludewig, M. Microbiological safety and sensory quality of culti-vated mushrooms (Pleurotus eryngii, Pleurotus ostreatus, and Lentinula edodes) at retail level and post-retail storage. Foods 2021, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Im, J.-H.; Yu, H.-W.; Park, C.-H.; Kim, J.-W.; Shin, J.-H.; Jang, K.-Y.; Park, Y.-J. Phenylalanine Ammonia-Lyase: A Key Gene for Color Discrimination of Edible Mushroom Flammulina velutipes. J. Fungi 2023, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, S.; Kitazawa, H.; Wang, Y. Storage temperature effect on quality related with cell wall metabolism of shiitake mushrooms (Lentinula edodes) and its modeling. Food Packag. Shelf Life 2022, 32, 100865. [Google Scholar] [CrossRef]
- Iqbal, T.; Rodrigues, F.A.S.; Mahajan, P.V.; Kerry, J.P. Effect of time, temperature, and slicing on respiration rate of mushrooms. J. Food Sci. 2009, 74, 6. [Google Scholar] [CrossRef] [PubMed]
- NP 1421; Foodstuffs Derived from Fruits and Vegetables: Determination of Acidity. Instituto Português da Qualidade: Lisbon, Portugal, 1977.
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase Activity and Color of Blanched and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Castañón, X.; Argaiz, A.; López-Malo, A. Effect of storage temperature on the microbial and color stability of banana purée with addition of vanillin or potassium sorbate. Food Sci. Technol. Int. 1999, 5, 51–58. [Google Scholar] [CrossRef]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. ISO: Geneva, Switzerland, 2013.
- ISO 17410; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. ISO: Geneva, Switzerland, 2001.
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds. ISO: Geneva, Switzerland, 2008.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
- Wu, P.-R.; Hwang, S.-G.; Chen, C.-L.; Lin, H.-L. Effects of Storage Duration and Temperature on Browning and Quality of Postharvest Bamboo Shoots. Horticulturae 2024, 10, 616. [Google Scholar] [CrossRef]
- Zan, X.; Shao, Z.-Y.; Yang, Y.; Jia, W.; Sun, L.; Cui, F.-J.; Li, W.; Zhu, H.-A.; Sun, W.-J.; Zhang, J.-S. Improving overall postharvest quality of straw mushroom using an accessible and low-cost strategy. J. Food Compos. Anal. 2024, 128, 106036. [Google Scholar] [CrossRef]
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh mushroom preservation techniques. Foods 2021, 10, 2126. [Google Scholar] [CrossRef]
- Claassen, G.D.H.; Kirst, P.; Van, A.T.T.; Snels, J.C.M.A.; Guo, X.; van Beek, P. Integrating time-temperature dependent deterioration in the economic order quantity model for perishable products in multi-echelon supply chains. Omega 2024, 125, 103041. [Google Scholar] [CrossRef]
- Niu, Y.; Yun, J.; Bi, Y.; Wang, T.; Zhang, Y.; Liu, H.; Zhao, F. Predicting the shelf life of postharvest Flammulina velutipes at various temperatures based on mushroom quality and specific spoilage organisms. Postharvest Biol. Technol. 2020, 167, 111235. [Google Scholar] [CrossRef]
- Zheng, C.; Li, J.; Liu, H.; Wang, Y. Review of postharvest processing of edible wild-grown mushrooms. Food Res. Int. 2023, 173, 113223. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.M.; Simões, A.S.; Miranda, C.; Sales, H.; Pontes, R.; Nunes, J. Microbiological assessment of white button mushrooms with an edible film coating. Foods 2023, 12, 3061. [Google Scholar] [CrossRef]
- Bermúdez-Gómez, P.; Fernández-López, J.; Pérez-Clavijo, M.; Viuda-Martos, M. Evaluation of sample size influence on chemical characterization and in vitro antioxidant properties of flours obtained from mushroom stems coproducts. Antioxidants 2024, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; Ros-Chumillas, M.; Navarro-Martínez, A.; Barón, M.; Navarro-Segura, L.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Martínez-Hernández, G.B. Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life. Foods 2021, 10, 1196. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Oliveira, F.A.R.; Macedo, I. Effect of temperature and humidity on the transpiration rate of the whole mushrooms. J. Food Eng. 2008, 84, 281–288. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, L.; Xia, Q.; Yi, K.; Li, Y. Novel Post-Harvest Preservation Techniques for Edible Fungi: A Review. Foods 2024, 13, 1554. [Google Scholar] [CrossRef] [PubMed]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 108, 128–139. [Google Scholar] [CrossRef]
- Allegretti, O.; Travan, L.; Cividini, R. Drying techniques to obtain white beech. In Proceedings of the COST E53 Meeting: Quality Control for Wood and Wood Products, Bled, Slovenia, 21–23 April 2009; pp. 7–13. [Google Scholar]
- Suhaili, M.; Nor-Khaizura, M.-A.R.; Nur Hanani, Z.A.; Ismail-Fitry, M.R.; Samsudin, N.; Jambari, N. Assessment of microbiological safety and physicochemical changes of grey oyster mushroom (Pleurotus sajor-caju) during storage at 4 °C and 25 °C. Sains Malays. 2021, 50, 2993–3002. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Abdelazez, A.; Helal, M. Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus bisporus) during Storage. Coatings 2021, 11, 149. [Google Scholar] [CrossRef]
- Liufang, Y.; Wu, Y.; Zhou, H.; Qu, H.; Yang, H. Recent advances in the application of natural products for postharvest edible mushroom quality preservation. Foods 2024, 13, 2378. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, S.; Buescher, R.W.; Kim, K.S. Textural changes in mushrooms (Agaricus bisporus) associated with tissue ultrastructure and composition. J. Food Sci. 2000, 65, 1404–1408. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Wang, Y. Effect of alginate/nano-Ag coating on microbial and physicochemical characteristics of shiitake mushroom (Lentinus edodes) during cold storage. Food Chem. 2013, 141, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cui, Y.; Wang, X.; Cong, J.; Yu, J.; Yan, S.; Bai, J.; Xu, H.; Li, M. Electron-beam generated X-Ray irradiation could retard the senescence of postharvest Hericium erinaceus via regulating reactive oxygen metabolism. Food Biosci. 2024, 59, 104002. [Google Scholar] [CrossRef]
- Instituto Nacional de Saúde Doutor Ricardo Jorge. Interpretação de Resultados de Ensaios Microbiológicos em Alimentos Prontos para Consumo e em Superfícies do Ambiente de Preparação e Distribuição Alimentar: Valores-Guia; INSA IP: Lisbon, Portugal, 2019; Available online: https://repositorio.insa.pt//handle/10400.18/5610 (accessed on 20 November 2024).
- Simón, A.; González-Fandos, E.; Tobar, V. The sensory and microbiological quality of fresh sliced mushroom (Agaricus bisporus L.) packaged in modified atmospheres. Int. J. Food Sci. Technol. 2005, 40, 943–952. [Google Scholar] [CrossRef]
- Manthou, E.; Tarlak, F.; Lianou, A.; Ozdemir, M.; Zervakis, G.I.; Panagou, E.Z.; Nychas, G.-J.E. Prediction of indigenous Pseudomonas spp. growth on oyster mushrooms (Pleurotus ostreatus) as a function of storage temperature. LWT-Food Sci. Technol. 2019, 111, 506–512. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- González, G.C.; Rugolo, M.; Finimundy, T.C.; Ohaco, E.; Pildain, M.B.; Barroetaveña, C. Impact of Air- and Freeze-Drying Methods on Total Phenolic Content and Antioxidant Activity of Fistulina antarctica and Ramaria patagonica Fructification. Appl. Sci. 2023, 13, 8873. [Google Scholar] [CrossRef]
- Atila, F. Comparative evaluation of the antioxidant potential of Hericium erinaceus, Hericium americanum and Hericium coralloides. Acta Sci. Pol. Hortorum Cultus 2019, 18, 97–106. [Google Scholar] [CrossRef]
- Alkin, M.; Söğüt, E.; Seydim, A.C. Determination of bioactive properties of different edible mushrooms from Turkey. J. Food Meas. Charact. 2021, 15, 3608–3617. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szablewska, K.; Niedzielski, P.; Mleczek, M. The effect of drying temperature on bioactive compounds and antioxidant activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. J. Food Sci. Technol. 2020, 57, 513–525. [Google Scholar] [CrossRef]
- Song, T.; Zhang, Z.; Liu, S.; Chen, J.; Cai, W. Effect of Cultured Substrates on the Chemical Composition and Biological Activities of Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 1183–1190. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Bakir, T.; Karadeniz, M.; Unal, S. Investigation of antioxidant activities of Pleurotus ostreatus stored at different temperatures. Food Sci. Nutr. 2018, 6, 1040–1044. [Google Scholar] [CrossRef]
- Tan, X.; Sun, J.; Xu, Z.; Li, H.; Hu, J.; Ning, H.; Qin, Z.; Pei, H.; Sun, T.; Zhang, X. Effect of heat stress on production and in-vitro antioxidant activity of polysaccharides in Ganoderma lucidum. Bioprocess Biosyst. Eng. 2018, 41, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Şahin, H.; Malkoç, M. Wild edible mushrooms as a natural source of phenolics and antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Chun, S.; Gopal, J.; Muthu, M. Antioxidant activity of mushroom extracts/polysaccharides—Their antiviral properties and plausible anti-COVID-19 properties. Antioxidants 2021, 10, 1899. [Google Scholar] [CrossRef]
- Mishra, K.K.; Pal, R.S.; Bhatt, J.C. Comparison of antioxidant properties in cap and stipe of Lentinula edodes—A medicinal mushroom. Emir. J. Food Agric. 2015, 27, 562–569. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Ribeiro, B.; Lopes, R.; Andrade, P.B.; Seabra, R.M.; Gonçalves, R.F.; Baptista, P.; Quelhas, I.; Valentão, P.C. Comparative study of phytochemicals and antioxidant potential of wild edible mushroom caps and stipes. Food Chem. 2008, 110, 47–56. [Google Scholar] [CrossRef]

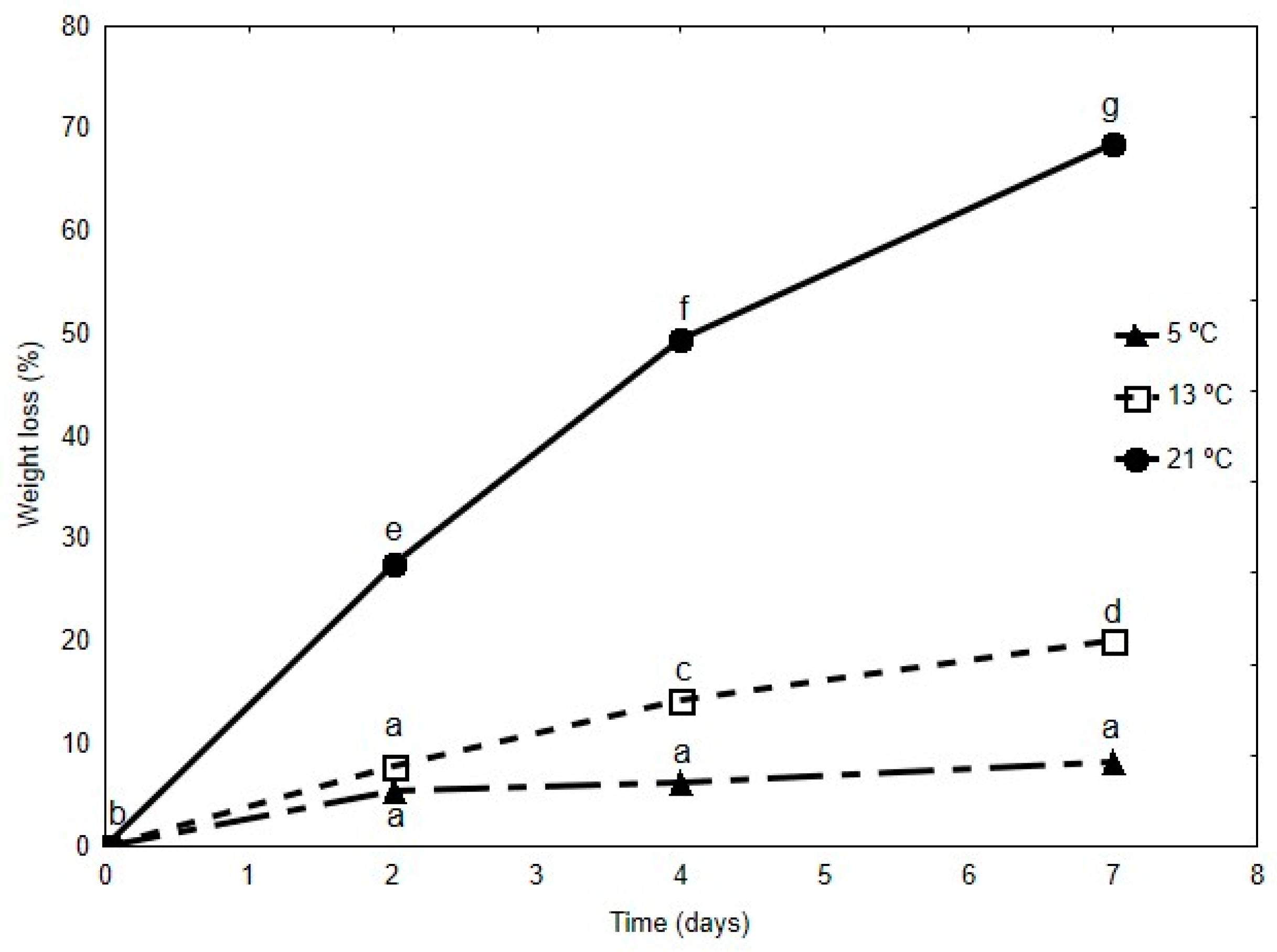
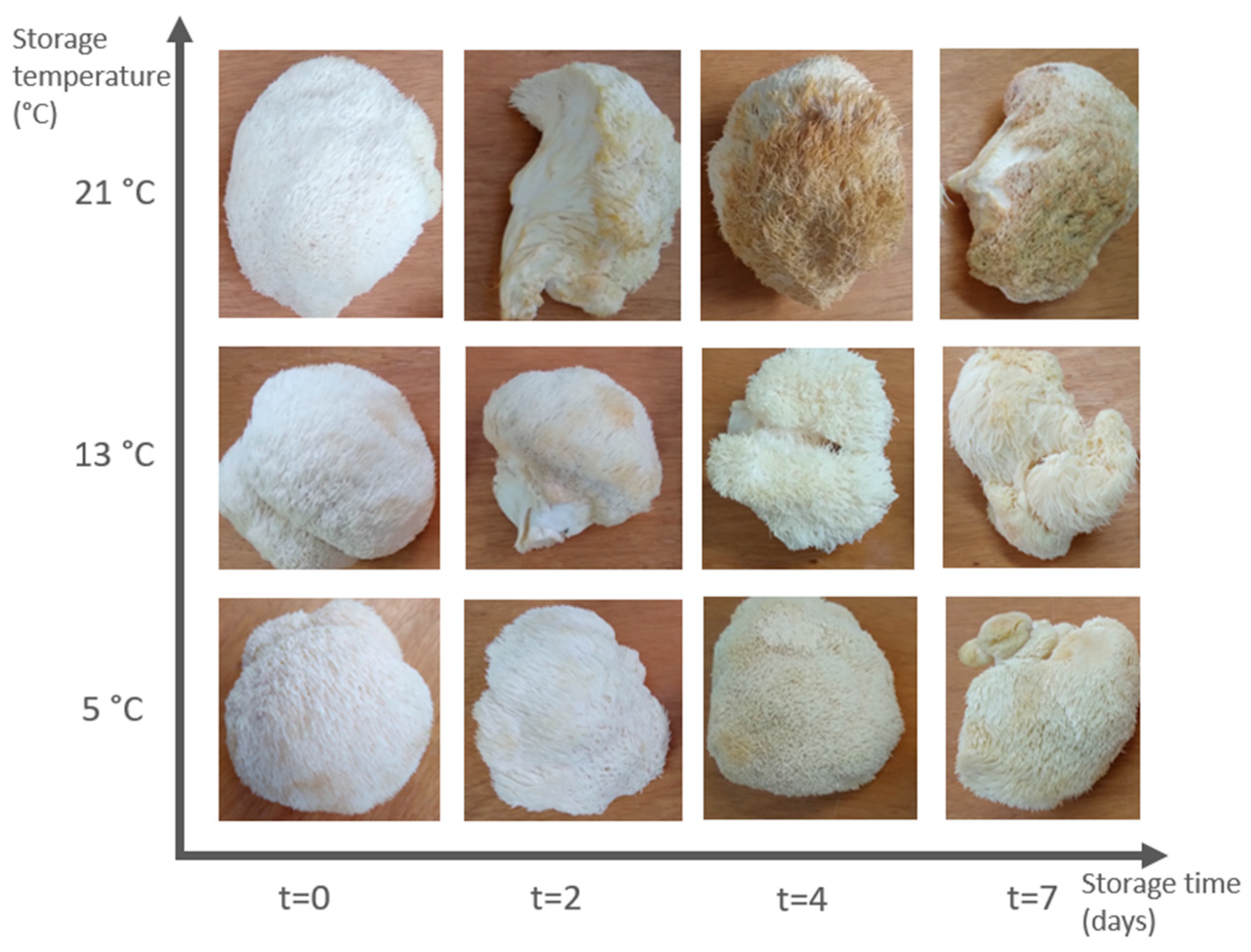
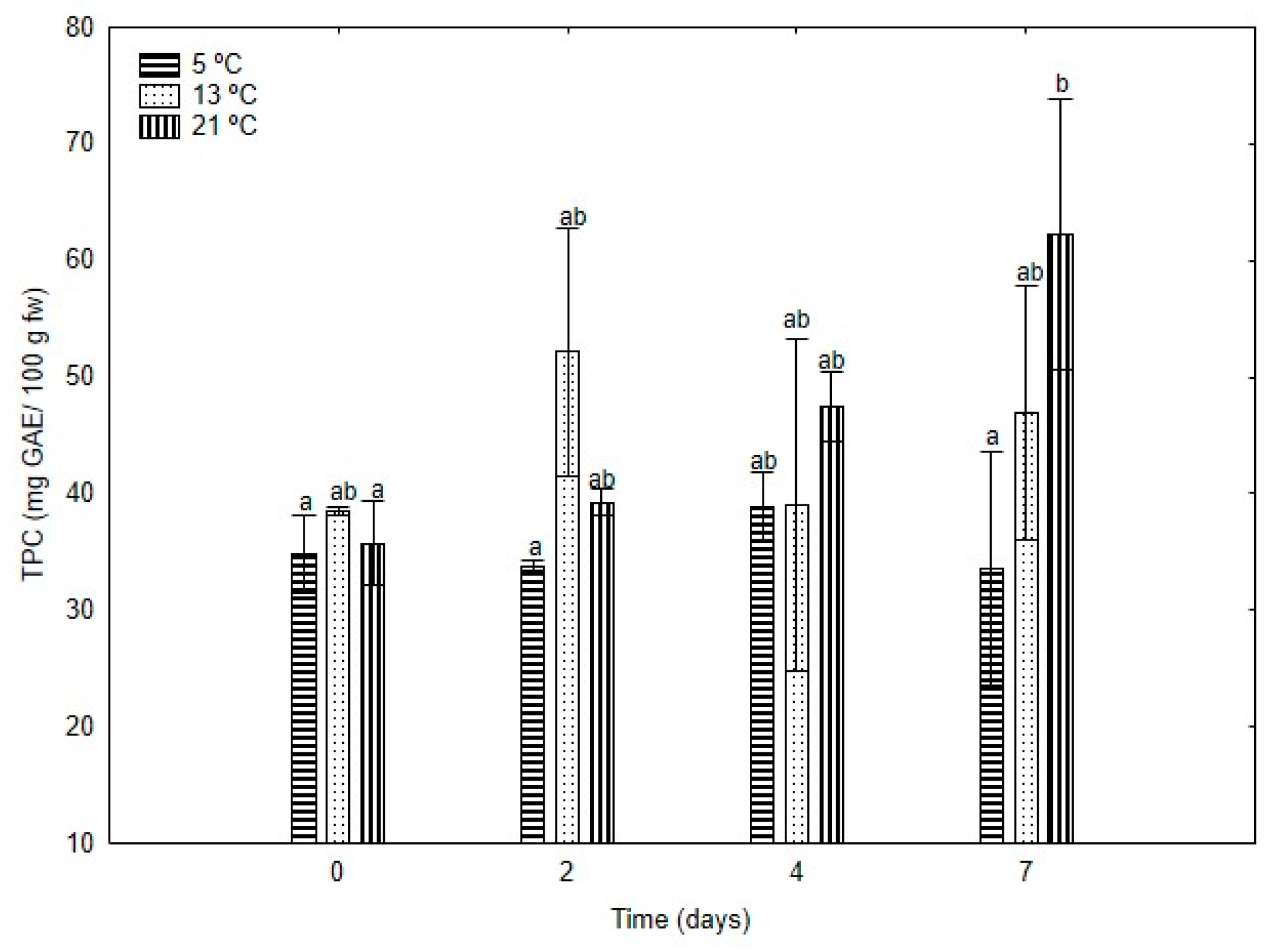
 5 °C;
5 °C;  13 °C;
13 °C;  21 °C. Different letters indicate significant differences (p < 0.05).
21 °C. Different letters indicate significant differences (p < 0.05).
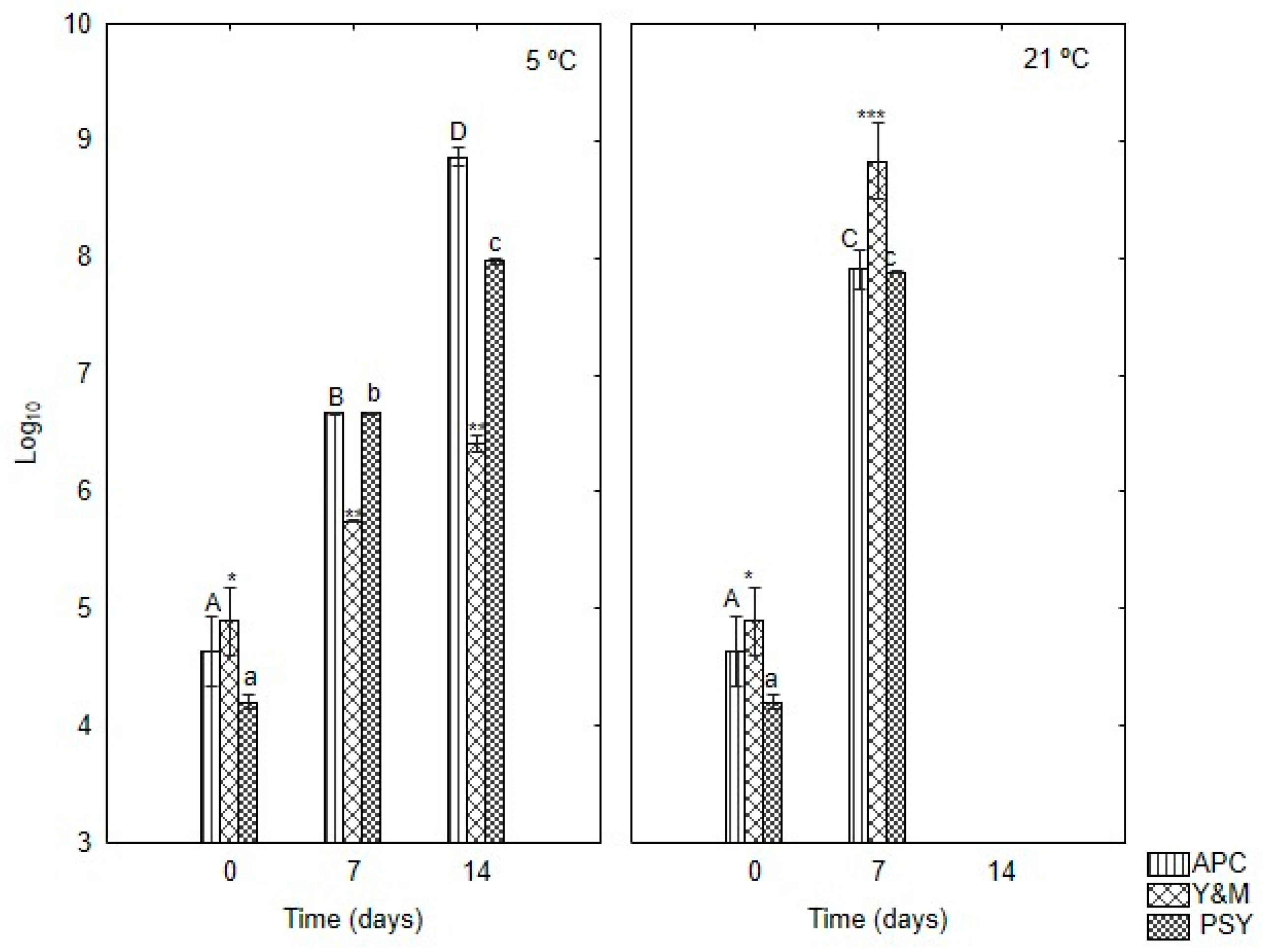
 5 °C;
5 °C;  13 °C;
13 °C;  21 °C. Different letters indicate significant differences (p < 0.05).
21 °C. Different letters indicate significant differences (p < 0.05).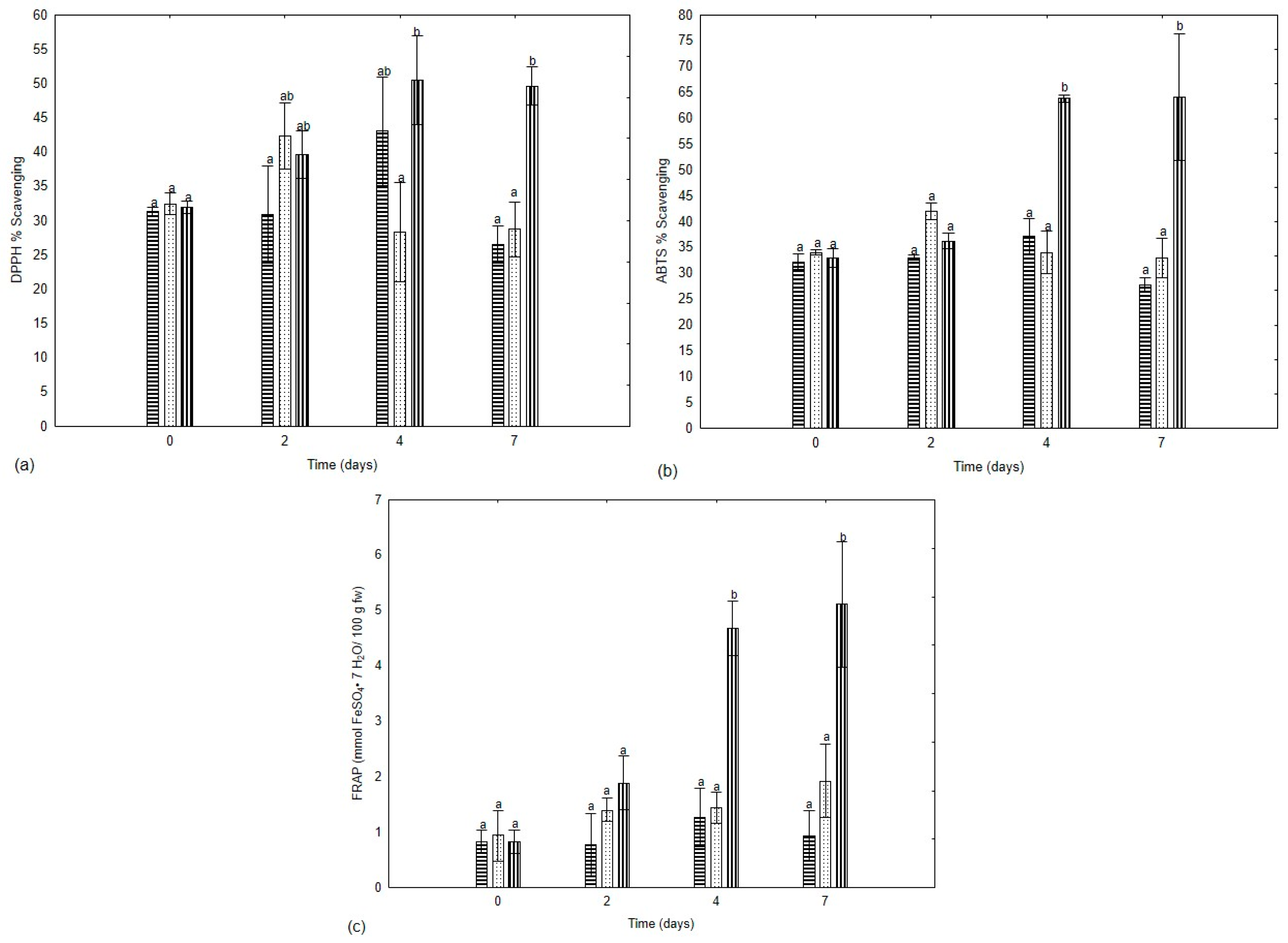
| Storage Temperature (°C) | Days | MC (% w/w) | pH | TA (% CA) |
|---|---|---|---|---|
| 5 | 0 | 90.7 ± 0.2 a | 5.70 ± 0.08 bcd | 0.17 ± 0.00 bf |
| 2 | 89.3 ± 1.1 a | 5.43 ± 0.05 a | 0.19 ± 0.01 bcf | |
| 4 | 87.7 ± 1.2 a | 5.57 ± 0.19 abcd | 0.24 ± 0.01 acd | |
| 7 | 90.0 ± 0.9 a | 5.46 ± 0.04 ac | 0.24 ± 0.01 acd | |
| 13 | 0 | 90.3 ± 0.3 a | 5.73 ± 0.05 bd | 0.17 ± 0.00 b |
| 2 | 89.9 ± 2.6 a | 5.61 ± 0.07 abcd | 0.24 ± 0.04 acde | |
| 4 | 89.2 ± 3.0 a | 5.51 ± 0.06 acd | 0.21 ± 0.00 bcdf | |
| 7 | 90.6 ± 6.4 a | 5.43 ± 0.04 a | 0.30 ± 0.01 ae | |
| 21 | 0 | 90.4 ± 0.3 a | 5.79 ± 0.06 b | 0.10 ± 0.01 b |
| 2 | 87.1 ± 1.1 a | 5.68 ± 0.19 abcd | 0.26 ± 0.01 ade | |
| 4 | 81.2 ± 4.5 b | 5.57 ± 0.08 abcd | 0.28 ± 0.02 ae | |
| 7 | 79.0 ± 4.2 b | 5.82 ± 0.09 b | 0.31 ± 0.03 e |
| Parameters | Temperature (°C) | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 7 | ||
| L* | 5 | 81.88 ± 2.39 ab | 83.28 ± 1.16 ab | 81.27 ± 4.45 ab | |
| 13 | 83.56 ± 3.10 ab | 84.76 ± 4.69 ab | 86.38 ± 4.72 b | 84.98 ± 2.39 ab | |
| 21 | 83.67 ± 3.69 ab | 79.16 ± 9.85 ab | 76.64 ± 7.23 a | ||
| a* | 5 | −0.72 ± 0.47 b | −0.19 ± 0.39 ab | −0.33 ± 1.08 ab | |
| 13 | −0.19 ± 0.62 ab | −0.24 ± 0.76 ab | −0.37 ± 0.43 ab | −0.10 ± 0.63 ab | |
| 21 | −0.18 ± 0.31 ab | 1.69 ± 2.81 bc | 2.50 ± 2.14 c | ||
| b* | 5 | 15.08 ± 3.87 a | 17.28 ± 3.26 ab | 17.14 ± 5.93 ab | |
| 13 | 18.03 ± 2.64 ab | 15.28 ± 4.13 a | 16.13 ± 5.11 a | 18.53 ± 3.90 ab | |
| 21 | 19.89 ± 4.92 ab | 25.01 ± 7.55 b | 24.78 ± 4.43 b | ||
| BI | 5 | 19.54 ± 6.24 a | 22.68 ± 5.20 a | 23.62 ± 11.04 a | |
| 13 | 22.58 ± 3.97 abc | 19.68 ± 6.69 a | 20.54 ± 7.64 a | 24.28 ± 6.91 ab | |
| 21 | 26.96 ± 8.82 abc | 42.79 ± 21.21 c | 42.35 ± 14.71 bc | ||
| TCD | 5 | - | 5.78 ± 3.19 ab | 3.04 ± 1.88 a | 6.76 ± 4.06 abc |
| 13 | 5.42 ± 4.39 ab | 6.13 ± 4.76 abc | 4.48 ± 1.89 ab | ||
| 21 | 5.41 ± 3.49 ab | 13.49 ± 7.18 c | 11.84 ± 6.08 bc | ||
| Firmness (N) | 5 | 45.5 ± 5.3 abc | 50.3 ± 10.1 abc | 58.2 ± 12.7 abc | |
| 13 | 51.2 ± 23.5 abc | 42.3 ± 11.2 abc | 37.6 ± 4.8 ab | 43.9 ± 15.6 abc | |
| 21 | 43.0 ± 7.8 abc | 22.5 ± 3.7 a | 63.8 ± 29.5 bc | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Vida, M.; Ramos, A.C.; Lidon, F.J.; Reboredo, F.H.; Gonçalves, E.M. Storage Temperature Effect on Quality and Shelf-Life of Hericium erinaceus Mushroom. Horticulturae 2025, 11, 158. https://doi.org/10.3390/horticulturae11020158
Silva M, Vida M, Ramos AC, Lidon FJ, Reboredo FH, Gonçalves EM. Storage Temperature Effect on Quality and Shelf-Life of Hericium erinaceus Mushroom. Horticulturae. 2025; 11(2):158. https://doi.org/10.3390/horticulturae11020158
Chicago/Turabian StyleSilva, Mafalda, Manuela Vida, Ana Cristina Ramos, Fernando J. Lidon, Fernando H. Reboredo, and Elsa M. Gonçalves. 2025. "Storage Temperature Effect on Quality and Shelf-Life of Hericium erinaceus Mushroom" Horticulturae 11, no. 2: 158. https://doi.org/10.3390/horticulturae11020158
APA StyleSilva, M., Vida, M., Ramos, A. C., Lidon, F. J., Reboredo, F. H., & Gonçalves, E. M. (2025). Storage Temperature Effect on Quality and Shelf-Life of Hericium erinaceus Mushroom. Horticulturae, 11(2), 158. https://doi.org/10.3390/horticulturae11020158









