Genome-Wide Identification and Expression Analysis of UBP Genes in Peppers (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growing Conditions, and Stress Treatments
2.2. Data Preparation
2.3. Identification and Sequence Analysis of UBP Gene Family Members in Peppers
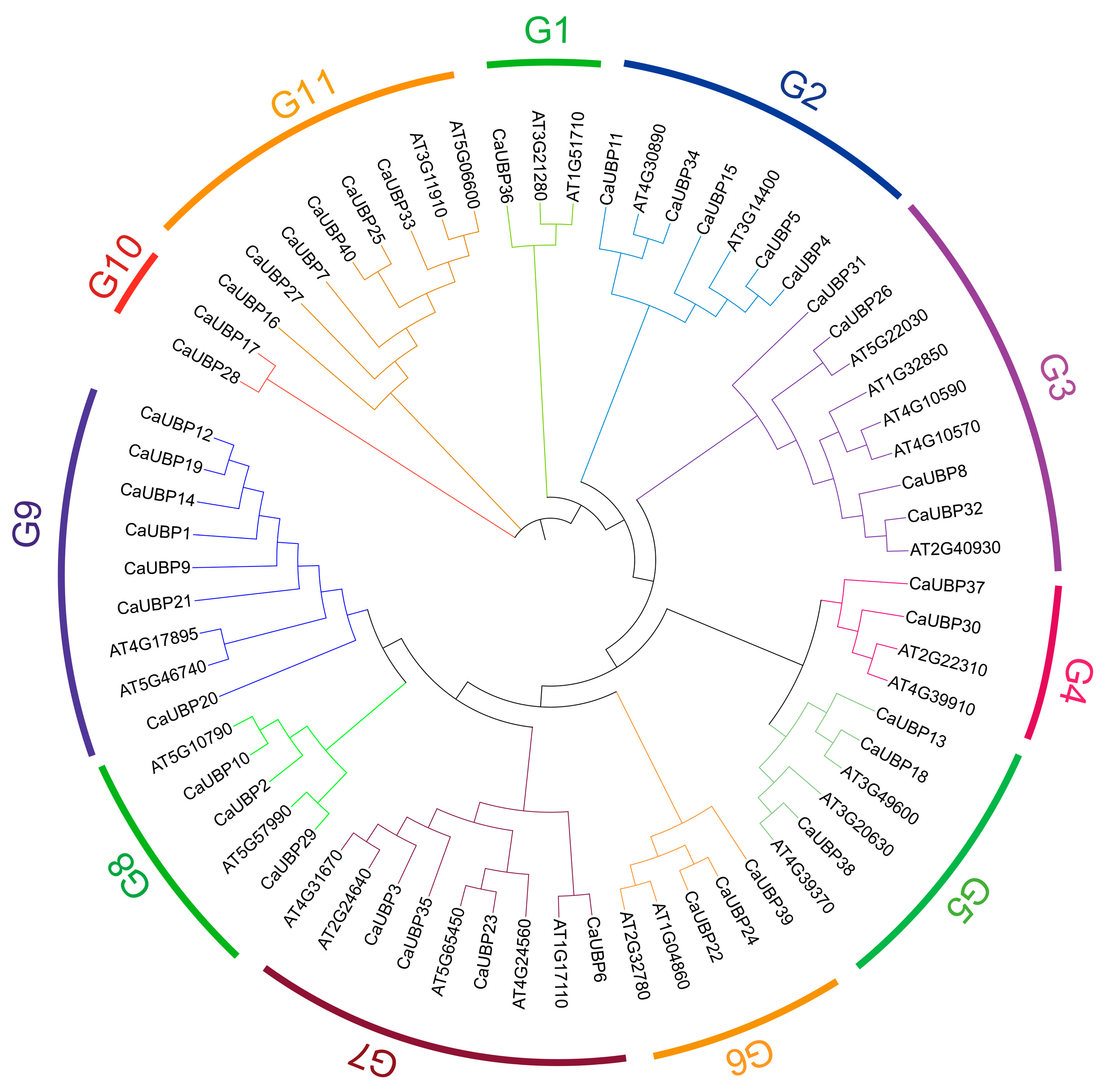
2.4. Phylogenetic Tree Construction
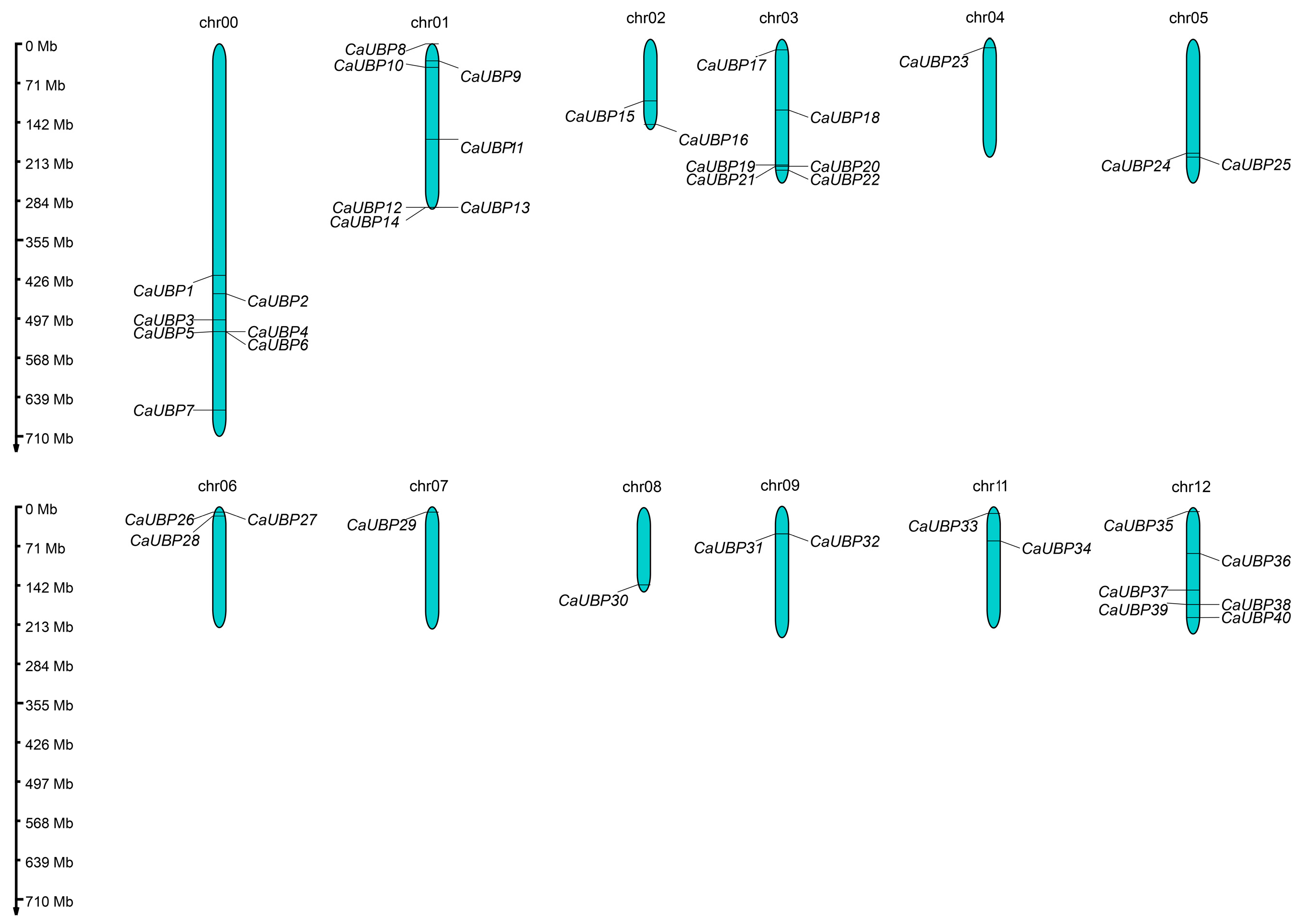
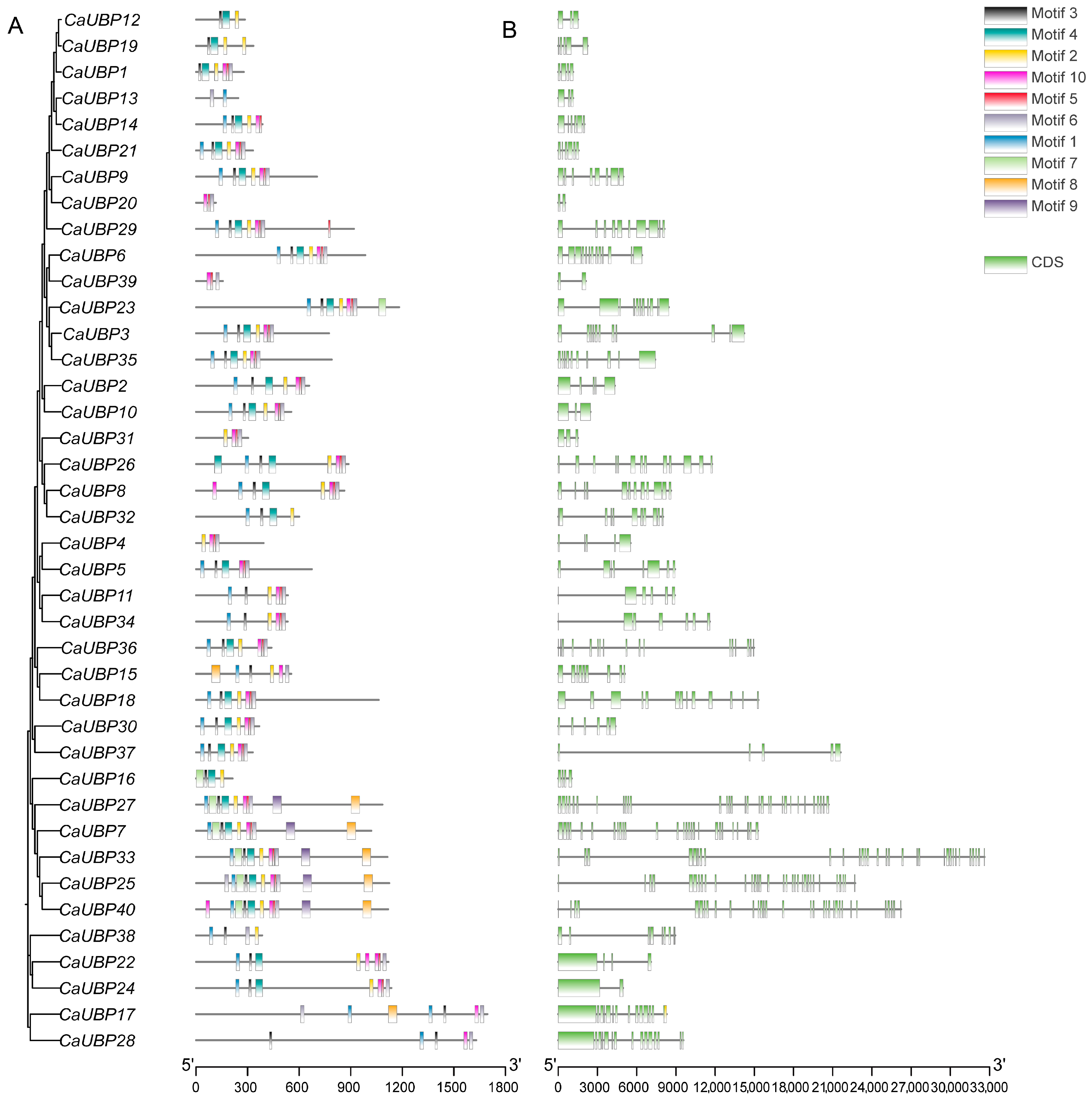
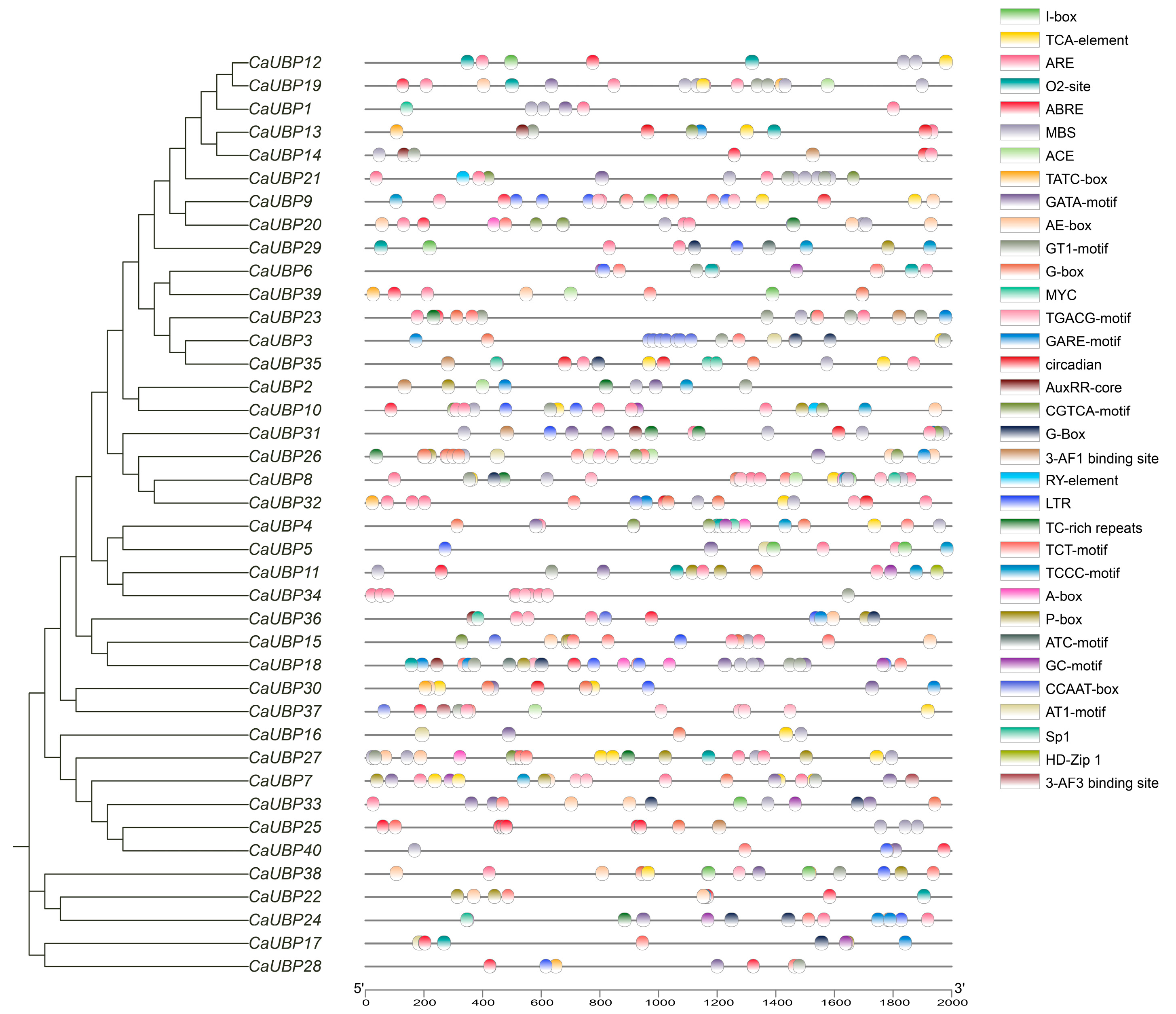
2.5. Analysis of the Chromosomal Mapping, Gene Structure, Conserved Motif, and Cis-Acting Element of UBP Members in Peppers
2.6. Collinearity Analysis of the UBP Gene Family
2.7. GO and Protein–Protein Interaction Network Analysis
2.8. Expression Profile Analysis of UBP Family Members
2.9. RT-qPCR Analysis
3. Results
3.1. UBP Gene Family Member Identification, Physicochemical Characterization, and Subcellular Localization in Peppers
3.2. Chromosomal Location, Gene Structure, and Conserved Motif Analysis of CaUBPs
3.3. Phylogenetic Analysis
3.4. Analysis of Cis-Acting Elements in the Promoter Region of CaUBP Genes
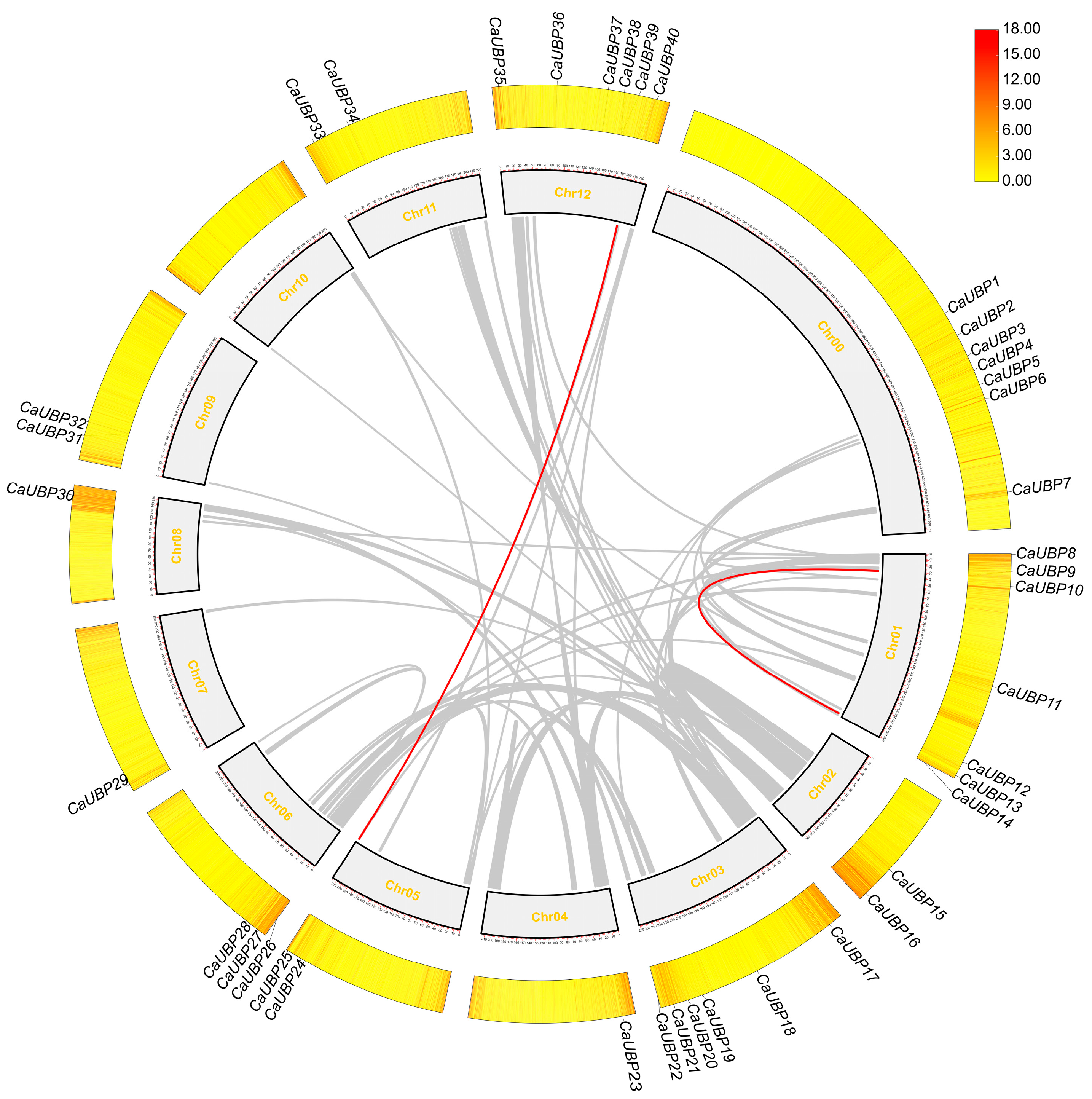
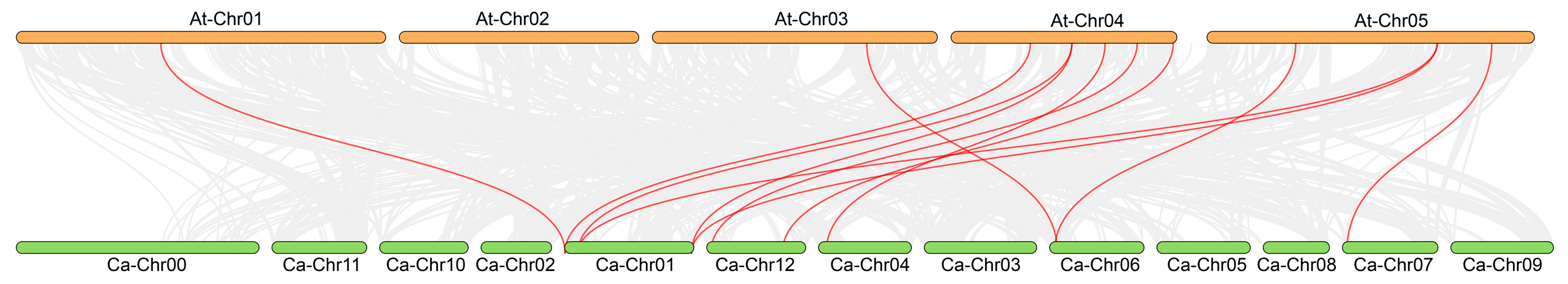
3.5. Collinearity Analysis
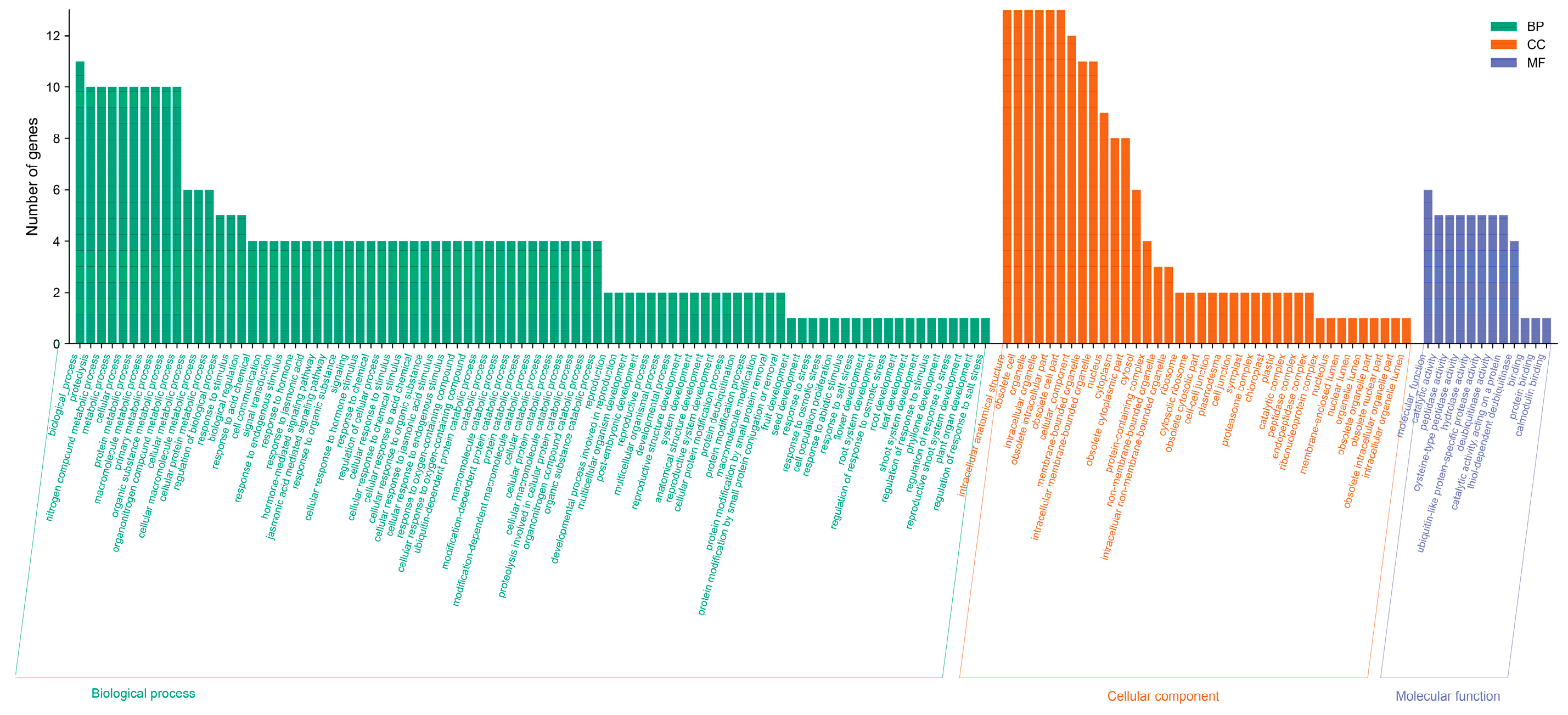
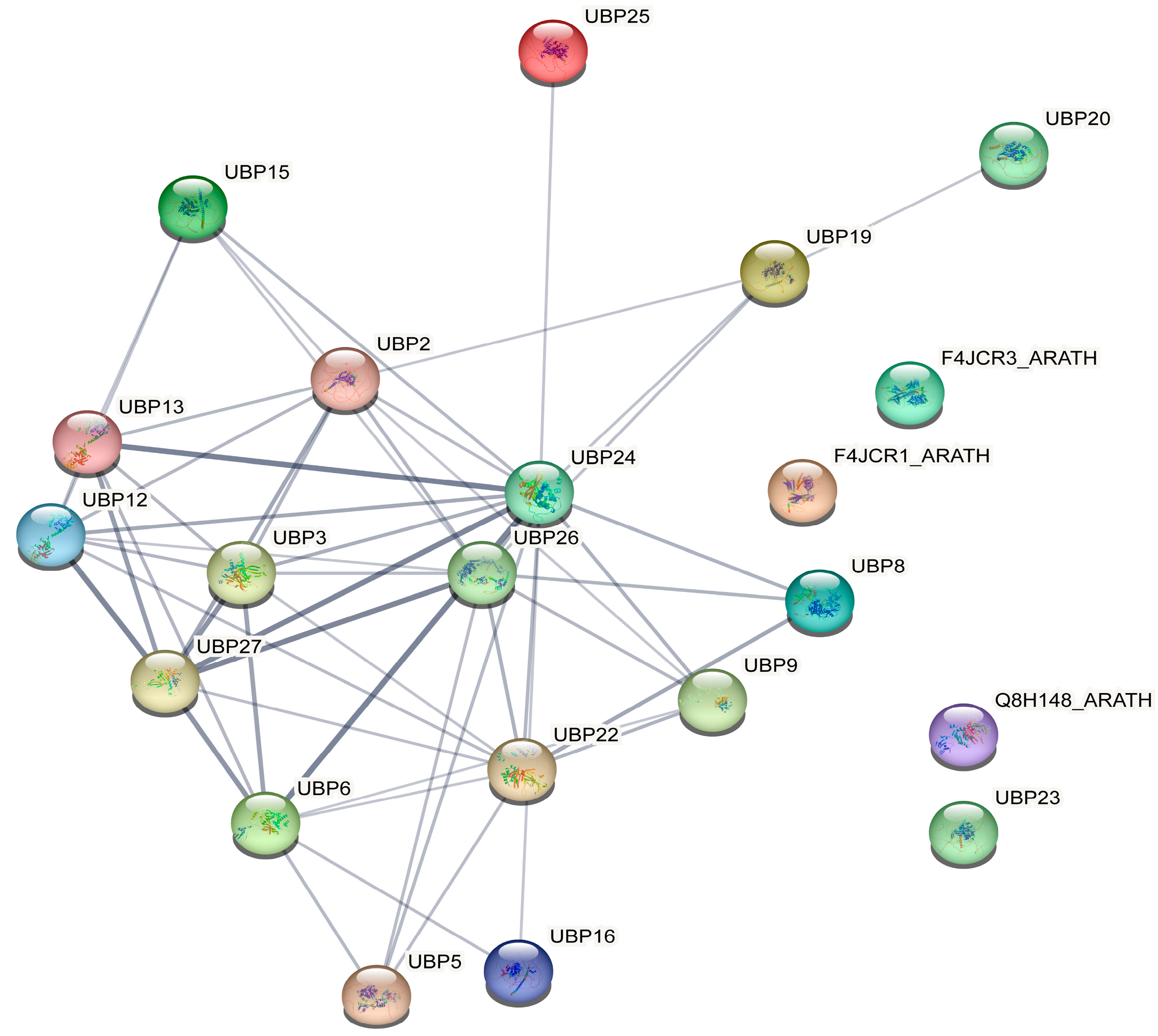
3.6. GO Analysis and Protein–Protein Interaction Network Analysis
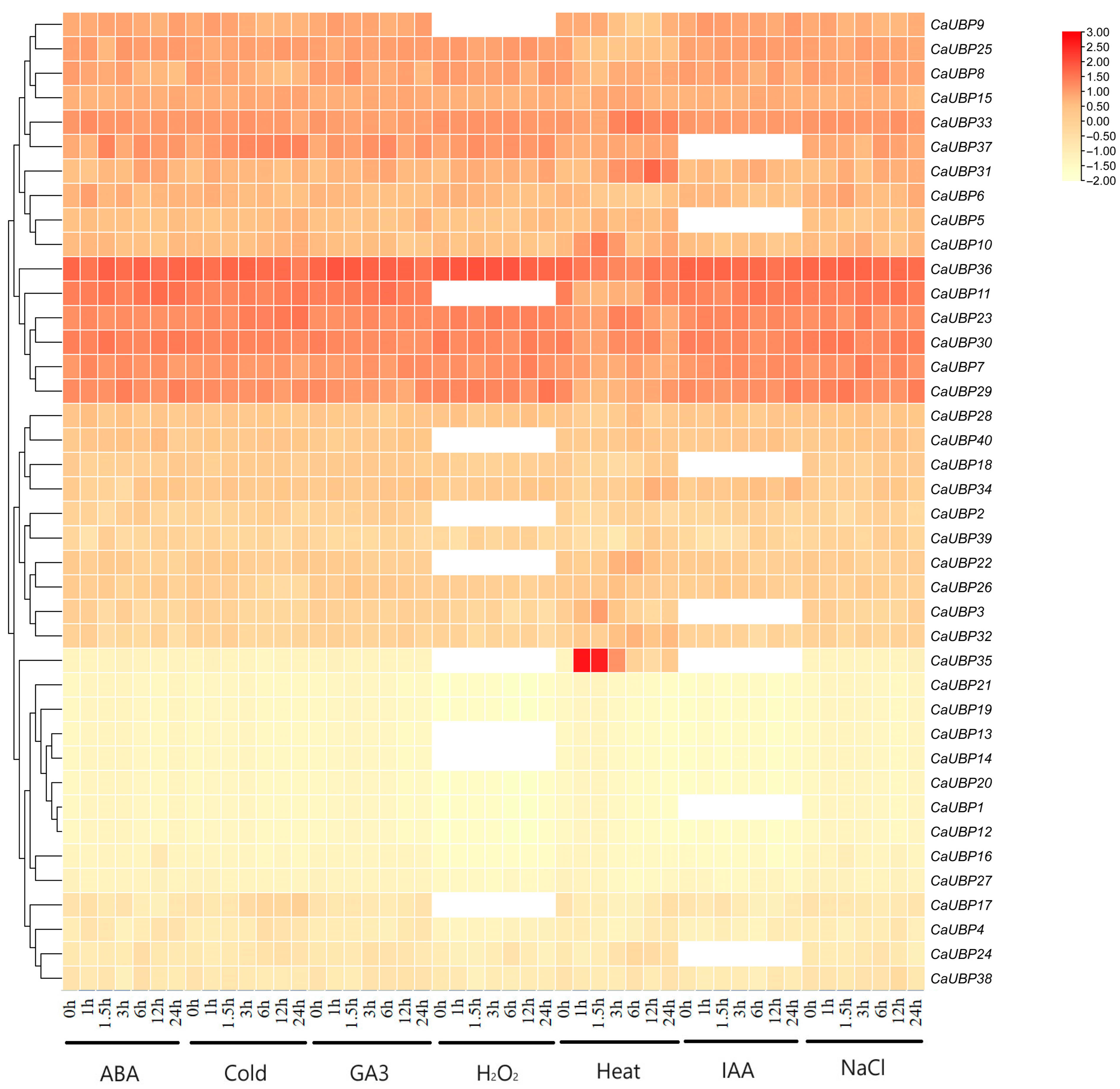
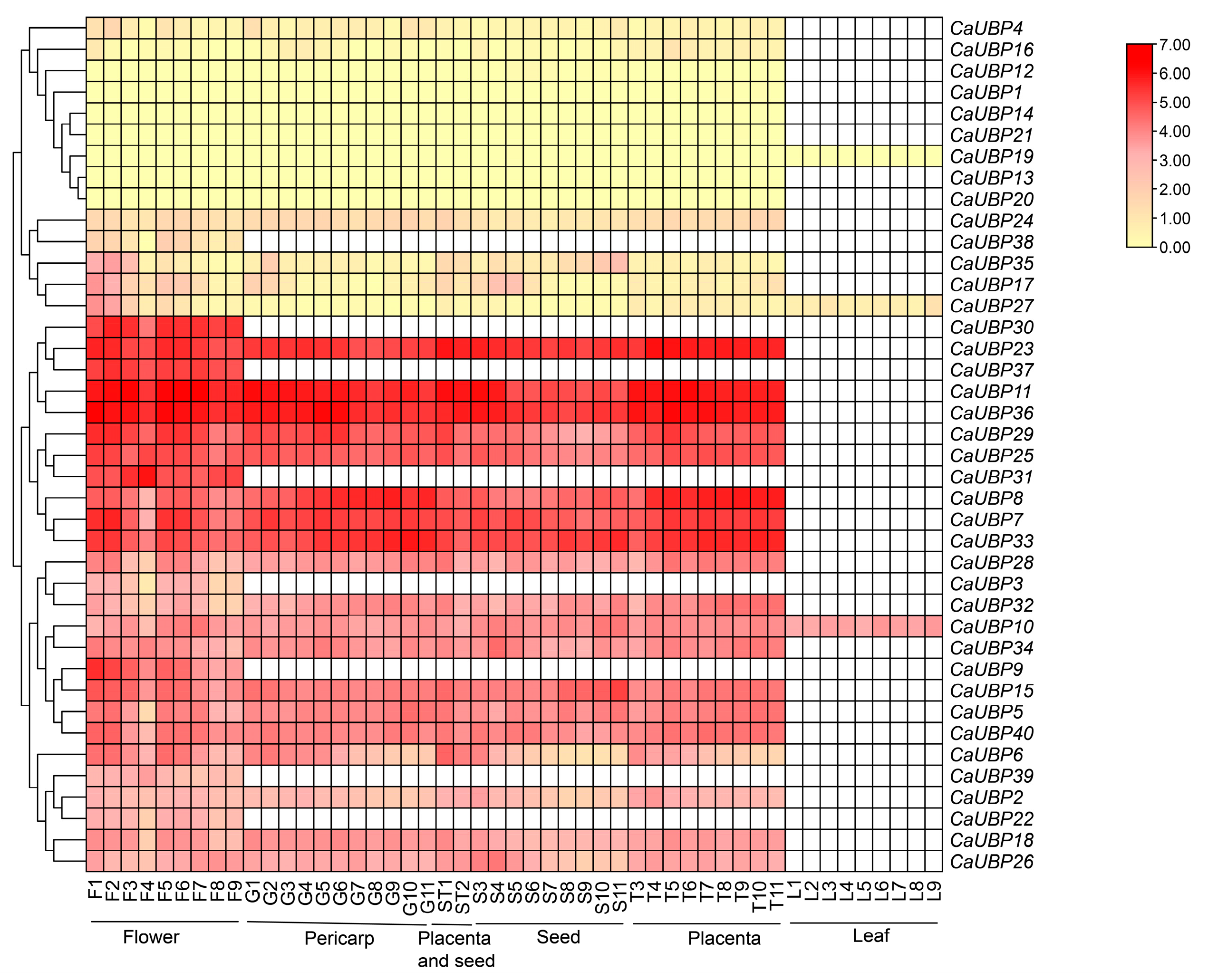
3.7. CaUBP Family Expression Analysis
3.8. Analysis of the Relative Expression of the CaUBP Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DUBs | Deubiquitinating Enzymes |
| UBP | Ubiquitin-specific Protease UBP |
| USPs | ubiquitin-specific proteases |
| OTUs | ovarian tumor proteases |
| UCHs | ubiquitin carboxy-terminal hydrolases |
| JAMMs | enzymes with JAB1/MPN/Mov34 motifs |
| MJDs | Machado–Joseph disease protein domain proteases |
| GRAVY | grand average of hydropathicity |
References
- Kalinowska, K.; Nagel, M.K.; Goodman, K.; Cuyas, L.; Anzenberger, F.; Alkofer, A.; Paz-Ares, J.; Braun, P.; Rubio, V.; Otegui, M.S.; et al. Arabidopsis ALIX is required for the endosomal localization of the deubiquitinating enzyme AMSH3. Proc. Natl. Acad. Sci. USA 2015, 112, E5543–E5551. [Google Scholar] [CrossRef] [PubMed]
- Avvakumov, G.V.; Walker, J.R.; Xue, S.; Finerty, P.J., Jr.; Mackenzie, F.; Newman, E.M.; Dhe-Paganon, S. Amino-terminal dimerization, NRDP1-rhodanese interaction, and inhibited catalytic domain conformation of the ubiquitin-specific protease 8 (USP8). J. Biol. Chem. 2006, 281, 38061–38070. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, P.; Song, L.; Jeffrey, P.D.; Chenova, T.A.; Wilkinson, K.D.; Cohen, R.E.; Shi, Y. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005, 24, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, P.; Li, M.; Li, W.; Yao, T.; Wu, J.W.; Gu, W.; Cohen, R.E.; Shi, Y. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 2002, 111, 1041–1054. [Google Scholar] [CrossRef]
- Haq, S.; Ramakrishna, S. Deubiquitylation of deubiquitylases. Open Biol. 2017, 7, 17700116. [Google Scholar] [CrossRef]
- Komander, D.; Clague, M.J.; Urbé, S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Wang, L.; Zhang, L.; Jiang, L. Evolution and function of ubiquitin-specific proteases (UBPs): Insight into seed development roles in plants. Int. J. Biol. Macromol. 2022, 221, 796–805. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Zhang, H.; He, H.; Ma, L.; Deng, X.W. Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J. Cell Mol. Biol. 2008, 55, 844–856. [Google Scholar] [CrossRef]
- Xu, M.; Jin, P.; Liu, T.; Gao, S.; Zhang, T.; Zhang, F.; Han, X.; He, L.; Chen, J.; Yang, J. Genome-wide identification and characterization of UBP gene family in wheat (Triticum aestivum L). PeerJ 2021, 9, e11594. [Google Scholar] [CrossRef]
- Fu, W.; Fan, D.; Liu, S.; Bu, Y. Genome-wide identification and expression analysis of Ubiquitin-specific protease gene family in maize (Zea mays L). BMC Plant Biol. 2024, 24, 4. [Google Scholar] [CrossRef]
- Yan, N.; Doelling, J.H.; Falbel, T.G.; Durski, A.M.; Vierstra, R.D. Vierstra. The ubiquitin-specific protease family from Arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol. 2000, 124, 1828–1843. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, W.; Li, N.; Zhang, W.; Liu, C.; Li, C.; Li, Y. Ubiquitin-specific protease14 interacts with ultraviolet-b insensitive4 to regulate endoreduplication and cell and organ growth in Arabidopsis. Plant Cell 2016, 28, 1200–1214. [Google Scholar] [PubMed]
- Liu, F.; Yu, H.; Deng, Y.; Zheng, J.; Liu, M.; Ou, L.; Yang, B.; Dai, X.; Ma, Y.; Feng, S.; et al. PepperHub, an Informatics Hub for the Chili Pepper Research Community. Mol. Plant 2017, 10, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; de Luna Ruiz, J.J.; Coppens d’ Eeckenbrugge, G.; Hijmans, R.J.; Gepts, P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef]
- Yi, P.; Xia, W.; Wu, R.C.; Lonard, D.M.; Hung, M.C.; O’ Malley, B.W. SRC-3 coactivator regulates cell resistance to cytotoxic stress via TRAF4-mediated p53 destabilization. Genes Dev. 2013, 27, 274–287. [Google Scholar] [CrossRef]
- Oh, K.H.; Yang, S.W.; Park, J.M.; Seol, J.H.; Iemura, S.; Natsume, T.; Murata, S.; Tanaka, K.; Jeon, Y.J.; Chung, C.H. Control of AIF-mediated cell death by antagonistic functions of CHIP ubiquitin E3 ligase and USP2 deubiquitinating enzyme. Cell Death Differ. 2011, 18, 1326–1336. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, H.; Zhang, M.; Gao, Y.; Li, L.; Gao, Y.; Li, M.; Yang, Y.; Guo, Y.; Li, X. Ubiquitin-specific protease 24 negatively regulates abscisic acid signalling in Arabidopsis thaliana. Plant Cell Environ. 2016, 39, 427–440. [Google Scholar] [CrossRef]
- Hernandez-Garcia, C.M.; Finer, J.J. A novel cis-acting element in the GmERF3 promoter contributes to inducible gene expression in soybean and tobacco after wounding. Plant Cell Rep. 2016, 35, 303–316. [Google Scholar] [CrossRef]
- Gu, Z.; Steinmetz, L.M.; Gu, X.; Scharfe, C.; Davis, R.W.; Li, W.H. Role of duplicate genes in genetic robustness against null mutations. Nature 2003, 421, 63–66. [Google Scholar] [CrossRef]
- Bowers, J.E.; Chapman, B.A.; Rong, J.; Paterson, A.H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 2003, 422, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, J.; Yang, Y.; Chen, C.; Liu, Y.; Jin, X.; Chen, L.; Li, X.; Deng, X.W.; Schumaker, K.S.; et al. Ubiquitin-specific protease16 modulates salt tolerance in Arabidopsis by regulating Na+/H+ antiport activity and serine hydroxymethyltransferase stability. Plant Cell 2012, 24, 5106–5122. [Google Scholar] [CrossRef] [PubMed]
- Auesukaree, C.; Damnernsawad, A.; Kruatrachue, M.; Pokethitiyook, P.; Boonchird, C.; Kaneko, Y.; Harashima, S. Genome-wide identification of genes involved in tolerance to various environmental stresses in Saccharomyces cerevisiae. J. Appl. Genet. 2009, 50, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.M.; Janse, D.M.; Tanay, A.; Shamir, R.; Church, G.M. A global view of pleiotropy and phenotypically derived gene function in yeast. Mol. Syst. Biol. 2005, 1, 2005.0001. [Google Scholar] [CrossRef]
- Du, L.; Li, N.; Chen, L.; Xu, Y.; Li, Y.; Zhang, Y.; Li, C.; Li, Y. The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin-specific protease UBP15/SOD2 in Arabidopsis. Plant Cell 2014, 26, 665–677. [Google Scholar] [CrossRef]
- Kahana, A. The deubiquitinating enzyme Dot4p is involved in regulating nutrient uptake. Biochem. Biophys. Res. Commun. 2001, 282, 916–920. [Google Scholar] [CrossRef]
- Doelling, J.H.; Yan, N.; Kurepa, J.; Walker, J.; Vierstra, R.D. The ubiquitin-specific protease UBP14 is essential for early embryo development in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2001, 27, 393–405. [Google Scholar] [CrossRef]
- An, Z.; Liu, Y.; Ou, Y.; Li, J.; Zhang, B.; Sun, D.; Sun, Y.; Tang, W. Regulation of the stability of RGF1 receptor by the ubiquitin-specific proteases UBP12/UBP13 is critical for root meristem maintenance. Proc. Natl. Acad. Sci. USA 2018, 115, 1123–1128. [Google Scholar] [CrossRef]
- Derkacheva, M.; Liu, S.; Figueiredo, D.D.; Gentry, M.; Mozgova, I.; Nanni, P.; Tang, M.; Mannervik, M.; Köhler, C.; Hennig, L. H2A deubiquitinases UBP12/13 are part of the Arabidopsis polycomb group protein system. Nat. Plants 2016, 2, 16126. [Google Scholar] [CrossRef]
- Wu, R.; Shi, Y.; Zhang, Q.; Zheng, W.; Chen, S.; Du, L.; Lu, C. Genome-Wide Identification and Characterization of the UBP Gene Family in Moso Bamboo (Phyllostachys edulis). Int. J. Mol. Sci. 2019, 20, 4309. [Google Scholar] [CrossRef]
- Nassrallah, A.; Rougée, M.; Bourbousse, C.; Drevensek, S.; Fonseca, S.; Iniesto, E.; Ait-Mohamed, O.; Deton-Cabanillas, A.F.; Zabulon, G.; Ahmed, I.; et al. DET1-mediated degradation of a SAGA-like deubiquitination module controls H2Bub homeostasis. Elife 2018, 7, e37892. [Google Scholar] [CrossRef] [PubMed]
- Doelling, J.H.; Phillips, A.R.; Soyler-Ogretim, G.; Wise, J.; Chandler, J.; Callis, J.; Otegui, M.S.; Vierstra, R.D. The ubiquitin-specific protease subfamily UBP3/UBP4 is essential for pollen development and transmission in Arabidopsis. Plant Physiol. 2007, 145, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, J.; Cai, J.; Patil, S.B. Ubiquitin-Specific Proteases function in plant development and stress responses. Plant Mol. Biol. 2017, 94, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Jung, C.; Seo, J.S.; Kim, J.K.; Chua, N.H. The Deubiquitinating Enzymes UBP12 and UBP13 Positively Regulate MYC2 Levels in Jasmonate Responses. Plant Cell 2017, 29, 1406–1424. [Google Scholar] [CrossRef]

| Gene Name | Forward Primers (5’→3’) | Reverse Primers (5’→3’) |
|---|---|---|
| CaUBP3 | AGAATCTACCCTCGGCGAAT | TGCTGGACATGAGAACTTGC |
| CaUBP17 | ACCCTGACATGGTTGAAAGC | CAAAAGCAAGACCCTGAAGC |
| CaUBP27 | GATGCTCGAATCATGGGAGT | AGAACAACCATTGCCTCCAC |
| CaUBP32 | GACAAGACCTACGGGAGCAG | AAGCCAGTTCACCAACCATC |
| CaUBP34 | AGAAGACGTTCCAACCACG | TCGCCGTCCAGCTCGACCAG |
| CaUBP35 | CAGCGGTCCTTGATGAATTT | CAGCATCCCCATGAATCTCT |
| CaUBP38 | CAGCGGTCCTTGATGAATTT | CAGCATCCCCATGAATCTCT |
| β-Actin | CCACCTCTTCACTCTCTGCTCT | ACTAGGAAAAACAGCCCTTGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Wang, T.; Huang, J.; Xu, J.; Ruan, Y.; Liang, Y.; Wang, J. Genome-Wide Identification and Expression Analysis of UBP Genes in Peppers (Capsicum annuum L.). Horticulturae 2025, 11, 458. https://doi.org/10.3390/horticulturae11050458
Chang X, Wang T, Huang J, Xu J, Ruan Y, Liang Y, Wang J. Genome-Wide Identification and Expression Analysis of UBP Genes in Peppers (Capsicum annuum L.). Horticulturae. 2025; 11(5):458. https://doi.org/10.3390/horticulturae11050458
Chicago/Turabian StyleChang, Xuerui, Tiantian Wang, Jiaxin Huang, Jia Xu, Yangyang Ruan, Yanping Liang, and Jing Wang. 2025. "Genome-Wide Identification and Expression Analysis of UBP Genes in Peppers (Capsicum annuum L.)" Horticulturae 11, no. 5: 458. https://doi.org/10.3390/horticulturae11050458
APA StyleChang, X., Wang, T., Huang, J., Xu, J., Ruan, Y., Liang, Y., & Wang, J. (2025). Genome-Wide Identification and Expression Analysis of UBP Genes in Peppers (Capsicum annuum L.). Horticulturae, 11(5), 458. https://doi.org/10.3390/horticulturae11050458






