Abstract
Rhizopus stolonifer causes soft rot disease in strawberry and is considered one of the most destructive pathogens affecting strawberries worldwide. This study investigated the efficacy of three atmospheric non-thermal plasmas (NTPs) consisting of gliding arc (GA), Tesla coil (TC) and dielectric barrier discharge (DBD) for controlling R. stolonifer infection. Fungal mycelial discs were exposed to these plasmas for 10, 15 or 20 min, whereas conidial suspensions were treated for 1, 3, 5 or 7 min. Morphological alterations following non-thermal plasma exposure were studied using scanning electron microscopy (SEM). Exposure to GA and DBD plasmas for 20 min completely inhibited mycelial growth. SEM analysis revealed significant structural damage to the mycelium, sporangia and sporangiospores of treated samples compared to untreated controls. Complete inhibition of sporangiospore germination was achieved with treatments for at least 3 min for all NTPs. Pathogenicity assays on strawberry fruit showed that 15 min exposure to any of the tested NTPs completely prevented the development of soft rot disease. Importantly, NTP treatments did not adversely affect the external or internal characteristics of treated strawberries. These findings suggest that atmospheric non-thermal plasmas offer an effective approach for controlling R. stolonifer infection in strawberries, potentially providing a non-chemical alternative for post-harvest disease management.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) is an economically important small fruit crop that is widely cultivated in many countries of the world. Strawberry is highly popular and appreciated by consumers for its unique taste and flavour as well as its health benefits [1]. However, strawberry has a limited post-harvest shelf life, often because of soft rot caused by Rhizopus stolonifer [2,3].
Rhizopus stolonifer is known to be a devastating post-harvest pathogen and is becoming one of the limiting factors for strawberry production worldwide due to its fast growth and wide array of hosts [4]. This soft rot disease is usually associated with handling damage. The first signs of infection may be the leakage of juice from packed fruit, and on inspection, a white mouldy growth with black fruiting bodies is often seen [5]. The soft rot disease can spread rapidly from one infected fruit to the next healthy fruit, resulting in the extensive breakdown of the commodity [3].
To mitigate the economic impact of R. stolonifer infections, effective management of soft rot disease becomes essential, particularly during rainy seasons and in high-humidity environments [6]. While conventional control strategies primarily rely on systemic fungicide applications at field level, post-harvest disease management employs different approaches. Low-temperature storage serves as a primary intervention after harvest, as the rapid cooling of fruit effectively suppresses fungal development and inhibit sporangiospore germination. This has turned out to be the most efficient disease control measure available [7].
Non-thermal plasmas (NTPs), commonly referred to as cold plasmas in applied sciences, constitute the fourth stage of matter, characterised by a pronounced thermal disequilibrium between highly energetic electrons and substantially cooler heavy particles, including ions and neutral atoms [8]. This distinct thermodynamic property differentiates NTPs from thermal plasma systems, where all constituent components exist in thermal equilibrium. This unique thermal incongruity enables NTPs to initiate various chemical and physical processes that would otherwise require significantly elevated temperatures, thereby offering considerable advantages for applications involving thermally sensitive materials and biological systems [9].
NTPs operate at near-ambient temperatures, rendering them particularly suitable for the treatment of thermally sensitive biological materials such as seeds, seedlings and fresh produce without inducing thermal degradation [10]. The antimicrobial efficacy of NTPs derive from a multifaceted mechanism involving the generation of reactive oxygen and nitrogen species (RONS), ultraviolet radiation emission and the production of charged particles, collectively creating a hostile environment capable of inactivating a diverse spectrum of plant pathogens including phytopathogenic bacteria, fungi and viruses [11].
The formation of RONS by NTPs is typically caused by a strong electric field, exciting and ionising air molecules, leading to their formation. These species include molecules such as hydroxyl radicals (OH•), superoxide (O2−) and nitric oxide (NO) in the gas phases [12]. Short-lived species like hydroxyl radicals and singlet oxygen (1O2) have brief lifespans, while long-lived species, such as hydrogen peroxide (H2O2), nitrite (NO2−) and nitrate (NO3−), persist for longer. These reactive species are well known and play a role in many biomedical and agricultural processes [13].
In the context of escalating concerns regarding the environmental persistence of chemical pesticides and the emergence of resistant pathogen populations, the NTP technology represents a promising, environmentally sustainable alternative that aligns with contemporary integrated plant disease management strategies [14]. NTPs are recognised as a food-safe intervention due to its demonstrated efficacy in inactivating foodborne pathogens through mechanisms that do not generate persistent toxic residues or substantially compromise the nutritional integrity or organoleptic properties of food products [15]. This approach offers significant advantages compared to conventional chemical treatments by maintaining food quality parameters simultaneously with substantial reductions in microbial load, positioning it as a promising alternative for food-safety applications where the preservation of product characteristics is important [16].
The efficacy of NTPs against fungi primarily stems from their ability to compromise spore and mycelial cell-membrane integrity, triggering cytoplasmic leakage and the subsequent loss of cellular contents [17]. Additionally, NTP treatment induces multiple destructive mechanisms within fungal cells, including protein and DNA degradation through oxidative damage, ultimately culminating in apoptosis [18]. The oxidative stress is postulated to be the primary mediator of microbial decontamination through multiple mechanisms inducing oxidative stress, compromising membrane integrity, denaturing proteins and degrading nucleic acids, culminating in the inactivation of diverse microorganisms including bacteria, fungi and viruses [9,19]. The distinctive antimicrobial efficacy of NTPs is derived from the synergistic interaction between short-lived reactive radicals and persistent oxidative species, conferring broad-spectrum antimicrobial activity [11].
For the prevention and control of post-harvest diseases in fruit crops, NTPs have been explored as an alternative, chemical-free approach for managing post-harvest fungal pathogens and have been applied to a variety of fungi and fruit types [20] such as controlling Botrytis cinerea, causing grey mould in grapes and strawberries [21,22], Penicillium expansum, causing blue mould disease in apples [23] and Colletotrichum asianum, causing anthracnose disease in mango [24].
Previous studies have demonstrated the efficacy of cold plasma technology for the microbial decontamination of strawberries. Research has shown that plasma treatments effectively reduce bacterial and fungal contamination while maintaining fruit quality parameters including firmness, pH, colour, taste and antioxidant activity [15,25,26]. SEM analysis revealed structural damages to fungal hyphae and a shelf-life extension of 2–3 days has been achieved under refrigeration [26,27]. These studies highlight the potential of NTPs for strawberry preservation, emphasising the need to optimise treatment parameters for effective microbial control without compromising fruit quality.
Three plasma sources were tested, each offering distinct advantages for fungal control applications: gliding arc (GA) plasma provides high-energy discharge with dense production of reactive oxygen and nitrogen species (RONS) for aggressive decontamination, Tesla coil (TC) plasma delivers moderate-energy output with a balanced generation of RONS, suitable for intermediate treatments and dielectric barrier discharge (DBD) plasma offers gentle, low-energy discharge predominantly producing reactive oxygen species for preserving product integrity [28]. This selection enables the comprehensive evaluation of plasma types across the spectrum of fungal inactivation efficiency, treatment selectivity and host tissue compatibility, facilitating the optimisation of plasma applications in post-harvest disease management. Therefore, this study evaluated the efficacy of various non-thermal plasma treatments (GA, TC and DBD) for inactivating R. stolonifer, the causal agent of soft rot disease in strawberry.
2. Materials and Methods
2.1. Source of Pathogen
Rhizopus stolonifer was isolated from diseased strawberries using a single-sporangiospore isolation technique. The isolate was morphological and molecularly identified [29]. The fungus was cultured on potato dextrose agar (PDA, Himedia, Maharashtra, India) at room temperature (25–28 °C) for 3 days prior to use.
2.2. Plasma Device and Properties
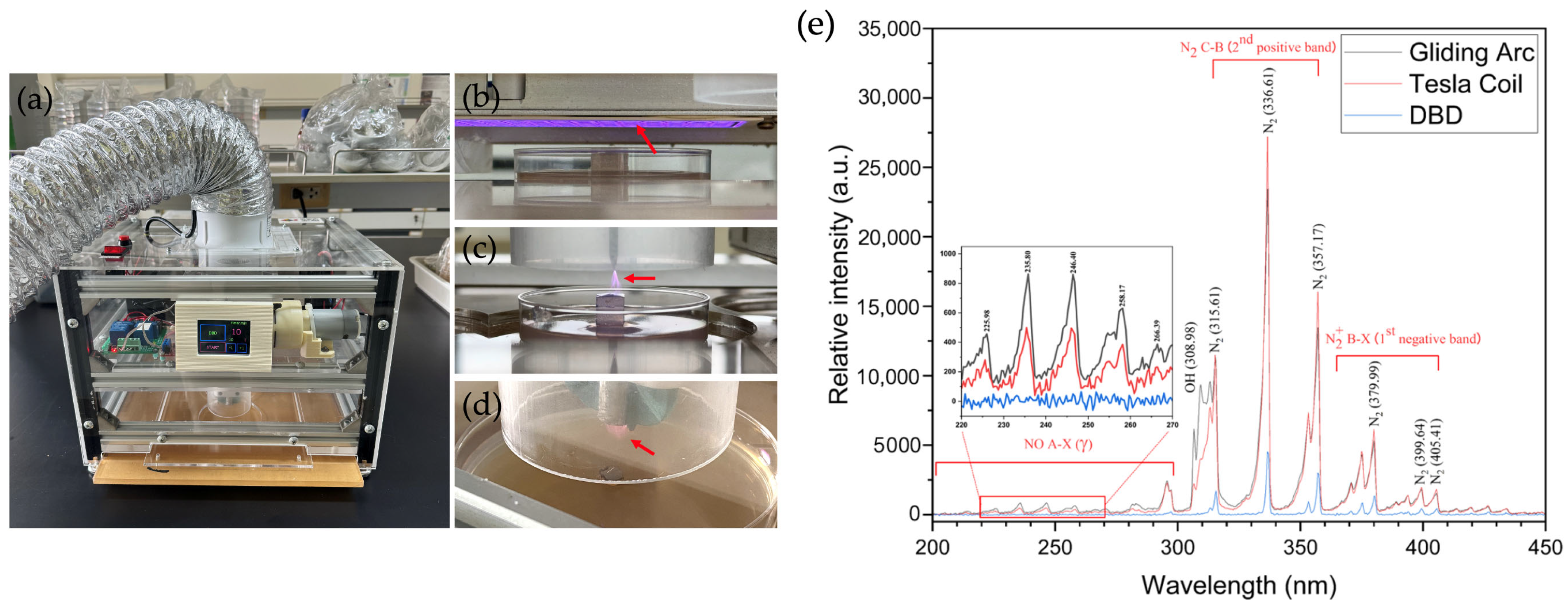
Ambient air reactive oxygen and nitrogen species (RONS) were generated using gliding arc (GA), Tesla coil (TC) and dielectric barrier discharge (DBD) plasmas within a semi-closed frame, as described earlier [28]. The GA and TC plasmas were each powered by a 20 W radio frequency (RF) self-resonant power supply, providing a sinusoidal AC output with a peak voltage of 6–10 kV at a frequency of 700–900 kHz. In contrast, the DBD plasma was powered by a continuous 18 W AC high-voltage source, delivering 7.6 kV peak-to-peak, at a frequency of 18.2 kHz. The planar mesh-powered electrode for the DBD plasma was mounted on an aluminium ceramic sheet (1.0 mm thickness) with active lateral dimensions of 5.3 cm × 11.3 cm. The technical setup for the three plasma devices is shown in Figure 1 together with their optical emission spectra. In particular, the air-plasma spectra were examined using optical emission spectroscopy equipped with an Exemplar LS-Smart CCD spectrometer (BRC115P-U, B&W Tek, Inc., Delaware, NJ, USA) at a resolution of 1.5 nm over a broad wavelength range of 200 to 800 nm. The light emitted from the plasma volume was obtained through a SMA-905 optical fibre (FC-UV400-2-ME, Avantes B.V., Apeldoorn, The Netherlands) with a diameter of 2.5 mm and the integration time was set to 300 ms. These three air-plasma sources were employed, because GA produces a high-electron-density volume plasma and transitions occur at a relatively low gas temperature. TC, which is achieved through a resonant transformer circuit, facilitates efficient energy transfer from the air-plasma to the discharge and DBD with a dielectric barrier limits the current flow, preventing a continuous arc and leading to a multitude of micro-discharges where high-energy electrons in the plasma volume cause chemical reactions without significantly heating the bulk gas (Figure 1e).
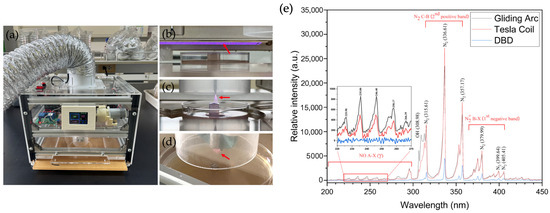
Figure 1.
Atmospheric non-thermal plasma devices. (a) The plasma device, (b) dielectric barrier discharge (DBD) plasma, (c) Tesla coil (TC) plasma, (d) gliding arc (GA) plasma and (e) the optical emission spectra of the three plasma devices, GA, TC and DBD, in the range from 200 to 450 nm. The red arrows indicate the generated plasma.
Thus, ambient air molecules (N2, O2 and water vapour) were ionised and dissociated by the strong electric fields, leading to the generation of RONS with high-energy electrons in the plasma phase. The ionisation and dissociation reactions are presented below (Equation (1)). However, the direct electron impact dissociation of N2 occurs much more slowly than that of O2. Therefore, atomic oxygen (O•) is the primary intermediate product. Electron collisions with N2 preferentially result in the formation of various excited N2 molecules, whereas the generation of RONS occurs during the plasma propagation phase (Equations (2)–(7)) [30]. In humid air, electron impact can also produce atomic oxygen (O•) and hydroxyl radicals (OH•) through dissociative excitation (Equation (8)). Both O• and OH• are transient chemical species with significantly stronger oxidative properties than neutral oxidants such as ozone.
e− + N2 → e− + N2* (N2 (C) or N2 (B)),
N2* + O2 → N2 + O• + O•,
O• + O2 + M → O3 + M,
N• + O2 → NO + O•,
O• + N2 → NO + N•,
NO + O• +M → NO2 + M,
O3 + NO → O2 + NO2,
e− + H2O → e− + H + OH•,
2.3. Inhibition of Mycelial Growth
To inhibit mycelial growth in vitro, a cork borer was used to stamp out discs of fungal cultures (0.75 cm in diameter) from a PDA plate after three days of incubation. The resulting mycelial discs were subjected to GA, TC or DBD plasmas. The distance between the plasma sources and the objects was fixed at 1.0 cm. Due to the types of plasma, GA and TC plasmas were applied through direct exposure, whereas DBD plasma was applied indirectly. Treatments were applied for 10, 15 or 20 min, with treatments conducted in triplicate. The operating room temperature was 25 °C and air humidity was 50% relative humidity (RH). The treated mycelial discs were placed on 9 cm PDA plates and incubated at 25–28 °C for 3 days until the fungal mycelia in the untreated control fully developed. The radial growth of non-thermal plasma (NTP)-treated mycelia was compared with the control, following the calculation method proposed earlier [31].
The radial growth of NTP-treated mycelia was compared with the untreated control by calculating the mean diameter (dc) of the colony in the control sample and the mean diameter (dt) of the colony in the treated sample.
2.4. Inhibition of Sporangiospore Germination
To evaluate the effect of GA, TC and DBD plasmas on the inhibition of sporangiospore germination, sporangiospores were collected from a cultured PDA plate and adjusted to a concentration of 106 sporangiospores/mL using a haemocytometer. A 50 µL sporangiospore suspension was placed on a water agar (WA) plug (1 cm2 in size), placed on a slide culture and exposed to GA, TC or DBD plasmas for 1, 3, 5 or 7 min. The operating room temperature was 25 °C and the air humidity was 50% RH. The slide culture was then incubated at 25–28° and sporangiospore germination was assessed at 24 h post-incubation. Untreated sporangiospores served as a control. Each treatment included three replications (20 conidia per replication) and the experiment was performed three times. The percentage of conidial germination inhibition was calculated using Equation (10) from [32].
where gc is the number of germinated conidia in the untreated control and gt is the number of germinated conidia for each plasma treatment.
2.5. Fungal Morphology Studied by Scanning Electron Microscopy (SEM)
Fungal mycelia and sporangiospores were prepared for observation by scanning electron microscopy, following the method outlined by Supakitthanakorn et al. [33]. Mycelial discs exposed to individual GA, TC or DBD plasma treatments for 10, 15 or 20 min, along with the untreated control and sporangiospores treated with GA, TC or DBD plasma for 1, 3, 5 or 7 min were fixed in 2.5% (v/v) glutaraldehyde diluted in 0.01 M phosphate-buffered saline (PBS) at 4 °C overnight. Subsequently, the samples were washed three times in 0.01 M PBS (10 min each). Afterwards, the samples were dehydrated in a graded series of ethanol concentrations (30, 50, 70, 80, 90 and 100%), each for 15 min. The ethanol in the samples was then replaced using a critical point dryer K850 (Quorum Technologies, Sussex, UK). Following this, the samples were glow-discharged and SEM observations were carried out using a JSM-IT200 (JEOL Ltd., Tokyo, Japan).
2.6. Inactivation of Fungal Pathogenicity
Fungal mycelial discs were exposed to each NTP for 10, 15 or 20 min and then placed on strawberry fruit, which were surface-disinfected using 10% (v/v) Clorox (Clorox, Oakland, CA, USA). Untreated mycelial discs were used as a positive control and fungal-free discs as a negative control. Each treatment contained nine pieces of fruit and the experiment was repeated three times. The fruit were incubated at room temperature (25–28 °C) under controlled light conditions with a 12 h light/12 h dark cycle and observed daily. Disease incidence (DI), disease severity (DS) and disease severity index (DSI) were recorded. Disease incidence was recorded and calculated according to [5] (Equation (11)), as follows:
Disease severity was evaluated at 2, 3, 4, 5 and 6 dpi by a scale of scores composed of five levels (0 = absence of symptoms; 1 = >0 to 25% area covered by fungal growth; 2 = 26 to 50%; 3 = 51 to 75%; 4 = >76% area covered by fungal growth). From these numbers, Disease Index (DI) was calculated according to [5] (Equation (12)):
in which ni is the number of infected fruit in the corresponding scale of scores and N is the total number of fruit.
2.7. Visual Effects of Non-Thermal Plasma on Characteristics of Strawberry
Strawberries were sliced to a uniform thickness of 1.0 cm, encompassing both external and internal tissues. The slices were exposed to all NTP devices for durations of 10, 15 or 20 min. Visual characteristics consisting of discolouration, dehydration and surface roughness of both external and internal tissues were assessed immediately following plasma treatment.
2.8. Statistical Analyses
Data in this study were analysed using Statistix version 8 (Analytical Software, Tallahassee, FL, USA). Analysis of variance (factors plasma type and exposure duration) followed by a least significant difference (LSD) test at p < 0.05 was performed. All experiments were performed three times and the results from independent, representative experiments are presented.
3. Results
3.1. Inhibition of Mycelial Growth
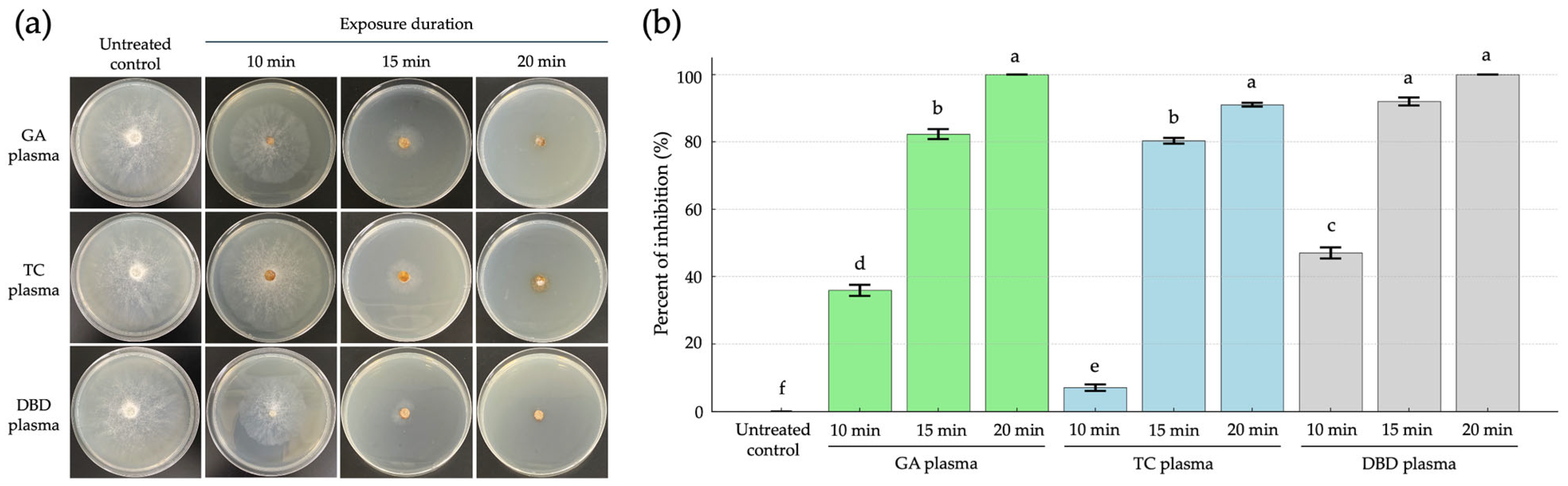
The inhibitory effect of GA, TC and DBD plasmas was evaluated against R. stolonifer under varying exposure durations (10, 15 or 20 min), as compared to untreated controls at 5 days post-incubation (dpi). The NTP treatments significantly inhibited fungal growth on strawberries. The 20 min exposures of both GA and DBD plasmas showed the highest inhibition, reaching 100% inhibition and were statistically grouped together, indicating no significant difference between them (Figure 2a,b). Similarly, TC plasma at 20 min also exhibited strong inhibitory effects (90%). At 15 min, a marked suppression of mycelial growth was evident for all NTP exposures with 71, 71 and 86% inhibition from GA, TC and DBD plasma exposure, respectively, suggesting increased sensitivity to the NTP treatments with prolonged exposure (Figure 2a,b).
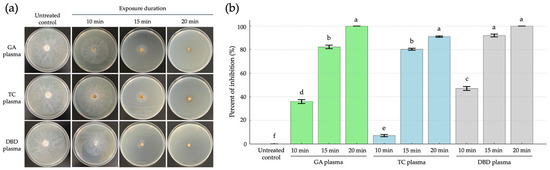
Figure 2.
The inhibition of mycelial growth of Rhizopus stolonifer at 5 days post incubation (dpi) through atmospheric non-thermal plasmas after exposure for 10, 15 or 20 min. (a) Mycelial growth of R. stolonifer on PDA. (b) The percent inhibition of mycelial growth of R. stolonifer. Average from three replications. Error bars represent standard error of means. Means marked with different letters are significantly different at p < 0.05, using the LSD test.
In contrast, shorter exposure times, especially TC plasma for 10 min, showed the lowest inhibition (7%), which was significantly different from all other treatments (Figure 2a,b). The untreated control exhibited extensive mycelial growth, indicating normal fungal development in the absence of NTP exposure (Figure 2a,b). Overall, increasing the plasma exposure time improved the antifungal efficacy, with GA and DBD plasmas being more effective than TC plasma at equivalent durations. These findings suggest that GA and DBD plasma treatments, particularly at 20 min, are highly effective in inhibiting the mycelial growth of R. stolonifer.
3.2. Inhibition of Sporangiospore Germination
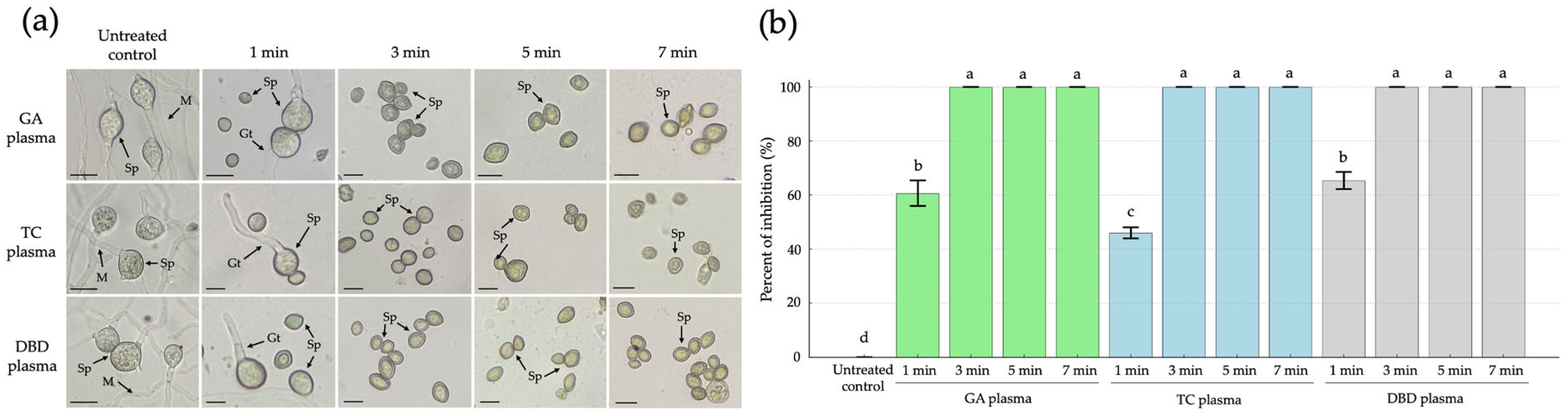
The microscope images illustrate the morphological effects of NTP treatments on R. stolonifer sporangiospores over various exposure durations (1, 3, 5 and 7 min) compared to the untreated control. In the untreated control, characteristic fungal structures such as mature sporangiospores (Sp), germ tubes (Gt) and intact mycelia (M) were clearly visible, indicating active fungal growth and viability (Figure 3a). Following 1 min exposure to GA, TC or DBD plasmas, sporangiospores remained largely intact, although a reduction in sporangiospore germination was observed with 61, 46 and 65% inhibition of germination from GA, TC and DBD plasma exposure, respectively (Figure 3a,b). After 3, 5 and 7 min of exposure, no visible germ tubes or mycelial growth were observed with a 100% inhibition of sporangiospore germination (Figure 3a,b).
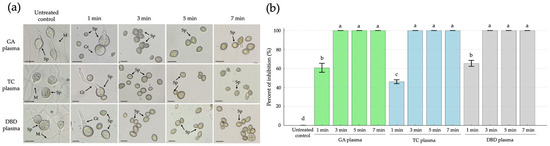
Figure 3.
The effects of atmospheric non-thermal gliding arc (GA), Tesla coil (TC) and dielectric barrier discharge (DBD) plasmas on inhibition of sporangiospore germination of Rhizopus stolonifer after 1, 3, 5 or 7 min exposure to each plasma, observed at 24 h after incubation. (a) Sporangiospore germination observed by light microscopy. (b) Percentage inhibition of sporangiospore germination. Sp = sporangiospores; Gt = germ tube; M = mycelia. The scale bar is 10 µM. Error bars represent standard error of means. Means marked with different letters are significantly different at p < 0.05, using the LSD test.
3.3. Fungal Morphology with SEM
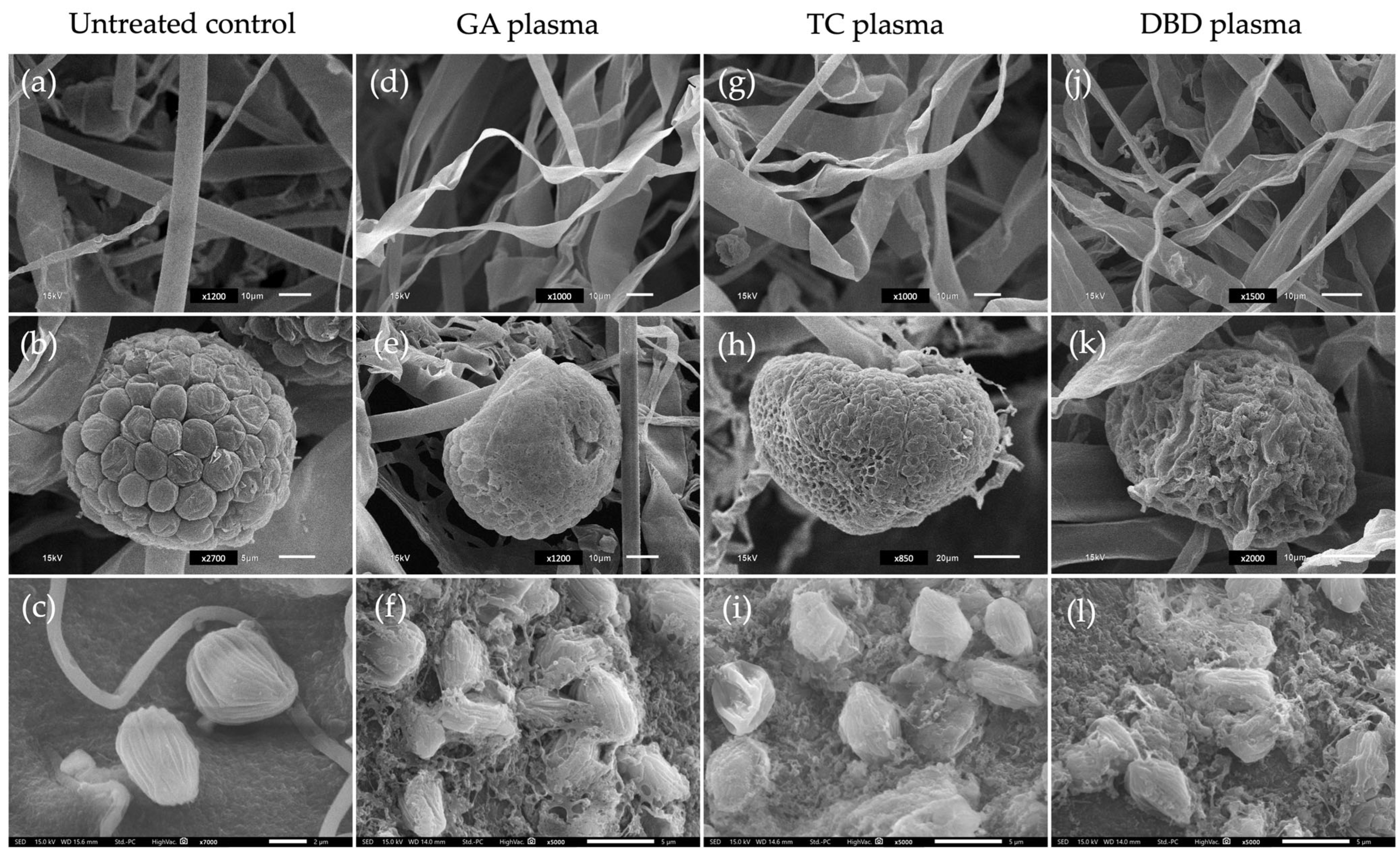
SEM images illustrate the morphological effects of three types of non-thermal plasma treatments GA, TC and DBD on fungal mycelia, sporangia and sporangiospores compared to an untreated control. For the untreated control, mycelia displayed smooth surfaces with intact structures at 24 h after incubation (Figure 4a). In contrast, plasma-treated mycelia exhibited various degrees of structural deformation. Notably, GA and TC treatments caused the shrinkage and twisting of mycelia, while DBD plasma showed a more pronounced disruption and collapse of the mycelial structures, suggesting higher oxidative or structural damage (Figure 4d,g,j).
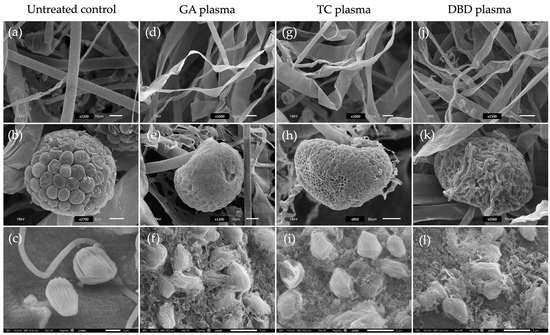
Figure 4.
Scanning electron micrographs of mycelia (top row) and sporangia (middle row) of Rhizopus stolonifer after exposure to three atmospheric non-thermal plasmas (NTPs) for 20 min and sporangiospores (bottom row) treated to NTPs for 7 min. (a–c) Untreated control. (d–f) Gliding arc (GA) plasma. (g–i) Tesla coil (TC) plasma. (j–l) Dielectric barrier discharge (DBD) plasma. Scale bars: 2 µm (c); 5 µm (b,f,i,l); 10 µm (a,d,g,j,e,k); and 20 µm (h).
For sporangia, the untreated control sample revealed well-organised and intact surfaces (Figure 4b). NTP treatments led to significant morphological changes. Exposure to GA and TC plasmas caused a moderately wrinkled and collapsed surface (Figure 4e,h). The DBD plasma caused the most severe damage, with the surface appearing highly deformed and ruptured (Figure 4k). In the case of sporangiospores, the untreated control presented intact, ovoid sporangiospores with smooth surfaces (Figure 4c). NTP-treated sporangiospores showed increasing levels of damage. GA and TC exposure lead to surface roughening and minor deformation (Figure 4f,i), whereas DBD plasma resulted in extensive sporangiospore wall disruption and disintegration, likely affecting viability (Figure 4l).
3.4. Reduction in Fungal Pathogenicity
The NTP treatments of GA and DBD plasma at 15 or 20 min exposure were the most effective non-thermal plasma in inhibiting the pathogenicity of R. stolonifer. These treatments markedly suppressed fungal growth, with little to no visible mycelial development even at 6 dpi (Figure 5). GA plasma at 10 min of exposure showed partial effectiveness, with minor fungal growth observed at later time points, whereas GA at 15 or 20 min almost completely inhibited fungal colonisation (Figure 5). Similarly, DBD plasma at 15 or 20 min exposure effectively maintained fruit characteristics, indicating strong antifungal activity (Figure 5). TC plasma, however, was overall less effective. At 10 or 15 min exposure, fungal growth appeared as early as 2–3 dpi, with extensive colonisation by 4 dpi, resembling the untreated, inoculated fruit (positive control, Figure 5). Only the 20 min TC exposure delayed and reduced fungal development, indicating that longer exposure times are needed for this plasma type to achieve significant control.
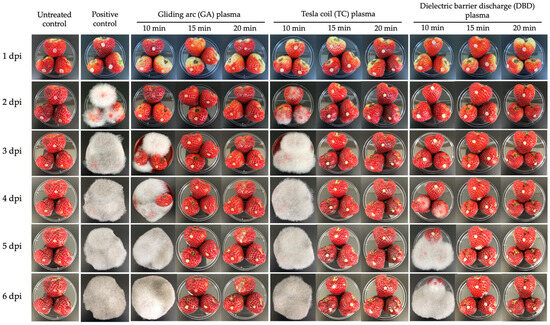
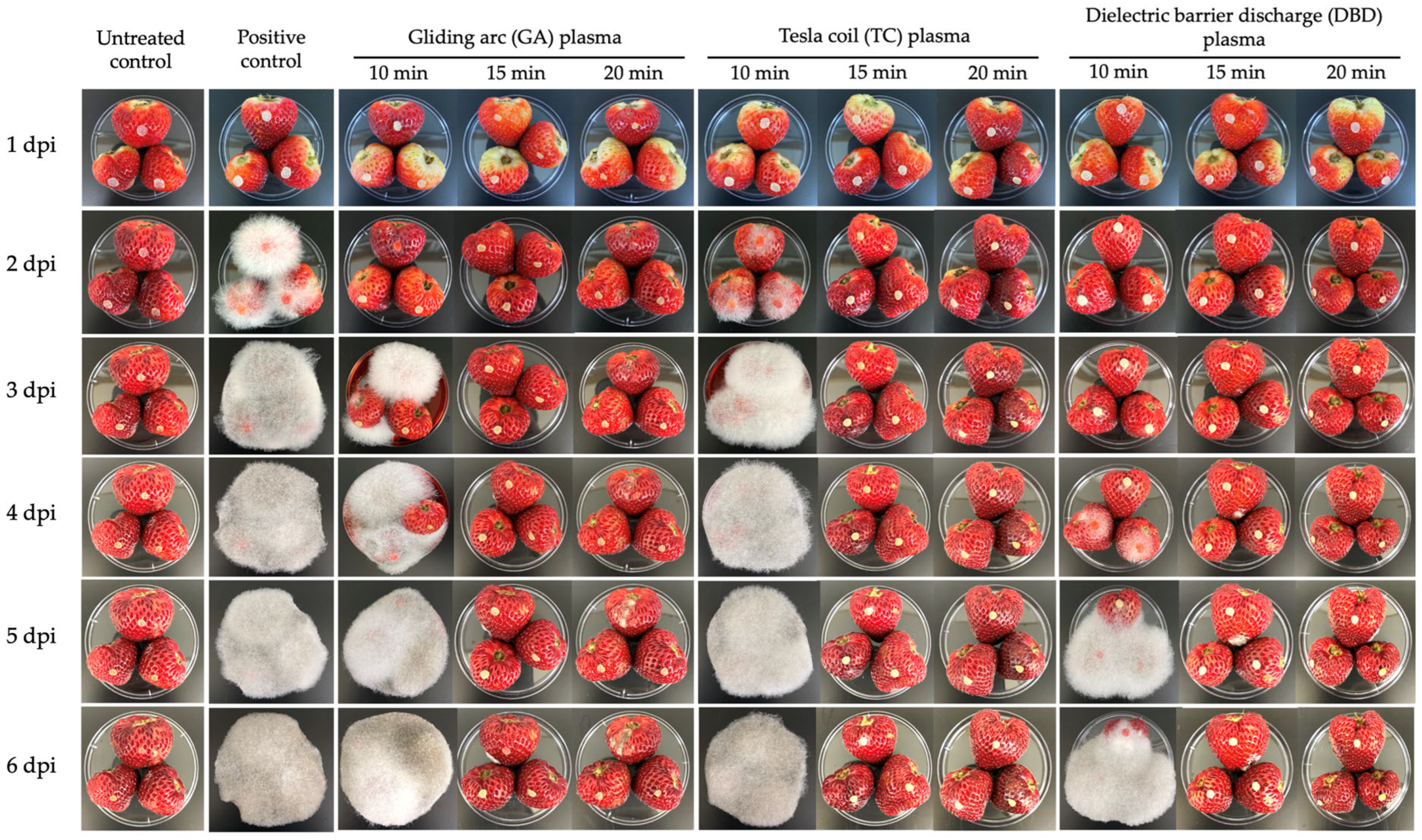
Figure 5.
Inhibition of Rhizopus stolonifer pathogenicity on strawberries was assessed after exposure to atmospheric non-thermal gliding arc (GA), Tesla coil (TC) and dielectric barrier discharge (DBD) plasmas for 10, 15 or 20 min. Photographs were taken at 1, 2, 3, 4, 5 and 6 days post-inoculation (dpi). Positive control: untreated, inoculated fruit.
The untreated, uninoculated control remained healthy throughout the experiment, showing no signs of fungal infection, confirming the sterility of the environment (Figure 5). In contrast, the untreated positive control (inoculated without plasma treatment) showed rapid and severe fungal growth beginning at 2 dpi, with full fruit coverage by white mycelium by 3 dpi, which persisted and intensified to 6 dpi. This demonstrates the aggressive nature of R. stolonifer under untreated conditions (Figure 5).
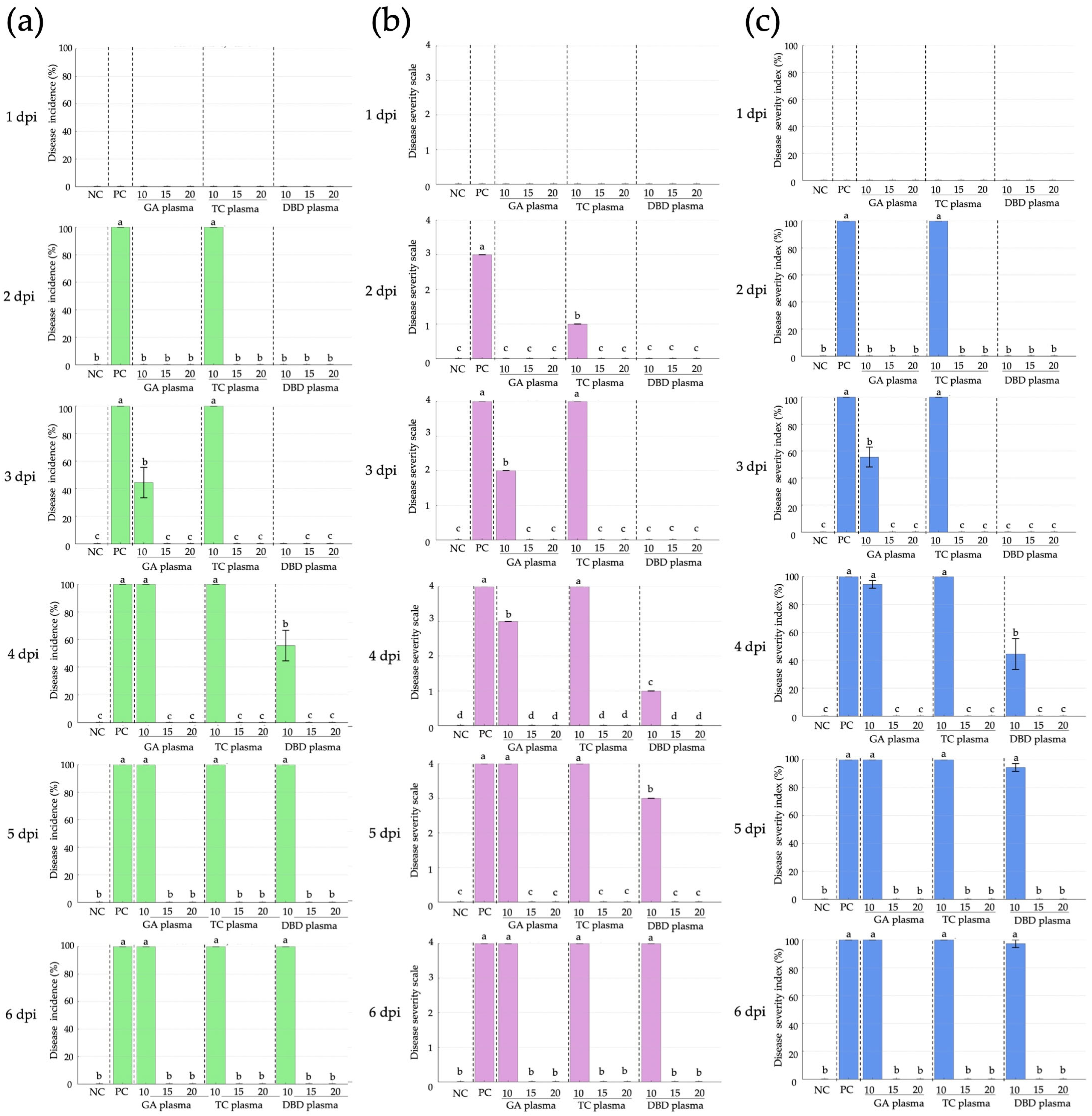
The effect of NTP treatments on the disease incidence of soft rot disease in strawberries caused by R. stolonifer was evaluated for 6 days (Figure 5 and Figure 6). Treatments consisted of GA, TC and DBD plasmas applied for 10, 15 or 20 min, along with untreated and untreated, inoculated controls. At 1 dpi, no disease symptoms were observed for any treatment. From 2 dpi, the untreated, inoculated control fruit exhibited 100% disease incidence (Figure 5 and Figure 6), confirming the successfulness of R. stolonifer infection. Both GA and TC plasma treatments applied for 10 min significantly reduced disease incidence at 2 and 3 dpi, with GA at 10 min exposure exhibiting the greatest reduction. Conversely, DBD plasma demonstrated the most consistent and effective disease control. At 2 and 3 dpi, no disease symptoms were detected for any DBD-treated fruit. At 4 dpi, DBD 10 min displayed moderate disease incidence (56%), whereas DBD at 15 or 20 min exposure demonstrated complete disease suppression throughout the 6-day evaluation period (Figure 5 and Figure 6). These findings suggest that all plasma treatments, particularly the longer exposure durations (15 or 20 min), provided the most effective inhibition of soft rot disease progression.
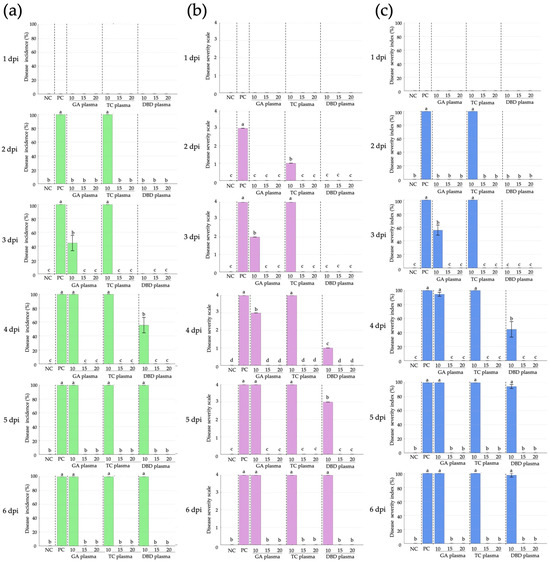
Figure 6.
Disease incidence, disease severity and disease severity index (DSI) of Rhizopus stolonifer soft rot disease of strawberry after exposure to atmospheric non-thermal gliding arc (GA), Tesla coil (TC) and dielectric barrier discharge (DBD) plasmas for 10, 15 or 20 min. (a) Disease incidence. (b) Disease severity. (c) Disease severity index. NC: untreated, uninoculated control, PC: inoculated, untreated control. Within each graph, means marked with different letters are significantly different at p < 0.05, using the LSD test.
For disease severity, at 1 dpi, no disease symptoms were recorded for any treatment (Figure 5 and Figure 6). From 2 dpi, the untreated, inoculated control fruit showed a progressive increase in disease severity, reaching the maximum score of 4 by 4 dpi and maintaining this level until the end of the experiment (Figure 5 and Figure 6). In the GA and TC plasma treatment, a notable reduction in disease severity was observed at 2 and 3 dpi, particularly for the 10 min treatment, which showed the lowest severity scores at these time points. However, the disease severity scores for the GA and TC treatments reached the maximum level (4) by 4 dpi, comparable to the untreated, inoculated control (Figure 5 and Figure 6). DBD plasma treatment provided superior suppression of disease severity at all time points. No symptoms were observed at 2 and 3 dpi for any DBD treatment (Figure 5 and Figure 6). From 4 dpi, DBD treatments of 15 or 20 min consistently maintained a disease severity score of 0, whereas the 10 min treatment exhibited a moderate increase in disease severity, beginning at 4 dpi, yet remaining lower than all other plasma treatments (Figure 5 and Figure 6).
For the disease severity index (DSI), no symptoms were observed for any treatment at 1 dpi (Figure 5 and Figure 6). By 2 dpi, both the inoculated, untreated control and TC treatments reached a DSI of 100%, whereas the GA and DBD treatments remained at DSI 0% (Figure 5 and Figure 6). At 3 dpi, the inoculated, untreated control and TC plasma with 10 min exposure durations gave DSI 100%, whereas GA plasma at 10 min gave DSI 56%. However, treatments with exposure times exceeding 15 min exhibited DSI 0% (Figure 5 and Figure 6). By 4 dpi, DSI for GA plasma at 10 min reached DSI 94%, whereas all NTP treatments at 15–20 min achieved complete control. DBD plasma at 10 min moderately suppressed symptoms, with 15–20 min treatments achieving complete inhibition (Figure 5 and Figure 6). At 5–6 dpi, all 10 min exposures for all NTPs and the inoculated, untreated control reached DSI 100%, while longer exposures of 15 or 20 min maintained DSI 0% (Figure 5 and Figure 6). DBD plasma demonstrated the highest efficacy, achieving full disease suppression at 15 min exposure.
3.5. Effects of NTPs on Characteristics of Strawberries
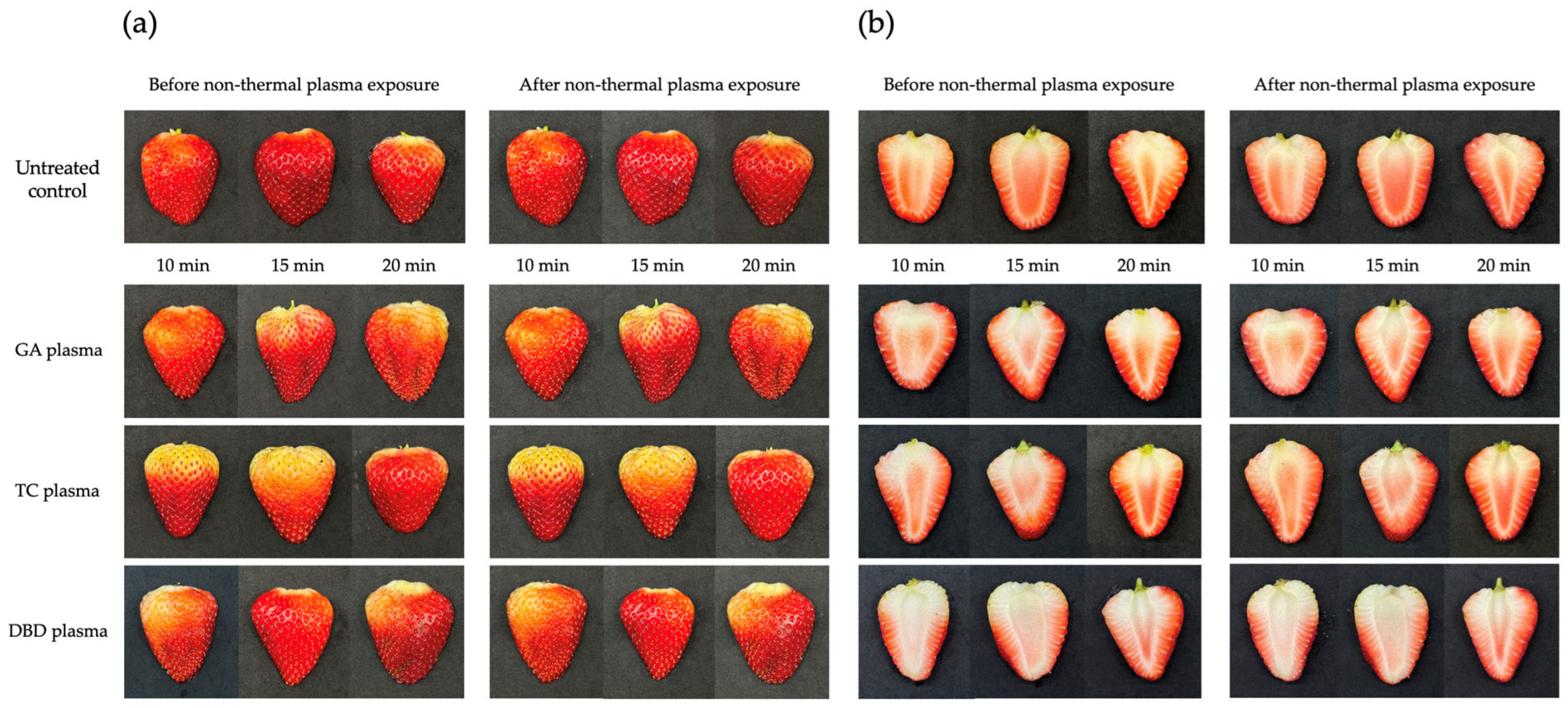
The visual effects of the non-thermal plasmas GA, TC and DBD on strawberries at various exposure durations (10, 15 or 20 min) are shown in Figure 7. Untreated strawberries served as controls. For all NTP treatments, the external appearance of the fruit remained relatively unchanged and the fruit surface maintained their characteristic red colouration throughout the exposure period. These results suggest the non-toxicity of the non-thermal plasmas on both the external and internal characteristics of strawberries even after treatments for 10, 15 or 20 min.
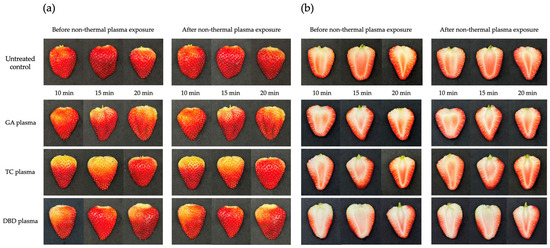
Figure 7.
Effects of atmospheric non-thermal gliding arc (GA), Tesla coil (TC) and dielectric barrier discharge (DBD) plasmas on external and internal characteristics of strawberry after exposure for 10, 15 or 20 min. (a) external characteristics of strawberries before (left) and after (right) exposure to NTPs. (b) internal characteristics of strawberries before (left) and after (right) exposure to NTPs.
4. Discussion
R. stolonifer is a common fungal pathogen responsible for a soft rot disease in strawberries, leading to rapid fruit decay during storage and transport [3]. Non-thermal plasmas (NTPs) are increasingly being explored as an effective method for controlling post-harvest diseases in fruits and vegetables due to its strong antimicrobial properties and minimal impact on product quality [34,35]. To explore an alternative strategy for fungal inactivation and disease control, the efficacy of NTPs was investigated.
GA and DBD plasma treatments completely inhibited mycelial growth after exposure for 20 min, but for TC plasma, exposure for 20 min could not completely inhibit mycelial growth and there is a need to prolong the exposure period for complete inhibition. This result is consistent with a previous study [29], which reported that TC plasma exhibited the lowest antifungal efficacy against Cercospora lactucae-sativae.
The duration of plasma treatment required to inhibit mycelial growth and sporangiospore germination may vary depending on the fungal species. For example, plasma exposure for 3 min reduced the mycelial growth of Fusarium graminearum and F. oxysporum f. sp. lycopersici [36], whereas exposure for 5–10 min inhibited spore germination by up to 100% in Penicillium digitatum, Fusarium fujikuroi, Colletotrichum gloeosporioides and Cercospora lactucae-sativae [29,37,38]. In the current study, the duration of plasma exposure required for the complete inhibition of mycelial growth was 20 min, whereas complete inhibition of sporangiospore germination was achieved with 3 min exposure. The treatment time of NTPs for fungal inactivation is influenced by factors such as plasma type and power, gas composition, electrode distance, humidity and target surface properties. Short distances and oxygen-rich gases enhance reactive species oxygen like •OH, while humidity boosts aqueous RONS. Optimising these parameters is crucial to ensure effective fungal control without compromising product quality [39].
It was observed that the plasma treatments required a longer exposure time to effectively inhibit fungal mycelial growth compared to fungal sporangiospores. This can be attributed to the greater structural complexity, increased cell-wall thickness and the dense hyphal network of mycelia, which act as physical and biochemical barriers against the reactive species generated by plasma [14,17]. In contrast, sporangiospores, due to their smaller size and higher surface-to-volume ratio, are more readily inactivated under similar plasma conditions. These changes may primarily be due to oxidative stress induced by short-lived RONS like hydroxyl radicals (•OH) and singlet oxygen (1O2) and long-lived species like hydrogen peroxide (H2O2) and nitrite (NO2−), which degrade lipid membranes and denature proteins as observed under SEM [40].
SEM observations after non-thermal plasma treatment revealed that DBD plasma induced the most pronounced structural damage to all fungal components ranging from mycelia, sporangia to sporangiospores, followed by the GA and TC treatments. These results suggest that DBD plasma is potentially the most effective in disintegrating the structural integrity and viability of fungal propagules, supporting its potential use in antifungal applications. DBD plasma was shown to significantly reduce Penicillium expansum, the causal agent of blue mould in apples, without altering the physicochemical characteristics of the fruit [21,41]. Similarly, DBD plasma treatment inhibited the growth of Botrytis cinerea, a major post-harvest pathogen in strawberries, thereby extending shelf life and maintaining fruit quality [18].
The optical emission spectroscopy analysis revealed that GA plasma produced the highest concentrations of RONS, followed by TC and DBD plasmas. While these compositional differences correlate with antifungal efficacy and treatment intensity, DBD plasma demonstrated superior performance against R. stolonifer due to its plate-type configuration, providing uniform coverage, whereas GA and TC are point-source design and resulted in incomplete treatment coverage. However, prolonged exposure times minimised these configuration-based differences, suggesting that treatment duration can compensate for plasma source limitations.
The reduction in fungal pathogenicity after non-thermal plasma treatment showed that DBD plasma achieved the greatest reduction after 10 min exposure, compared to GA and TC plasmas. However, after 15 or 20 min exposure, the inactivation results did not differ significantly among the non-thermal plasma treatments. GA and TC plasmas, being point or small-volume plasma sources, may not be suitable for scaling up or for applications involving large numbers of samples. In contrast, DBD plasma, due to its high flexibility and adaptability, is particularly well-suited for treating fruit products of various shapes and sizes. By modifying the reactor design and discharge parameters, DBD systems can be tailored to provide effective treatment for a wide range of agricultural products [42]. Furthermore, the DBD plasma technology is cheaper and easier to install than GA and TC.
The post-treatment evaluation of strawberry external and internal characteristics following NTP exposure revealed only negligible alterations, primarily manifested as minor dehydration effects attributable to extended plasma treatment durations. These findings demonstrate the substantial potential of non-thermal plasma technology for the effective surface disinfection of strawberries while preserving critical quality parameters and commercial acceptability. The minimal impact on fruit integrity suggests that NTP treatment represents a viable approach for post-harvest pathogen control that maintains the balance between antimicrobial efficacy and product quality preservation. However, the effectiveness of NTP treatment can vary depending on the type of plasma system, the specific pathogen targeted and the physical and biochemical characteristics of fruits [11,19].
In NTP-treated strawberries, the technology demonstrated dual benefits by significantly reducing the microbial burden while concurrently extending shelf life, without adversely affecting critical quality parameters including fruit firmness, chromaticity or nutritional composition when applied under optimised operational conditions [43,44]. However, an excessive duration of exposure or elevated plasma intensity can lead to detrimental effects, including surface dehydration and oxidative degradation, underscoring the critical importance of precise parameter optimisation including treatment duration, carrier gas composition and electrode-to-sample distance [45]. The efficacy of NTP technology is therefore dependent on the careful calibration of these operational variables to achieve optimal pathogen inactivation while minimising potential quality deterioration [46].
Furthermore, NTP technology presents a compelling environmentally sustainable and residue-free alternative to conventional chemical fungicides, positioning it as an attractive option for fresh produce preservation applications. The absence of chemical residues and the minimal environmental impact align with contemporary consumer preferences and regulatory trends toward reduced chemical inputs in food production [47]. Nevertheless, successful commercial implementation requires addressing key challenges including process scalability for industrial applications, the standardisation of treatment protocols and the navigation of regulatory frameworks for novel food-processing technologies [48]. These considerations represent critical areas requiring continued research and development to fully realise the commercial potential of NTP in post-harvest fruit preservation.
However, the NTP device used in this study had a fixed entrance and non-adjustable distance, which limited its application to whole strawberry fruit. As a result, only mycelial plugs and sliced strawberries could be examined. Therefore, further research will focus on scaling up the non-thermal plasma device to accommodate whole strawberries, enabling plasma exposure of the entire fruit to prevent and reduce fungal contamination.
5. Conclusions
This study demonstrates the potential of atmospheric NTPs, including GA, TC and DBD, as effective tools for controlling R. stolonifer, a causal agent of soft rot in strawberries. Plasma treatments, particularly GA and DBD, significantly inhibited fungal growth and induced extensive morphological damage to the mycelia, sporangia and sporangiospores, as confirmed by SEM observations. All NTP treatments effectively suppressed sporangiospore germination within a short exposure time and 15 min exposure was sufficient to completely prevent disease development in strawberry fruit without compromising fruit quality. These findings highlight the potential of NTPs as promising non-chemical alternatives for the post-harvest management of fungal infections in strawberries.
Author Contributions
Conceptualisation, D.B. and S.S.; methodology, S.S.; software, S.S.; validation, D.B. and S.S.; formal analysis, S.S.; investigation, S.S.; resources, D.B., H.J.L.J. and S.S.; data curation, D.B. and S.S.; writing—original draft preparation, D.B., H.J.L.J. and S.S.; writing—review and editing, D.B., H.J.L.J. and S.S.; visualisation, D.B. and S.S.; supervision, D.B. and H.J.L.J.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the Hub of Talents for Plasma Technology 2024 funded by the National Research Council of Thailand (NRCT), contract number N23E670083/2024.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors would like to thank Plasma and Beam Physics Research Facility, Faculty of Science, Chiang Mai University for providing the plasma device used in this study and Department of Plant Pathology, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University, Kamphaeng Saen Campus, for providing research facilities during the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DBD | Dielectric barrier discharge |
| GA | Gliding arc |
| NTP | Non-thermal plasma |
| SEM | Scanning electron microscopy |
| TC | Tesla coil |
References
- Aglave, B. Handbook of Plant Disease Identification and Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–70. [Google Scholar]
- Avis, T.J.; Martinez, C.; Tweddell, R.J. Effect of chlorine atmospheres on the development of Rhizopus rot (Rhizopus stolonifer) and gray mold (Botrytis cinerea) on stored strawberry fruits. Can. J. Plant Pathol. 2006, 28, 526–532. [Google Scholar] [CrossRef]
- Xu, X.; Agyare, S.; Browne, E.; Passey, T. Predicting infection of strawberry fruit by Mucor and Rhizopus spp. under protected conditions. Front. Hortic. 2024, 3, 1373717. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Liu, H.; Du, Y.; Jiao, W.; Sun, F.; Fu, M. Rhizopus stolonifer and related control strategies in postharvest fruit: A review. Heliyon 2024, 10, e29522. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Parisi, M.C.M.; Baggio, J.S.; Silva, P.P.M.; Paviani, B.; Spoto, M.H.F.; Gloria, E.M. Control of Rhizopus stolonifer in strawberries by the combination of essential T oil with carboxymethylcellulose. Int. J. Food Microbiol. 2019, 292, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Tsai, J.N.; Ann, P.J.; Chang, J.T.; Chen, P.R. First report of Rhizopus rot of strawberry fruit caused by Rhizopus stolonifer in Taiwan. Plant Dis. 2016, 101, 254. [Google Scholar] [CrossRef]
- Bautista-Banos, S.; Bosquez-Molina, E.; Barrera-Necha, L.L. Chapter 1—Rhizopus stolonifer (Soft Rot). In Postharvest Decay Control Strategies; Bautista-Banos, S., Ed.; Academic Press: London, UK, 2014; pp. 1–44. [Google Scholar]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.-D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from physical plasmas: Redox chemistry for biomedical therapy. Oxid. Med. Cell Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.; Yang, H.; Ma, L.; Guo, M.; Liu, Z. Cold plasma treatment improves seed germination and seedling growth of soybean. Sci. Rep. 2019, 9, 10245. [Google Scholar]
- Sera, B.; Sery, M.; Sera, J.; Gajdova, I. Effects of cold atmospheric plasma on plant growth and pathogen control: A review. Plants 2021, 10, 1896. [Google Scholar]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julák, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Lukes, P.; Locke, B.R.; Brisset, J.-L. Aqueous-phase chemistry of electrical discharge plasma in water and in gas–liquid environments. In Plasma Chemistry and Catalysis in Gases and Liquids; Fridman, A., Locke, B.R., Shah, M.M., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 243–308. [Google Scholar]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant disease control by non-thermal atmospheric-pressure plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Donnell, C.P.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2018, 80, 123–131. [Google Scholar] [CrossRef]
- Ma, R.; Jiao, Z. Inactivation of fungi and fungal toxins by cold plasma. In Applications of Cold Plasma in Food Safety, 1st ed.; Ding, T., Cullen, P., Yan, W., Eds.; Springer: Singapore, 2022; pp. 113–166. [Google Scholar]
- Panngom, K.; Lee, S.H.; Park, D.H.; Sim, G.B.; Kim, Y.H.; Uhm, S.H.; Park, G.; Choi, E.H. Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PLoS ONE 2014, 9, e99300. [Google Scholar] [CrossRef] [PubMed]
- Yudhistira, B.; Sulaimana, A.S.; Jumeri; Supartono, W.; Hsieh, C.-W. The use of low-pressure cold plasma optimization for microbial decontamination and physicochemical preservation of strawberries. J. Agric. Food Res. 2023, 14, 100844. [Google Scholar] [CrossRef]
- Siddique, S.S.; Hardy, G.E.S.J.; Bayliss, K.L. Cold plasma: A potential new method to manage postharvest diseases caused by fungal plant pathogens. Plant Pathol. 2018, 67, 1011–1021. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillium spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Khalaj, A.; Ahmadi, E.; Mirzaei, S.; Ghaemizadeh, F. Potential use of cold plasma treatment for disinfection and quality preservation of grape inoculated with Botrytis cinerea. Food Sci. Nutr. 2023, 12, 1818–1833. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Wang, X.; Wang, X. Investigation on the effect of cold plasma on the quality of apple during storage. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar]
- Wu, Y.; Cheng, J.-H.; Keener, K.M.; Sun, D.-W. Inhibitory effects of dielectric barrier discharge cold plasma on pathogenic enzymes and anthracnose for mango postharvest preservation. Postharvest Biol. Technol. 2023, 196, 112181. [Google Scholar] [CrossRef]
- Baier, M.; Foerster, J.; Schnabel, U.; Knorr, D.; Ehlbeck, J.; Herppich, W.B. Direct non-thermal plasma treatment for the sanitation of fresh strawberries. J. Food Eng. 2013, 116, 386–393. [Google Scholar]
- Zhou, R.; Zhou, R.; Zhang, X.; Yang, S.; Wang, Y. Effects of cold plasma on strawberry quality. J. Food Eng. 2016, 165, 17–25. [Google Scholar]
- Los, A.; Ziuzina, D.; Boehm, D.; Patil, S.; Bourke, P.; Cullen, P.J. Improving microbiological safety and quality of strawberries using cold plasma treatment. Int. J. Food Microbiol. 2020, 336, 108896. [Google Scholar]
- Supakitthanakorn, S.; Ruangwong, O.-U.; Boonyawan, D. Inactivation of Cercospora lactucae-sativa through application of non-thermal atmospheric pressure gliding arc, Tesla coil and dielectric barrier discharge plasmas. Appl. Sci. 2023, 13, 6643. [Google Scholar] [CrossRef]
- Supakitthanakorn, S.; Boonyawan, D. Inhibition of Rhizopus stolonifer causing soft rot disease of strawberry fruits through application of non-thermal atmospheric pressure dielectric barrier discharge (DBD) plasma. In Proceedings of the 7th Asian Conference on Plant Pathology, Changchun, China, 3–6 August 2025. [Google Scholar]
- Tarabova, B.; Lukes, P.; Janda, M.; Hensel, K.; Sikurova, L.; Machala, Z. Specificity of detection methods of nitrites and ozone in aqueous solutions activated by air plasma. Plasma Process. Polym. 2018, 15, 1800030. [Google Scholar] [CrossRef]
- Philippe, S.; Souaibou, F.; Guy, A.; Sebastien, D.T.; Boniface, Y.; Paulin, A.; Issaka, Y.; Dominique, S. Chemical composition and antifungal activity of essential oil of fresh leaves of Ocimum gratissimum from Benin against six mycotoxigenic fungi isolated from traditional cheese wagashi. Res. J. Biol. Sci. 2012, 1, 22–27. [Google Scholar]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G. Cold plasma seed treatments for sustainable agriculture: Prospects and challenges. Agriculture 2020, 10, 191. [Google Scholar]
- Supakitthanakorn, S.; Ruangwong, O.-U.; Sawangrat, C.; Srisuwan, W.; Boonyawan, D. Potential of nonthermal atmospheric-pressure dielectric barrier discharge plasma for inhibition of Athelia rolfsii causing southern blight disease in lettuce. Agriculture 2023, 13, 167. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, W.; Liu, R.; Xing, Y.; Yu, X.; Jiang, H. Cold plasma enzyme inactivation on dielectric properties and freshness quality in bananas. Innov. Food Sci. Emerg. Technol. 2021, 69, 102649. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Yang, Y.; Mulati, A.; Hong, J.; Wang, J. The effects of cold-plasma technology on the quality properties of fresh-cut produce: A review. Foods 2025, 14, 149. [Google Scholar] [CrossRef]
- Na, Y.H.; Park, G.; Choi, E.H.; Uhm, H.S. 2013. Effects of the physical parameters of a microwave plasma jet on the inactivation of fungal spores. Thin Solid Films 2013, 547, 125–131. [Google Scholar] [CrossRef]
- Kang, M.H.; Pengkit, A.; Choi, K.; Jeon, S.S.; Choi, H.W.; Shin, D.B.; Choi, E.H.; Uhm, S.H.; Park, G. Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS ONE 2015, 10, e0139263. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, C.; Hu, H.; Lei, J.; Han, L. Indirect treatment effects of water air MHCD jet on the inactivation of Penicillium digitatum suspension. IEEE Trans. Plasma Sci. 2016, 44, 2729–2737. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C.; Mushtaq, M.; Keener, K.M.; Bourke, P. Cold plasma in food and agriculture. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 2016; pp. 1–20. [Google Scholar]
- Ouf, S.A.; Basher, A.H.; Mohamed, A.A.H. Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J. Sci. Food Agric. 2015, 95, 3204–3210. [Google Scholar] [CrossRef]
- Nikzadfar, M.; Kazemi, A.; Abooei, R.; Abbaszadeh, R.; Firouz, M.S.; Akbarnia, A.; Rashvand, M. Application of cold plasma technology on the postharvest preservation of in-packaged fresh fruit and vegetables: Recent challenges and development. Food Bioprocess Technol. 2024, 17, 4473–4505. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, H.; Yang, X.; Cheng, J.-H. Versatile dielectric barrier discharge cold plasma for safety and quality control in fruits and vegetables products: Principles, configurations and applications. Food Res. Int. 2024, 196, 115117. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli in liquid media inside a sealed package. J. Appl. Microbiol. 2014, 116, 851–860. [Google Scholar] [CrossRef]
- Giannoglou, M.; Xanthou, Z.-M.; Chanioti, S.; Stergiou, P.; Christopoulos, M.; Dimitrakellis, P.; Efthimiadou, A.; Gogolides, E.; Katsaros, G. Effect of cold atmospheric plasma and pulsed electromagnetic fields on strawberry quality and shelf-life. Innov. Food Sci. Emerg. Technol. 2021, 68, 102631. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, Q.; Shi, W.; Yu, X.; Wang, S. Food preservation by cold plasma from dielectric barrier discharges in agri-food industries. Front. Nutr. 2022, 9, 1015980. [Google Scholar] [CrossRef]
- Harikrishna, S.; Anil, P.P.; Shams, R.; Dash, K.K. Cold plasma as an emerging nonthermal technology for food processing: A comprehensive review. J. Agri. Food Res. 2023, 14, 100747. [Google Scholar] [CrossRef]
- Sojithamporn, P.; Leksakul, K.; Sawangrat, C.; Charoenchai, N.; Boonyawan, D. Degradation of pesticide residues in water, soil, and food products via cold plasma technology. Foods 2023, 12, 4386. [Google Scholar] [CrossRef]
- Yawut, N.; Mekwilai, T.; Vichiansan, N.; Braspaiboon, S.; Leksakul, K.; Boonyawan, D. Cold plasma technology: Transforming food processing for safety and sustainability. J. Agri. Food Res. 2024, 18, 101383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).