Effects of Co-Inoculation of Indole-3-Acetic Acid-Producing and -Degrading Bacterial Endophytes on Plant Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. IAA-degrading Ability of the Bacterial Strains
2.3. Fate of IAA under Co-Cultivation of IAA-Producing and -Degrading Strains
2.4. Effect of Co-Inoculation of IAA-Producing and -Degrading Strains on Plants
2.5. Statistical Analysis
3. Results
3.1. IAA-Degrading Activity of the Bacterial Strains
3.2. Fate of IAA under Co-Cultivation of the IAA-producing and -degrading Strains
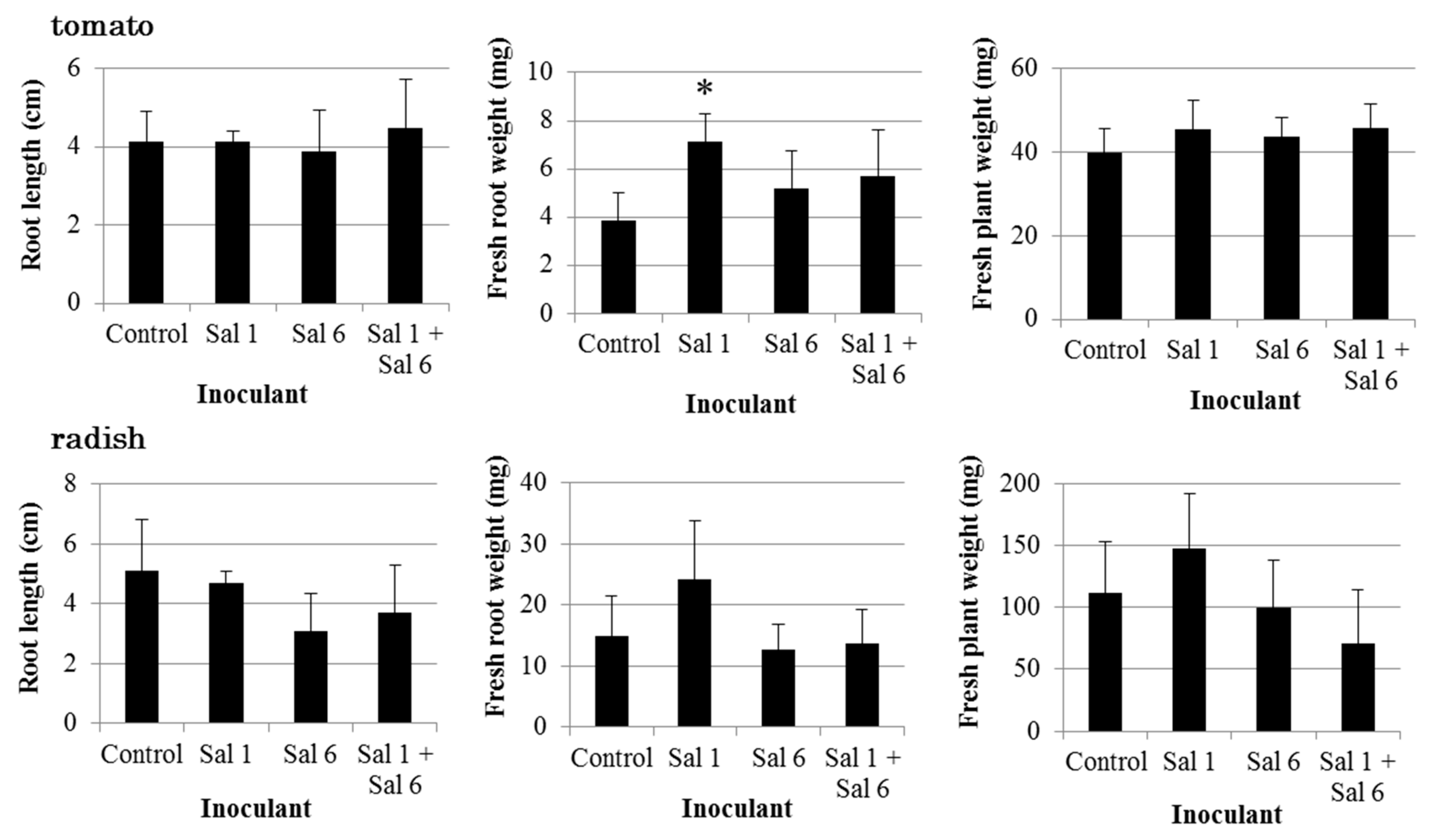
3.3. Effect of Inoculation of IAA-Producing and IAA-Degrading Strains on Plants
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tanimoto, E. Regulation of root growth by plant hormones—Roles for auxin and gibberellin. Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Tromas, A.; Perrot-Rechenmann, C. Recent pogress in auxin biology. Comptes Rendus Biol. 2010, 333, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Higashide, T.; Narukawa, M.; Shimada, Y.; Soeno, K. Suppression of elongation and growth of tomato seedlings by auxin biosynthesis inhibitors and modeling of the growth and environmental response. Sci. Rep. 2014, 4, 4556. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Doty, S.L. Characterization of bacterial endophytes of sweet potato plants. Plant Soil. 2009, 322, 197–207. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and Characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Elbeltagy, A.; Emara, H.M.; Abbas, I.H.; Hassan, M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci. 2013, 58, 195–201. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Reetha, S.; Bhuvaneshwari, G.; Thamizhiniyan, P.; Mycin, T.R. Isolation of indole acetic acid (IAA) producing rhizobacteria of Pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allim cepa. L). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 568–574. [Google Scholar]
- Susilowatia, D.N.; Riyanti, E.I.; Setyowati, M.; Mulya, K. Indole-3-acetic acid producing bacteria and its application on the growth of rice. In Proceedings of the AIP Conference, New York, NY, USA, 2018; Volume 2002, p. 020016. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Chaudhari, H.G.; Kasture, V.M.; Dhavale, D.D.; Chopade, B.A. Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J. Exp. Biol. 2009, 47, 993–1000. [Google Scholar] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Libbert, E.; Risch, H. Interactions between plants and epiphytic bacteria regarding their anxin metabolism, V. Isolation and identification of the IAA-producing and destroying bacteria from pea plants. Physiol. Plant. 1969, 22, 51–58. [Google Scholar] [CrossRef]
- Raczkowska-Błach, E.; Rózycki, H.; Strzelczyk, E.; Pokojska, A. Decomposition of indoleacetic acid (IAA) in soil and by bacterial strains isolated from soil and from the root zone of Scots pine (Pinus sylvestris L.). Microbiol. Res. 1995, 150, 265–270. [Google Scholar] [CrossRef]
- Gravel, V.; Martinez, C.; Antoun, H.; Tweddell, R.J. Antagonist microorganisms with the ability to control Pythium damping-off of tomato seeds in rockwool. BioControl 2005, 50, 771–786. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Dhungana, S.A.; Adachi, F.; Hayashi, S.; Puri, R.R.; Itoh, K. Plant growth promoting effects of Nepalese sweet potato endophytes. Horticulturae 2018, 4, 53. [Google Scholar] [CrossRef]
- Elbeltagy, A.; Nishioka, K.; Sato, T.; Suzuki, H.; Ye, B.; Hamada, T.; Isawa, T.; Mitsui, H.; Minamisawa, K. Endophytic colonization and in planta isolated from wild rice species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Weber, R.A. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. J. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Leveau, J.H.J.; Lindow, S.E. Utilization of the plant hormone indole-3-acetic acid for gowth by Pseudomonas putida strain 1290. Appl. Environ. Microbiol. 2005, 71, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Kurnasov, O.; Jablonski, L.; Polanuyer, B.; Dorrestein, P.; Begley, T.; Osterman, A. Aerobic tryptophan degradation pathway in bacteria: Novel kynurenine formamidase. FEMS Microbiol. Lett. 2003, 227, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Salcher, O.; Lingens, F. Metabolism of tryptophan by Pseudomonas aureofaciens and its relationship to pyrrolnitrin biosynthesis. J. Gen. Microbiol. 1980, 121, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Bouknight, R.R.; Sadoff, H.L. Tryptophan catabolism in Bacillus megaterium. J. Bacteriol. 1975, 121, 70–76. [Google Scholar] [PubMed]
- Cassan, F.; Vanderleyden, J.; Spaepen, S. Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J. Plant Growth Regul. 2014, 33, 440–459. [Google Scholar] [CrossRef]
- Prinsen, E.; Costacurta, A.; Michiels, K.; Vanderleyden, J.; Van Onckelen, H. Azospirillum brasilense indole-3-acetic acid biosynthesis: Evidence for a non-tryptophan dependent pathway. Mol. Plant Microbe Interact. 1993, 6, 609–615. [Google Scholar] [CrossRef]
- Rivera1, D.; Mora1, V.; Lopez1, G.; Rosas1, S.; Spaepen, S.; Vanderleyden, J.; Cassan1, F. New insights into indole-3-acetic acid metabolism in Azospirillum brasilense. J. Appl. Microbiol. 2018, 125, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, S.R.; Ronen, Z.; Amand, J. Growth in coculture stimulates metabolism of the phenylurea herbicide isoproturon by Sphingomonas sp. strain SRS2. Appl. Environ. Microbiol. 2002, 68, 3478–3485. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Shen, J.; Brady, S.R.; Muday, G.K.; Asami, T.; Yang, Z. Brassinosteroids Interact with Auxin to Promote Lateral Root Development in Arabidopsis. Plant Physiol. 2004, 134, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.C.F.; Costa, F.E.C.; Andreote, F.D.; Lacava, P.T.; Teixeira, M.A.; Assumpcao, L.C.; Araujo, W.L.; Azevedo, J.L.; Melo, I.S. Isolation of Micropropagated strawberry endophytic bacteria and assessment of their potential for plant growth promotion. World J. Microbiol. Biotechnol. 2009, 25, 189–195. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic in development of the host plant root system. Appl. Environ. Microbial. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
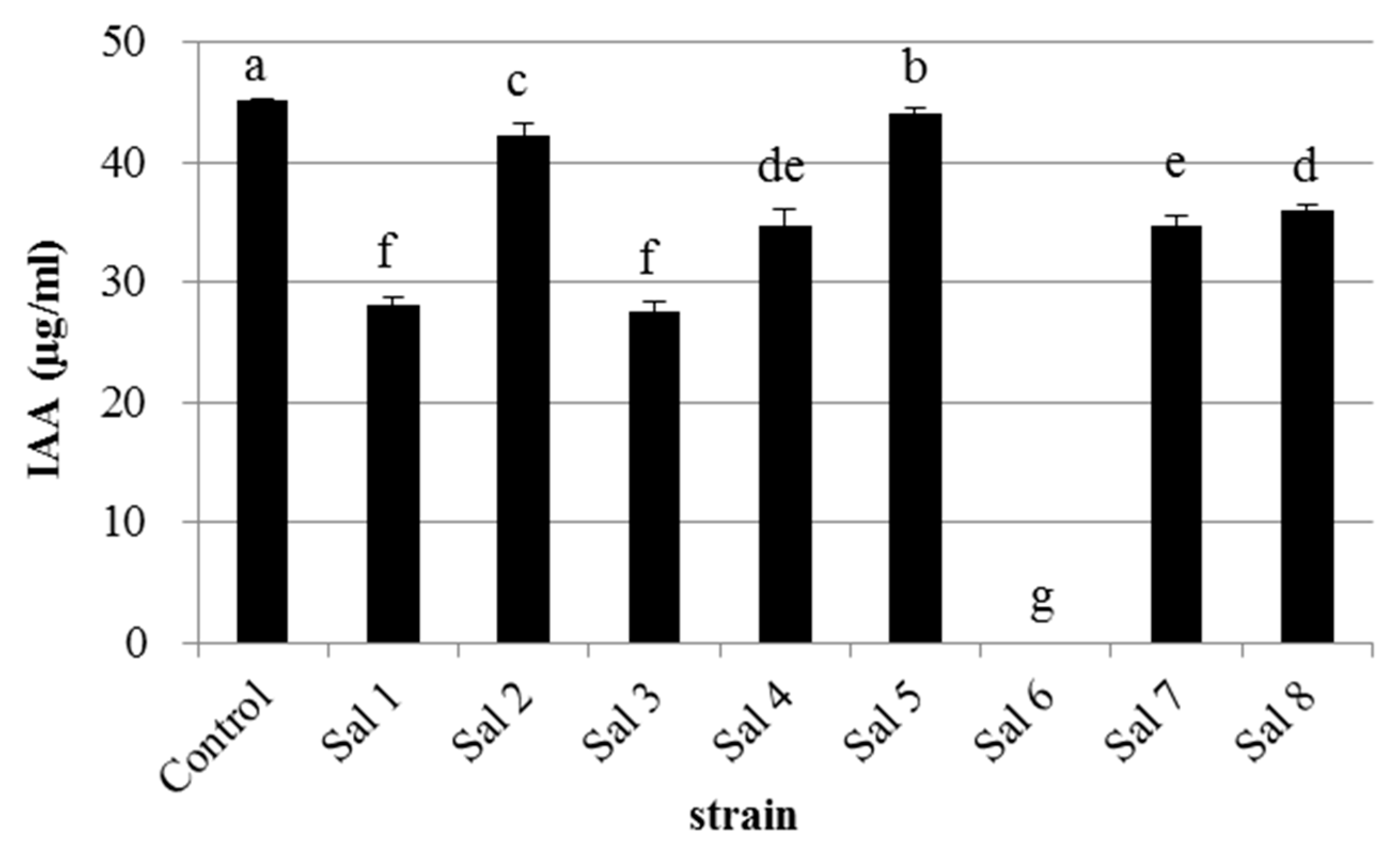



| Strain | * Most Similar Genus | Class | Accession Number | ** IAA-Producing Ability (μg/mL) |
|---|---|---|---|---|
| Sal 1 | Klebsiella sp. | Gammaproteobacteria | LC389410 | 65 |
| Sal 2 | Flavobacterium sp. | Flavobacteria | LC389415 | 0 |
| Sal 3 | Enterobacter sp. | Gammaproteobacteria | LC389433 | 40 |
| Sal 4 | Rhizobium sp. | Alphaproteobacteria | LC389434 | 20 |
| Sal 5 | Stenotrophomonas sp. | Gammaproteobacteria | LC389439 | 0 |
| Sal 6 | Herbaspirillum sp. | Betaproteobacteria | LC389442 | 0 |
| Sal 7 | Agrobacterium sp. | Alphaproteobacteria | LC389443 | 13 |
| Sal 8 | Microbacterium sp. | Actinobacteria | LC389445 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhungana, S.A.; Itoh, K. Effects of Co-Inoculation of Indole-3-Acetic Acid-Producing and -Degrading Bacterial Endophytes on Plant Growth. Horticulturae 2019, 5, 17. https://doi.org/10.3390/horticulturae5010017
Dhungana SA, Itoh K. Effects of Co-Inoculation of Indole-3-Acetic Acid-Producing and -Degrading Bacterial Endophytes on Plant Growth. Horticulturae. 2019; 5(1):17. https://doi.org/10.3390/horticulturae5010017
Chicago/Turabian StyleDhungana, Sabitri Adhikari, and Kazuhito Itoh. 2019. "Effects of Co-Inoculation of Indole-3-Acetic Acid-Producing and -Degrading Bacterial Endophytes on Plant Growth" Horticulturae 5, no. 1: 17. https://doi.org/10.3390/horticulturae5010017
APA StyleDhungana, S. A., & Itoh, K. (2019). Effects of Co-Inoculation of Indole-3-Acetic Acid-Producing and -Degrading Bacterial Endophytes on Plant Growth. Horticulturae, 5(1), 17. https://doi.org/10.3390/horticulturae5010017





