Hormonal Regulation of Early Fruit Development in European Pear (Pyrus communis cv. ‘Conference’)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites and Plant Material
2.2. Pollination and Hormonal Treatments
2.3. Hormonal Quantification
2.4. Gene Expression Analysis
2.5. Statistical Analyses
3. Results
3.1. Differences Between Fertilized and Intrinsic Parthenocarpic Fruits
3.1.1. Effects of Fertilization and Intrinsic Parthenocarpy on Fruit Parameters
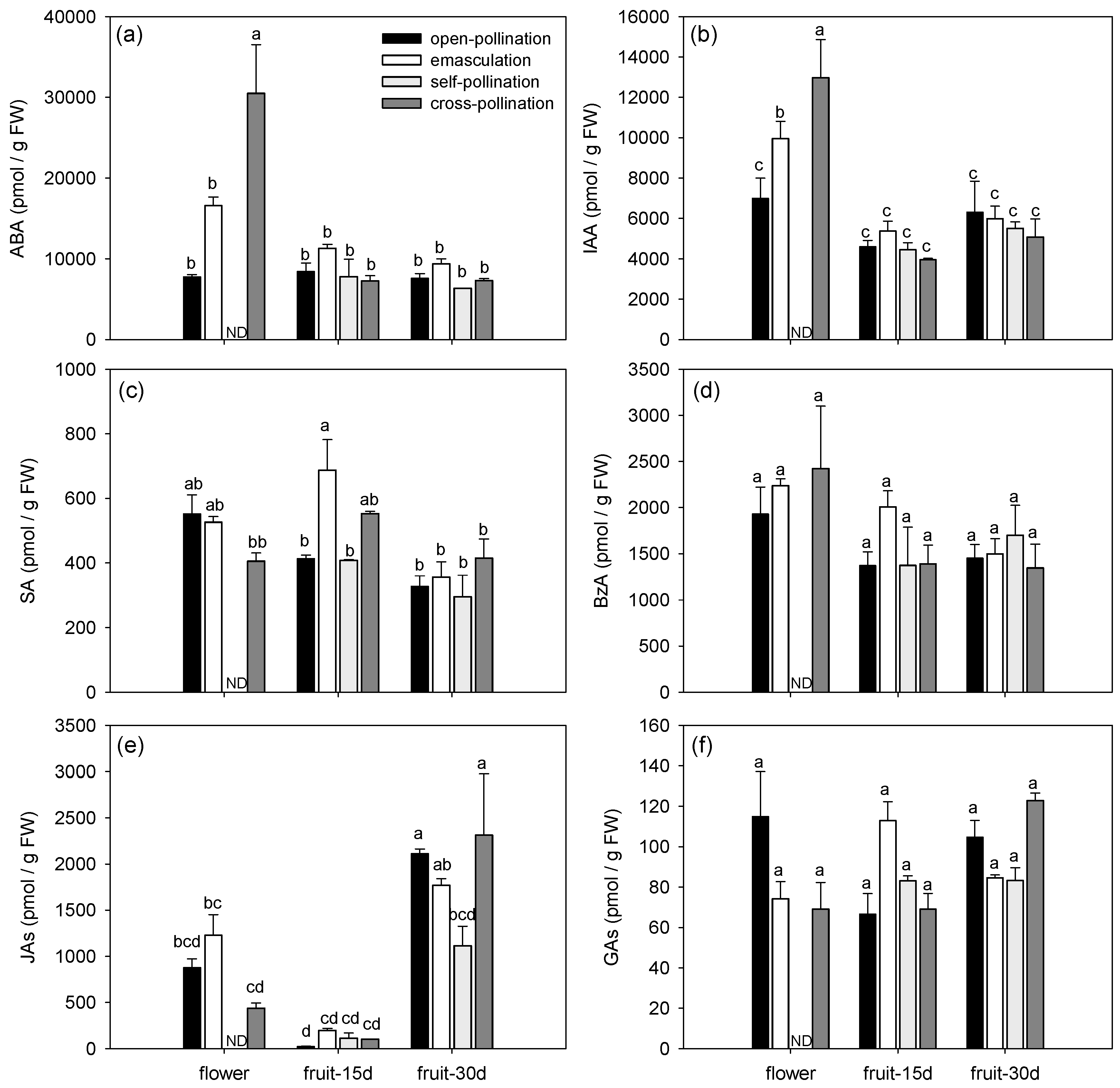
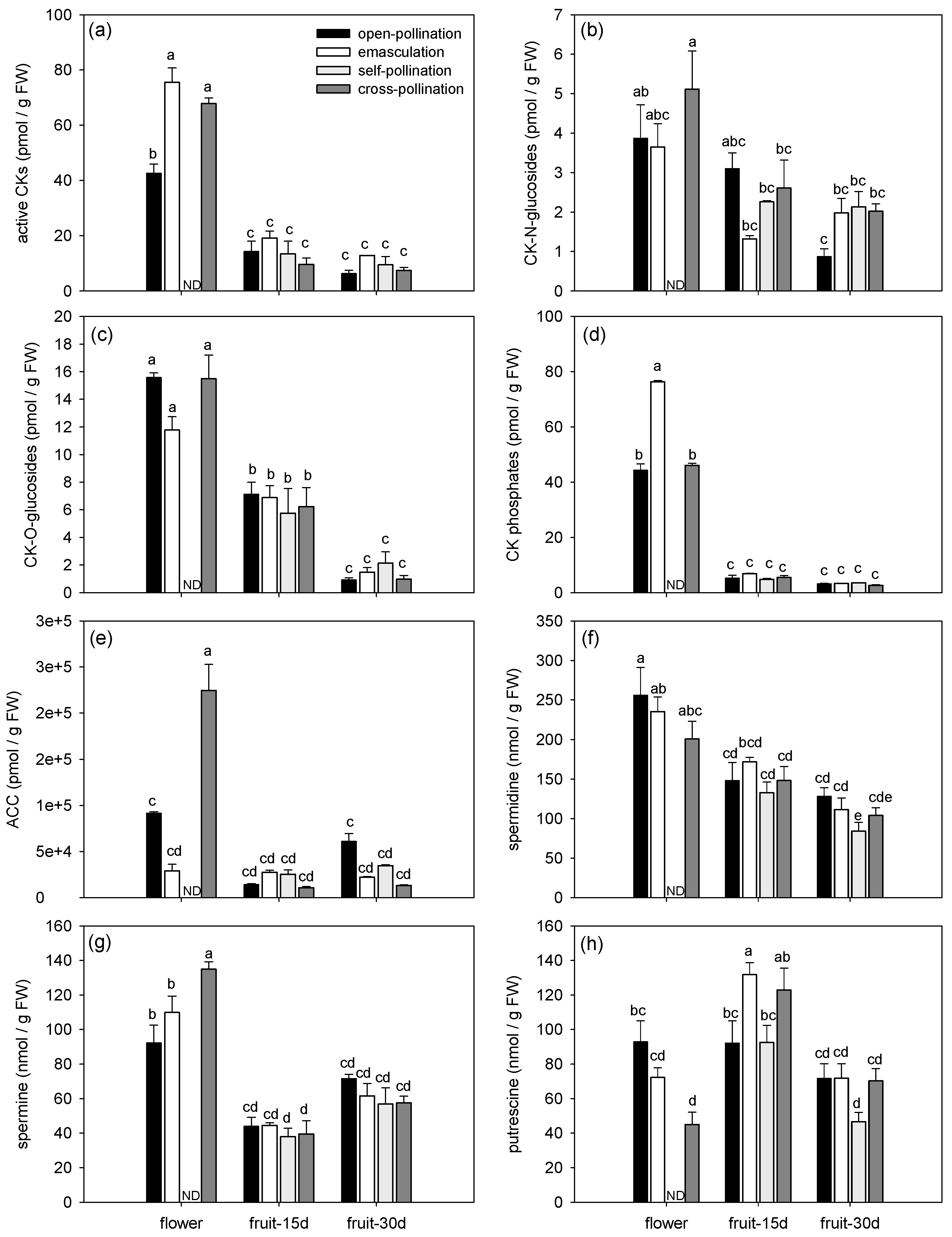
3.1.2. Effects of Fertilization and Intrinsic Parthenocarpy on Phytohormonal Profile
3.1.3. Effects of Fertilization and Intrinsic Parthenocarpy on Expression of Genes Involved in Phytohormone Metabolism
3.2. Differences Between Intrinsic and Extrinsic Parthenocarpic Fruits
3.2.1. Effects of Extrinsic Parthenocarpy on Fruit Parameters
3.2.2. Effects of Extrinsic Parthenocarpy on Phytohormonal Profile
3.2.3. Effects of Extrinsic Parthenocarpy on Expression of Genes Involved in Phytohormone Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Lu, Y.; Li, L.; Chu, Z.; Zhang, H.; Li, H.; Fernie, A.R.; Ouyang, B. Impairment of hormone pathways results in a general disturbance of fruit primary metabolism in tomato. Food Chem. 2019, 274, 170–179. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef]
- Huang, G.; Li, T.; Li, X.; Tan, D.; Jiang, Z.; Wei, Y.; Li, J.; Wang, A. Comparative transcriptome analysis of climacteric fruit of Chinese pear (Pyrus ussuriensis) reveals new insights into fruit ripening. PLoS ONE 2014, 9, e107562. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Liu, J.; Liu, F.; Zhai, R.; Zhu, C.; Wang, H.; Ma, F.; Xu, L. Histological, hormonal and transcriptomic reveal the changes upon gibberellin-induced parthenocarpy in pear fruit. Hortic. Res. 2018, 5, 1. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Xue, C.; Xu, L.; Sun, H.; Qin, M.-F.; Zhang, S.; Wu, J. Distinct transcriptome profiles reveal gene expression patterns during fruit development and maturation in five main cultivated species of pear (Pyrus L.). Sci. Rep. 2016, 6, 28130. [Google Scholar] [CrossRef]
- Oikawa, A.; Otsuka, T.; Nakabayashi, R.; Jikumaru, Y.; Isuzugawa, K.; Murayama, H.; Saito, K.; Shiratake, K. Metabolic profiling of developing pear fruits reveals dynamic variation in primary and secondary metabolites, including plant hormones. PLoS ONE 2015, 10, e0131408. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.J.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Azzi, L.; Deluche, C.; Gévaudant, F.; Frangne, N.; Delmas, F.; Hernould, M.; Chevalier, C. Fruit growth-related genes in tomato. J. Exp. Bot. 2015, 66, 1075–1086. [Google Scholar] [CrossRef]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant J. 1993, 5, 1439–1451. [Google Scholar]
- Srivastava, A.; Handa, A.K. Hormonal regulation of tomato fruit development: A molecular perspective. J. Plant Growth Regul. 2005, 24, 67–82. [Google Scholar] [CrossRef]
- Gorguet, B.; van Heusden, A.W.; Lindhout, P. Parthenocarpic fruit development in tomato. Plant Biol. 2005, 7, 131–139. [Google Scholar] [CrossRef]
- De Jong, M.; Mariani, C.; Vriezen, W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef]
- Obroucheva, N.V. Hormonal regulation during plant fruit development. Russ. J. Dev. Biol. 2014, 45, 11–21. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Lou, Y.; Wang, L.; Slovin, J.P.; Xu, W.; Wang, S.; Zhang, C. Proteomic analysis of the effects of gibberellin on increased fruit sink strength in Asian pear (Pyrus pyrifolia). Sci. Hortic. 2015, 195, 25–36. [Google Scholar] [CrossRef]
- Nyéki, A.; Soltész, M. The variation of seed content of fruits in pear varieties, also as function of different conditions of fertilization, as open pollination, natural autogamy and allogamy. Acta Hortic. 1998, 475, 237–250. [Google Scholar] [CrossRef]
- Quinet, M.; Jacquemart, A.-L. Difference between pollination and parthenocarpy in the “Conférence” pear production. Acta Hortic. 2015, 1094, 359–366. [Google Scholar] [CrossRef]
- Sugiyama, K.; Kami, D.; Muro, T. Induction of parthenocarpic fruit set in watermelon by pollination with bottle gourd (Lagenaria siceraria (Molina) Standl.) pollen. Sci. Hortic. 2014, 171, 1–5. [Google Scholar] [CrossRef]
- Joldersma, D.; Liu, Z. The making of virgin fruit: The molecular and genetic basis of parthenocarpy. J. Exp. Bot. 2018, 69, 955–962. [Google Scholar] [CrossRef]
- Jacquemart, A.-L.; Michotte-van der Aa, A.; Raspé, O. Compatibility and pollinator efficiency tests on Pyrus communis L. cv. ‘Conference’. J. Hortic. Sci. Biotechnol. 2006, 81, 827–830. [Google Scholar] [CrossRef]
- Quinet, M.; Jacquemart, A.-L. Cultivar placement affects pollination efficiency and fruit production in European pear (Pyrus communis) orchards. Eur. J. Agron. 2017, 91, 84. [Google Scholar] [CrossRef]
- Liu, J.; Zhai, R.; Liu, F.; Zhao, Y.; Wang, H.; Liu, L.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Melatonin induces parthenocarpy by regulating genes in gibberellin pathways of ‘Starkrimson’ Pear (Pyrus communis L.). Front. Plant Sci. 2018, 9, 946. [Google Scholar] [CrossRef]
- Niu, Q.; Wang, T.; Li, J.; Yang, Q.; Qian, M.; Teng, Y. Effects of exogenous application of GA4+7 and N-(2-chloro-4-pyridyl)-N′-phenylurea on induced parthenocarpy and fruit quality in Pyrus pyrifolia ‘Cuiguan’. Plant Growth Regul. 2015, 76, 251–258. [Google Scholar] [CrossRef]
- Zhang, C.; Lee, U.; Tanabe, K. Hormonal regulation of fruit set, parthenogenesis induction and fruit expansion in Japanese pear. Plant Growth Regul. 2008, 55, 231–240. [Google Scholar] [CrossRef]
- Deckers, T.; Schoofs, H. Improvement of fruit set on young pear trees cultivar conference with gibberellins. Acta Hortic. 2002, 596, 735–743. [Google Scholar] [CrossRef]
- De Jong, M.; Wolters-Arts, M.; Feron, R.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009, 57, 160–170. [Google Scholar] [CrossRef]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latché, A.; Pech, J.-C.; Bouzayen, M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef]
- Fos, M.; Proaño, K.; Nuez, F.; García-Martínez, J.L. Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol. Plant 2001, 111, 545–550. [Google Scholar] [CrossRef]
- Martínez-Bello, L.; Moritz, T.; López-Díaz, I. Silencing C19-GA 2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants. J. Exp. Bot. 2015, 66, 5897–5910. [Google Scholar] [CrossRef]
- Martí, C.; Orzáez, D.; Ellul, P.; Moreno, V.; Carbonell, J.; Granell, A. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007, 52, 865–876. [Google Scholar] [CrossRef]
- Harberd, N.P.; Belfield, E.; Yasumura, Y. The Angiosperm Gibberellin-GID1-DELLA growth regulatory mechanism: How an “Inhibitor of an Inhibitor” enables flexible response to fluctuating environments. Plant Cell 2009, 21, 1328–1339. [Google Scholar] [CrossRef]
- Stern, R.A.; Sapir, G.; Shafir, S.; Goldway, M. The appropriate management of honey bee colonies for pollination of Rosaceae fruit trees in warm climates. Middle East. Russ. J. Plant Sci. Biotechnol. 2007, 1, 13–19. [Google Scholar]
- Dobrev, P.I.; Kamınek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extractionq. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Djilianov, D.L.; Dobrev, P.I.; Moyankova, D.P.; Vankova, R.; Georgieva, D.T.; Gajdošová, S.; Motyka, V. Dynamics of endogenous phytohormones during desiccation and recovery of the resurrection plant species Haberlea rhodopensis. J. Plant Growth Regul. 2013, 32, 564–574. [Google Scholar] [CrossRef]
- Lefèvre, I.; Gratia, E.; Lutts, S. Discrimination between the ionic and osmotic components of salt stress in relation to free polyamine level in rice (Oryza sativa). Plant Sci. 2001, 161, 943–952. [Google Scholar] [CrossRef]
- Quinet, M.; Bataille, G.; Dobrev, P.I.; Capel, C.; Gómez, P.; Capel, J.; Lutts, S.; Motyka, V.; Angosto, T.; Lozano, R. Transcriptional and hormonal regulation of petal and stamen development by STAMENLESS, the tomato (Solanum lycopersicum L.) orthologue to the B-class APETALA3 gene. J. Exp. Bot. 2014, 65, 2243–2256. [Google Scholar] [CrossRef]
- R Core Team 2009. R: A Language and Environment for Statistical Computing, Vienna, Austria. 2012. Available online: http://www. R-project. org (accessed on 8 November 2017).
- Theron, K.I. Size matters: Factors influencing fruit size in pear. Acta Hortic. 2011, 909, 545–556. [Google Scholar] [CrossRef]
- Quinet, M.; Warzée, M.; Vanderplanck, M.; Michez, D.; Lognay, G.; Jacquemart, A.-L. Do floral resources influence pollination rates and subsequent fruit set in pear (Pyrus communis L.) and apple (Malus x domestica Borkh) cultivars? Eur. J. Agron. 2016, 77, 59–69. [Google Scholar] [CrossRef]
- Liu, J.-H.; Honda, C.; Moriguchi, T. Involvement of polyamine in floral and fruit development. Jpn. Agric. Res. Q. JARQ 2006, 40, 51–58. [Google Scholar] [CrossRef]
- Murase, K.; Hirano, Y.; Sun, T.; Hakoshima, T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 2008, 456, 459–463. [Google Scholar] [CrossRef]
- Vercammen, J.; Gomand, A. Fruit set of “Conference”: A small dose of gibberellins or Regalis. Acta Hortic. 2008, 800, 131–138. [Google Scholar] [CrossRef]
- Dreyer, C.; Theron, K.I. The efficacy of 6-benzyladenine, gibberellins4+7 and prohexadione-calcium to increase fruit size in ‘Forelle’ and ‘Abate Fetel’ pear. S. Afr. J. Plant Soil 2014, 31, 53–59. [Google Scholar] [CrossRef]
- Su, J.; Jia, B.; Jia, S.; Ye, Z.-F.; Heng, W.; Zhu, L.-W. Effect of plant growth regulators on calyx abscission, fruit quality, and auxin-repressed protein (ARP) gene expression in fruitlets of ‘Dangshansuli’ pear (Pyrus bretschneideri Rehd.). J. Hortic. Sci. Biotechnol. 2015, 90, 135–142. [Google Scholar] [CrossRef]


| Gene Name | Gene Function | Genbank Accession | Primer Sequences | Tm | Cycles |
|---|---|---|---|---|---|
| ACTIN | actin | AF386514 | TGCCTATGTAGGGGATGAGG GCTCAGCAGTTGTGGTGAAA | 55 °C | 30 |
| Auxin signaling | |||||
| PpARP1 | Auxin-repressed protein | KC422235 | AGGTAGCAACCTTGCCACCAAAT GATACCTAATCGTATTGCCATC | 55 °C | 30 |
| PpARP2 | Auxin-repressed protein | KC422236 | AGCGGTAACGGTGGATCAGCTCC CATCTCATGGATGATTTCGATTGGA | 55 °C | 30 |
| Gibberellin metabolism and signaling | |||||
| PcCPS | copalyl diphosphate synthase | KC153028 | GTGGCGTTAGTGGAGGATGT TCTCATGCAACACAGCACAA | 55 °C | 35 |
| PpKS | ent-kaurene synthase | JF441169 | CGATTGTCCTTCCAGCTCTC GTGCAGCACTTTGCTCATGT | 55 °C | 35 |
| MdKAO1 | ent-kaurene acid oxidase | KF437682 | CGCAGAAGGGCTTAACACTC CGGATTAGTGCGTTCCATCT | 58 °C | 35 |
| MdKAO2 | ent-kaurene acid oxidase | NM_001328825 | CGCAGAAGGGCTTAACACTC CGGATTAGTGCGTTCCATCT | 55 °C | 35 |
| GA20ox | gibberellin 20-oxidase | HQ833589 | CGAAAGCATCGCAACTTGTA GTGTGCTCATCGCCTCACTA | 55 °C | 37 |
| GA2ox | gibberellin 2-oxidase | XM_008347715 | TTGAGCAAAGGAATGTGCTG AGTGACGGCAGAGGTGCTAT | 55 °C | 37 |
| GA3ox | gibberellin 3-oxidase | JX308225 | GTGCATCAGCTGCCTTACAA CCAAGTAATTGGCCGGTAGA | 55 °C | 37 |
| GID1a | GA signal transduction factor | JF516247 | TGCCTATGATGATGGATGGA GCTCTTCGGGAACTTGACAG | 55 °C | 30 |
| GID1b | GA signal transduction factor | JN381497 | GGAAAGTCCCTGCCAACATA GCAGCCACTTTCTCGATTTC | 55 °C | 30 |
| GID1c | GA signal transduction factor | JN381498 | GCCTCCTCAACCGTGTTTAC GCAGCCACTTTCTCGATTTC | 55 °C | 30 |
| GID1d | GA signal transduction factor | JN381499 | GCCTATGATGATGGGTGGAC CTCTTCGGGAACTTGACAGC | 55 °C | 30 |
| DELLA | DELLA protein | JF304103 | CGTCCAGCAGAACAACTTCA AACTCGACGTGGATGGTTTC | 55 °C | 30 |
| Cytokinin metabolism | |||||
| PbCKX3 | cytokinin oxidase/dehydrogenase | XM_009354391 | GTGACGATCCAGAAGCCATT CGAGACCGGTGTAAGTCCAT | 58 °C | 35 |
| PbCKX5 | cytokinin oxidase/dehydrogenase | XM_009337490 | AGTGTTCAAGGGCATTTTGG CCGATGAAGGGCTGAAAATA | 53 °C | 35 |
| Polyamine metabolism | |||||
| PbSAMS | S-adenosylmethionine synthase | AF195233 | AACCAAGGTGGACAGGAGTG ACCCCTCTTCAGATCCAGGT | 55° | 30 |
| PbSAMS2lc | S-adenosylmethionine synthase | XM_009355137 | AACCAAGGTGGACAGGAGTG ACCCCTCTTCAGATCCAGGT | 55 °C | 28 |
| PbSAMDC | S-adenosylmethionine decarboxylase | JX624260 | GATCCTTCCAGATTCGGACA TGCTAGCAACATTGGAGTGC | 55 °C | 30 |
| PbSAMDC2 | S-adenosylmethionine decarboxylase | KC414856 | TCTTCGAGCCTGGACTGTTT CAATTTTGTTGTGCCACAGG | 55 °C | 30 |
| PbSAMDC3 | S-adenosylmethionine decarboxylase | KM670010 | TTTTCGAGCCGAGTGTCTTT CACAGCAAGGGAAATGGTTT | 55 °C | 30 |
| PbSAMDCla | S-adenosylmethionine decarboxylase | XM_009341666 | TCGCCTCCTTGACTTTGAGT GGCAGGAGATGAGAGTGAGG | 55 °C | 34 |
| PbSAMDClb | S-adenosylmethionine decarboxylase | XM_009375455 | CAGCTGAGTGCACCATTGTT AGCTACCTCCTCCGAAAAGC | 55 °C | 28 |
| PbSAMDCle | S-adenosylmethionine decarboxylase | XR_670231 | TCTTCGAGCCTGGACTGTTT CAATTTTGTTGTGCCACAGG | 55 °C | 33 |
| PbODC1 | ornithine decarboxylase | KP144199 | CCCAAATGTTCCTTGTTGCT AAAGCTGTTTCGGCAAAGAA | 58 °C | 35 |
| PbODC1l | ornithine decarboxylase-like | XM_009336895 | TTGCCGAAACAGCTTTCACATTGGT GCCGTTAAAGTTGGTTCCAGCAG | 58 °C | 35 |
| PbODC2l | ornithine decarboxylase-like | XM_009336872 | TTGCCGAAACGGCTTTCACAATGGT CCATTAAAGTTGGTTCCAATGG | 58 °C | 35 |
| Spmsl1 | spermine synthase-like | XM_009380504 | CGGTTGCTTGAGTAGCAAAT GATCGCCATAGTAAAATTTCC | 58 °C | 35 |
| Spmsl2 | spermine synthase-like | XM_009353722 | CGGTTGCTTGAGTAGCAGAT TAACATAATGTCGAATGGCT | 58 °C | 35 |
| Parameter | Initial Fruit Set (%) | Fruit Set at Harvest (%) | Fruit Weight (g) | Fruit Length (mm) | Fruit Size (mm) | Normal Seeds per Fruit | Aborted Seeds per Fruit |
|---|---|---|---|---|---|---|---|
| open-pollination | 86.8 ± 5.2a | 18.2 ± 6.1a | 138.6 ± 6.3a | 107.9 ± 2.1a | 57.8 ± 1.0a | 0.25 ± 0.09b | 1.95 ± 0.34a |
| emasculation | 56.7 ± 9.9b | 13.3 ± 2.1a | 120.3 ± 5.7a | 107.5 ± 2.5a | 51.4 ± 1.1b | 0.00 ± 0.00b | 0.97 ± 0.38b |
| self-pollination | 80.8 ± 4.4ab | 16.8 ± 6.1a | 118.7 ± 6.0a | 108.9 ± 2.2a | 51.1 ± 0.8b | 0.00 ± 0.00b | 0.38 ± 0.22b |
| cross-pollination | 62.6 ± 6.8ab | 18.5 ± 2.9a | 131.3 ± 5.5a | 99.8 ± 1.9b | 55.5 ± 0.9a | 1.91 ± 0.31a | 2.58 ± 0.36a |
| ANOVA | F3,25 = 3.59 P = 0.0277 | F3,25 = 0.33 P = 0.8048 | F3,191 = 2.43 P = 0.0669 | F3,191 = 4.26 P = 0.0061 | F3,191 = 5.85 P = 0.0008 | F3,103.9 = 13.28 P < 0.0001 | F3,86.7 = 17.67 P < 0.0001 |
| Parameter | Treatments | Flower and Fruit Stages | Interaction |
|---|---|---|---|
| ABA | F3,11 = 6.60, P = 0.0082 | F2,11 = 23.61, P = 0.0001 | F5,11 = 9.66, P = 0.0010 |
| IAA | F3,11 = 1.28, P = 0.3296 | F2,11 = 27.78, P < 0.0001 | F5,11 = 3.85, P = 0.0292 |
| SA | F3,11 = 4.53, P = 0.0266 | F2,11 = 12.92, P = 0.0013 | F5,11 = 3.75, P = 0.0316 |
| BzA | F3,11 = 0.58, P = 0.6396 | F2,11 = 5.00, P = 0.0286 | F5,11 = 0.68, P = 0.6490 |
| JAs | F3,11 = 2.34, P = 0.1300 | F2,11 = 59.83, P < 0.0001 | F5,11 = 3.19, P = 0.0504 |
| GAs | F3,11 = 0.88, P = 0.4810 | F2,11 = 3.05, P = 0.0885 | F5,11 = 6.73, P = 0.0042 |
| bioactive CKs | F3,11 = 11.93, P = 0.0009 | F2,11 = 280.64, P < 0.0001 | F5,11 = 7.35, P = 0.0030 |
| CK-N-glucosides | F3,11 = 1.71, P = 0.2231 | F2,11 = 18.96, P = 0.0003 | F5,11 = 1.84, P = 0.1859 |
| CK-O-glucosides | F3,11 = 0.70, P = 0.5710 | F2,11 = 127.94, P < 0.0001 | F5,11 = 1.81, P = 0.1922 |
| CK phosphates | F3,11 = 116.37, P < 0.0001 | F2,11 = 3757.22, P < 0.0001 | F5,11 = 117.34, P < 0.0001 |
| ACC | F3,11 = 18.47, P = 0.0001 | F2,11 = 95.31, P < 0.0001 | F5,11 = 38.02, P < 0.0001 |
| spermidine | F3,24 = 2.24, P = 0.1098 | F2,24 = 33.11, P <.0001 | F5,24 = 0.62, P = 0.6867 |
| spermine | F3,24 = 0.99, P = 0.4151 | F2,24 = 78.35, P < 0.0001 | F5,24 = 3.73, P = 0.0122 |
| putrescine | F3,24 = 4.26, P = 0.0151 | F2,24 = 28.62, P < 0.0001 | F5,24 = 3.98, P = 0.0090 |
| Parameter | Initial Fruit Set (%) | Fruit Set at Harvest (%) | Fruit Weight (g) | Fruit Length (mm) | Fruit Size (mm) | Normal Seeds per Fruit | Aborted Seeds per Fruit |
|---|---|---|---|---|---|---|---|
| open-pollination | 86.8 ± 5.2a | 18.2 ± 6.1a | 138.6 ± 6.3a | 107.9 ± 2.1a | 57.8 ± 1.0a | 0.25 ± 0.09b | 1.95 ± 0.34a |
| Intrinsic parthenocarpy | 80.8 ± 4.4ab | 16.8 ± 6.1a | 118.7 ± 6.0a | 108.9 ± 2.2a | 51.1 ± 0.8b | 0.00 ± 0.00b | 0.38 ± 0.22b |
| GA3 | 88.9 ± 4.1 a | 29.2 ± 3.1a | 133.6 ± 4.3 ab | 113.7 ± 1.8 a | 51.2 ± 0.8 b | 0.00 ± 0.00b | 0.14 ± 0.08 b |
| GA4/7 | 93.5 ± 2.0a | 24.6 ± 4.3 a | 117.5 ± 4.8 b | 111.6 ± 2.0 a | 49.9 ± 0.8 bc | 0.00 ± 0.00 b | 0.59 ± 0.23 b |
| 6BA | 79.2 ± 5.9 b | 17.4 ± 3.3 a | 120.8 ± 4.1 b | 107.7 ± 1.8 a | 48.1 ± 0.7 c | 0.03 ± 0.02 b | 0.14 ± 0.08 b |
| ANOVA | F4,24 = 2.46 P = 0.0726 | F4,24 = 1.34 P = 0.2837 | F4,344 = 3.54 P = 0.0076 | F4,344 = 1.86 P = 0.1168 | F4,344 = 8.12 P < 0.0001 | F4,85.17 = 2.76 P = 0.0089 | F4,146.4 = 7.71 P < 0.0001 |
| Parameter | Treatments | Fruit Stages | Interaction |
|---|---|---|---|
| ABA | F4,12 = 0.58, P = 0.6808 | F1,12 = 0.98, P = 0.3412 | F4,12 = 0.44, P = 0.7777 |
| IAA | F4,12 = 2.40, P = 0.1076 | F1,12 = 1.27, P = 0.2810 | F4,12 = 1.03, P = 0.4300 |
| SA | F4,12 = 8.08, P = 0.0021 | F1,12 = 50.73, P < 0.0001 | F4,12 = 4.54, P = 0.0182 |
| BzA | F4,12 = 2.36, P = 0.1118 | F1,12 = 1.22, P = 0.2914 | F4,12 = 1.18, P = 0.3673 |
| JAs | F4,12 = 9.03, P = 0.0013 | F1,12 = 285.97, P < 0.0001 | F4,12 = 9.23, P = 0.0012 |
| GAs | F4,12 = 17.84, P < 0.0001 | F1,12 = 16.18, P = 0.0017 | F4,12 = 16.84, P < 0.0001 |
| bioactive CKs | F4,12 = 0.35, P = 0.8365 | F1,12 = 16.26, P = 0.0017 | F4,12 = 0.54, P = 0.7078 |
| CK-N-glucosides | F4,12 = 1.18, P = 0.3684 | F1,12 = 24.24, P = 0.0004 | F4,12 = 2.96, P = 0.0649 |
| CK-O-glucosides | F4,12 = 0.10, P = 0.9799 | F1,12 = 41.97, P < 0.0001 | F4,12 = 0.34, P = 0.8471 |
| CK phosphates | F4,12 = 1.03, P = 0.4319 | F1,12 = 49.30, P < 0.0001 | F4,12 = 1.29, P = 0.3334 |
| ACC | F4,12 = 2.00, P = 0.1593 | F1,12 = 52.22, P < 0.0001 | F4,12 = 3.40, P = 0.0445 |
| spermidine | F4,12 = 3.18, P = 0.0356 | F1,12 = 54.63, P < 0.0001 | F4,12 = 2.34, P = 0.0900 |
| spermine | F4,12 = 1.37, P = 0.2813 | F1,12 = 17.31, P = 0.0005 | F4,12 = 0.92, P = 0.4732 |
| putrescine | F4,77 = 1.37, P = 0.0073 | F1,12 = 134.09, P < 0.0001 | F4,12 = 5.18, P = 0.0050 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinet, M.; Buyens, C.; Dobrev, P.I.; Motyka, V.; Jacquemart, A.-L. Hormonal Regulation of Early Fruit Development in European Pear (Pyrus communis cv. ‘Conference’). Horticulturae 2019, 5, 9. https://doi.org/10.3390/horticulturae5010009
Quinet M, Buyens C, Dobrev PI, Motyka V, Jacquemart A-L. Hormonal Regulation of Early Fruit Development in European Pear (Pyrus communis cv. ‘Conference’). Horticulturae. 2019; 5(1):9. https://doi.org/10.3390/horticulturae5010009
Chicago/Turabian StyleQuinet, Muriel, Christel Buyens, Petre I. Dobrev, Václav Motyka, and Anne-Laure Jacquemart. 2019. "Hormonal Regulation of Early Fruit Development in European Pear (Pyrus communis cv. ‘Conference’)" Horticulturae 5, no. 1: 9. https://doi.org/10.3390/horticulturae5010009
APA StyleQuinet, M., Buyens, C., Dobrev, P. I., Motyka, V., & Jacquemart, A.-L. (2019). Hormonal Regulation of Early Fruit Development in European Pear (Pyrus communis cv. ‘Conference’). Horticulturae, 5(1), 9. https://doi.org/10.3390/horticulturae5010009





