Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica juncea Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Desulfoglucosinolate Extraction and HPLC Analysis
2.3. HPLC Analysis of Phenolic Compounds
2.4. Determination of Total Anthocyanin Contents
2.5. Statistical Analysis
3. Results
3.1. Sprout Dry Weight and Phenotype Change
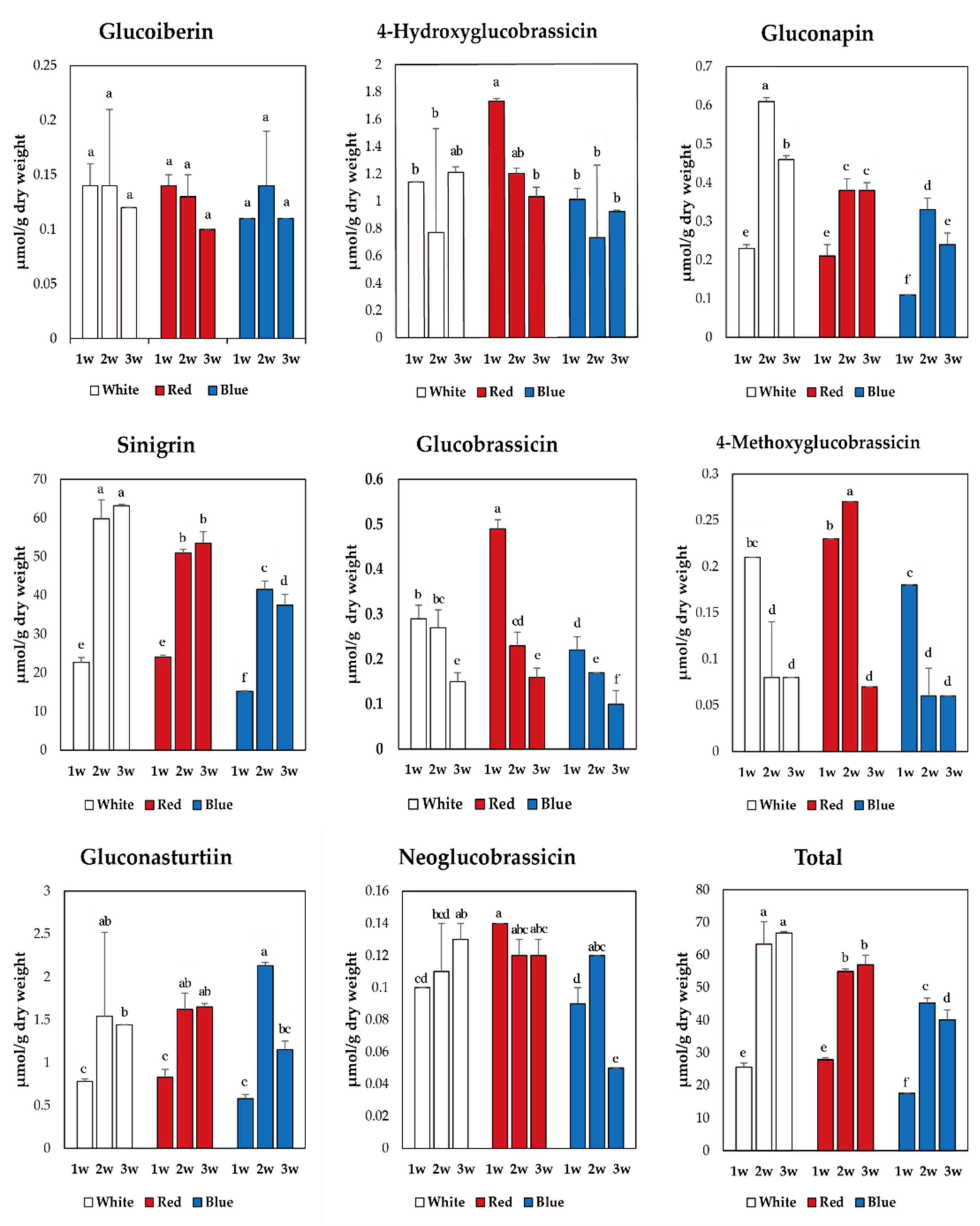
3.2. Glucosinolate Contents
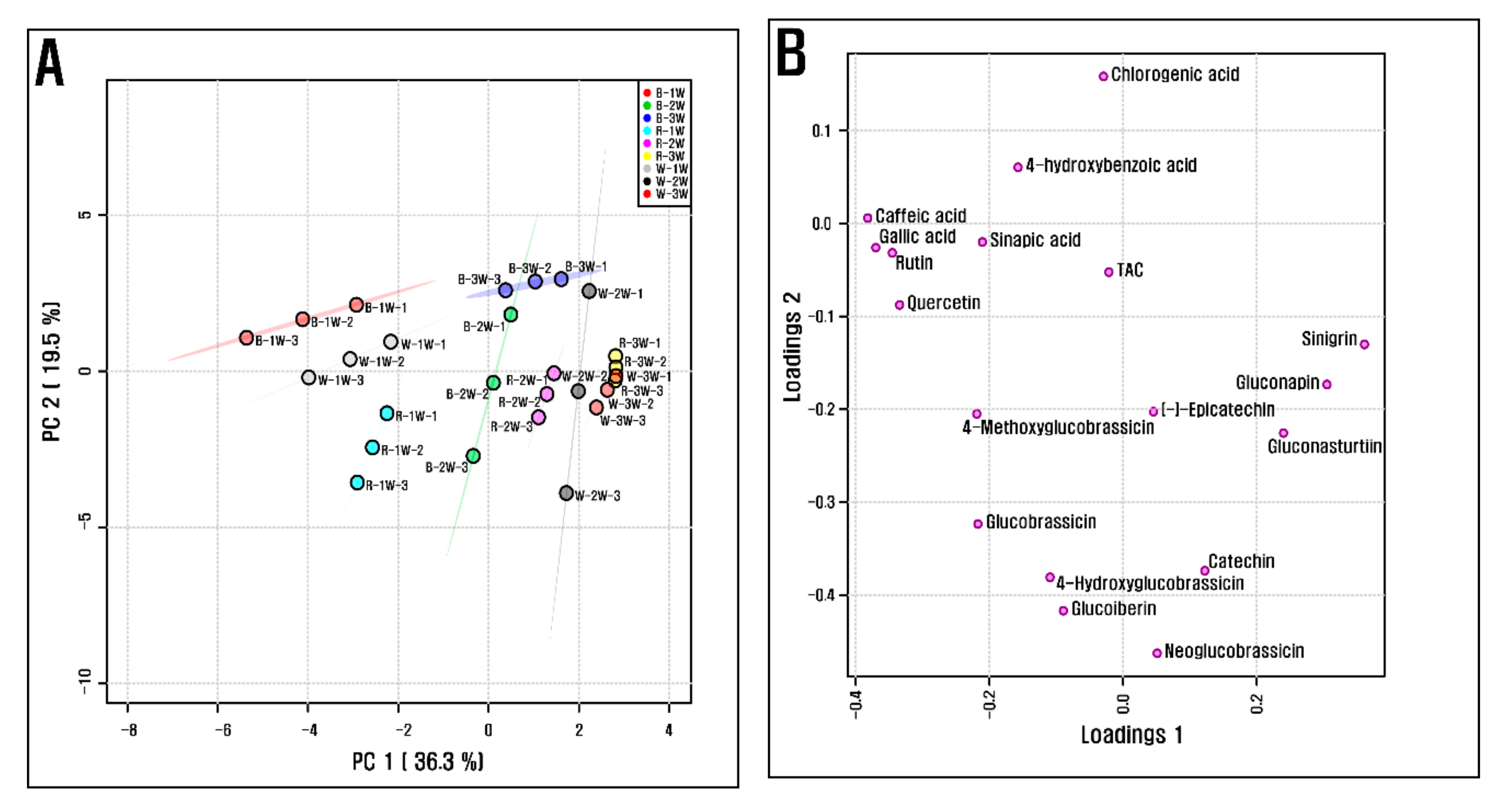
3.3. Total Anthocyanin, Total Chlorophyll, and Phenolic Contents
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manohar, P.R.; Pushpan, R.; Rohini, S. Mustard and its uses in Ayurveda. Indian J. Tradit. Know. 2009, 8, 400–404. [Google Scholar]
- Kim, H.Y.; Yokozawa, T.; Cho, E.J.; Cheigh, H.S.; Choi, J.S.; Chung, H.Y. In vitro and in vivo antioxidant effects of mustard leaf (Brassica juncea). Phytother. Res. 2003, 17, 465–471. [Google Scholar] [PubMed]
- Yu, J.C.; Jiang, Z.-T.; Li, R.; Chan, S.M. Chemical composition of the essential oils of Brassica juncea (L.) Coss. grown in different regions, Hebei, Shaanxi and Shandong, of China. J. Food Drug Anal. 2003, 11, 22–26. [Google Scholar]
- Moser, B.R.; Evangelista, R.L.; Jham, G. Fuel properties of Brassica juncea oil methyl esters blended with ultra-low sulfur diesel fuel. Renew. Energy 2015, 78, 82–88. [Google Scholar] [CrossRef]
- Kumar, A.; D’Souza, S.S.; Tickoo, S.; Salimath, B.P.; Singh, H.B. Antiangiogenic and proapoptotic activities of allyl isothiocyanate inhibit ascites tumor growth in vivo. Integr. Cancer Ther. 2009, 8, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.H.; Jung, J.W.; Lee, J.J.; Cho, Y.W.; Jang, C.G.; Jin, C.; Oh, T.H.; Ryu, J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007, 81, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Joardar, A.; Das, S. Effect of fatty acids isolated from edible oils like mustard, linseed or coconut on astrocytes maturation. Cell. Mol. Neurobiol. 2007, 27, 973–983. [Google Scholar] [CrossRef]
- Jung, H.A.; Woo, J.J.; Jung, M.J.; Hwang, G.S.; Choi, J.S. Kaempferol glycosides with antioxidant activity from Brassica juncea. Arch. Pharm. Res. 2009, 32, 1379–1384. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Choi, J.S.; Chung, H.Y. Antioxidant effects of isorhamnetin 3, 7-di-O-β-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Kroymann, J.; Mitchell-Olds, T. The glucosinolate-myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 2005, 8, 264–271. [Google Scholar] [CrossRef]
- Mithen, R.F.; Dekker, M.; Verkerk, R.; Rabot, S.; Johnson, I.T. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J. Sci. Food Agric. 2000, 80, 967–984. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.A.; Choi, J.H.; Choi, Y.S.; Han, D.J.; Kim, H.Y.; Shim, S.Y.; Chung, H.K.; Kim, C.J. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extracts on refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010, 84, 498–504. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Hypoglycemic and antihyperglycemic effect of Brassica juncea diet and their effect on hepatic glycogen content and the key enzymes of carbohydrate metabolism. Mol. Cell. Biochem. 2002, 241, 95–101. [Google Scholar] [CrossRef]
- Ye, X.; Ng, T.B. Isolation and characterization of juncin, an antifungal protein from seeds of Japanese Takana (Brassica juncea var. integrifolia). J. Agric. Food Chem. 2009, 57, 4366–4371. [Google Scholar] [CrossRef]
- Monsalve, R.I.; de la Pena, M.A.G.; Menendez-Arias, L.; Lopez-Otin, C.; Villalba, M.; Rodriguez, R. Characterization of a new oriental-mustard (Brassica juncea) allergen, Bra j IE: Detection of an allergenic epitope. Biochem. J. 1993, 293, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Thejass, P.; Kuttan, G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-α (TNF-α) production. Nitric Oxide 2007, 16, 247–257. [Google Scholar] [CrossRef]
- Yadav, S.P.; Vats, V.; Ammini, A.C.; Grover, J.K. Brassica juncea (Rai) significantly prevented the development of insulin resistance in rats fed fructose-enriched diet. J. Ethnopharmacol. 2004, 93, 113–116. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Secondary Metabolites in plant defense-mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Baskar, T.B.; Park, Y.E.; Park, J.S.; Lee, S.Y.; Park, S.U. In vitro antioxidant and antimicrobial properties of flower, leaf, and stem extracts of Korean mint. Antioxidants 2019, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Park, S.Y.; Lee, S.Y.; Kim, J.K.; Park, S.U. Analysis of metabolites in white flowers of Magnolia Denudata Desr. and violet flowers of Magnolia Liliiflora Desr. Molecules 2018, 23, 1558. [Google Scholar] [CrossRef] [Green Version]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; de Natale, A.; Pollio, A. Anti-cariogenic effects of polyphenols from plant stimulant beverages (cocoa, coffee, tea). Fitoterapia 2009, 80, 255–262. [Google Scholar] [CrossRef]
- Parker, R.O. Introduction to Plant Science; Taylor & Francis: Abingdon-On-Thames, UK, 2004. [Google Scholar]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.N.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Folta, K.M. Green light stimulates early stem elongation, antagonizing light-mediated growth inhibition. Plant Physiol. 2004, 135, 1407–1416. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in ‘Florida Broadleaf’mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Irradiance levels affect growth parameters and carotenoid pigments in kale and spinach grown in a controlled environment. Physiol. Plant. 2006, 127, 624–631. [Google Scholar] [CrossRef]
- Charron, C.S.; Sams, C.E. Glucosinolate content and myrosinase activity in rapid-cycling Brassica oleracea grown in a controlled environment. J. Am. Soc. Hortic. Sci. 2004, 129, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.; Arasu, M.V.; Park, S.; Byeon, D.H.; Chung, S.-O.; Park, S.U. LED lights enhance metabolites and antioxidants in chinese cabbage and kale. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Open Life Sci. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of light-emitting diodes on the accumulation of glucosinolates and phenolic compounds in sprouting canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kawaharada, C.; Jin, S.; Hashimoto, M.; Ishii, G.; Yamauchi, H. Structural elucidation of 4-(cystein-S-yl)butyl glucosinolate from the leaves of Eruca sativa. Biosci. Biotechnol. Biochem. 2007, 71, 114–121. [Google Scholar] [CrossRef] [Green Version]
- International Standards Organization (ISO). Part 1: Method Using High Performance Liquid Chromatography. In Rapeseed: Determination of Glucosinolates Content; (ISO 9167-1); International Standards Organization (ISO): Geneva, Switzerland, 1992; pp. 1–9. [Google Scholar]
- Park, C.H.; Bong, S.J.; Lim, C.J.; Kim, J.K.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red mizuna (Brassica rapa L. var. japonica). Foods 2020, 9, 1079. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Baskar, T.B.; Kim, J.K.; Park, S.U. Metabolic profiling and chemical-based antioxidant assays of green and red lettuce (Lactuca sativa). Nat. Prod. Commun. 2018, 13, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.K.; Goenadie, V.; Lee, H.W.; Liang, X.; Loh, C.S.; Ong, C.N.; Tan, H.T.W. Growth and glucosinolate profiles of a common Asian green leafy vegetable, Brassica rapa subsp. chinensis var. parachinensis (choy sum), under LED lighting. Sci. Hortic. 2020, 261, 108922. [Google Scholar] [CrossRef]
- Sun, B.; Tian, Y.X.; Chen, Q.; Zhang, Y.; Luo, Y.; Wang, Y.; Li, M.Y.; Gong, R.G.; Wang, X.R.; Zhang, F.; et al. Variations in the glucosinolates of the individual edible parts of three stem mustards (Brassica juncea). R. Soc. Open Sci. 2019, 6, 182054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuong, D.M.; Kim, J.K.; Bong, S.J.; Baek, S.A.; Jeon, J.; Park, J.S.; Park, S.U. Comparative analysis of glucosinolates and metabolite profiling of green and red mustard (Brassica juncea) hairy roots. 3 Biotech 2018, 8, 382. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Liu, D.; Chen, J.; Ye, X. Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chem. 2008, 108, 811–817. [Google Scholar] [CrossRef]
- Irtelli, B.; Navari-Izzo, F. Influence of sodium nitrilotriacetate (NTA) and citric acid on phenolic and organic acids in Brassica juncea grown in excess of cadmium. Chemosphere 2006, 65, 1348–1354. [Google Scholar] [CrossRef]
- Parikh, H.; Khanna, A. Pharmacognosy and phytochemical analysis of Brassica juncea seeds. Pharmacogn. J. 2014, 6, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Kim, Y.B.; Li, X.; Choi, S.R.; Park, S.; Park, J.S.; Lim, Y.P.; Park, S.U. Accumulation of Phenylpropanoids by white, blue, and red light irradiation and their organ-specific distribution in chinese cabbage (Brassica rapa ssp. pekinensis). J. Agric. Food Chem. 2015, 63, 6772–6778. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Moon, J.; Jeong, M.J.; Lee, S.I.; Lee, J.G.; Hwang, H.; Yu, J.; Kim, Y.-R.; Park, S.W.; Kim, J.A. Effect of LED mixed light conditions on the glucosinolate pathway in Brassica rapa. J. Plant Biotechnol. 2015, 42, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Thwe, A.A.; Kim, Y.B.; Li, X.; Seo, J.M.; Kim, S.J.; Suzuki, T.; Chung, S.O.; Park, S.U. Effects of light-emitting diodes on expression of phenylpropanoid biosynthetic genes and accumulation of phenylpropanoids in Fagopyrum tataricum sprouts. J. Agric. Food Chem. 2014, 62, 4839–4845. [Google Scholar] [CrossRef]
- Yeo, H.J.; Park, C.H.; Lee, K.B.; Kim, J.K.; Park, J.S.; Lee, J.W.; Park, S.U. Metabolic analysis of vigna unguiculata sprouts exposed to different light-emitting diodes. Nat. Prod. Commun. 2018, 13, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Szopa, A.; Starzec, A.; Ekiert, H. The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J. Photochem. Photobiol. B 2018, 179, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Agrawal, D.C.; Lee, M.R.; Lee, R.J.; Kuo, C.L.; Wu, C.R.; Tsay, H.S.; Chang, H.C. Influence of LED light spectra on in vitro somatic embryogenesis and LC-MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: A medicinal herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef] [Green Version]
- Cuong, D.; Ha, T.W.; Park, C.H.; Kim, N.S.; Yeo, H.J.; Chun, S.W.; Kim, C.; Park, S.U. Effects of LED lights on expression of genes involved in phenylpropanoid biosynthesis and accumulation of phenylpropanoids in wheat sprout. Agronomy 2019, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Taulavuori, K.; Julkunen-Tiitto, R.; Hyöky, V.; Taulavuori, E. Blue mood for superfood. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, L.B.; Leal-Costa, M.V.; Coutinho, M.A.; Moreira, N.d.S.; Lage, C.L.; Barbi, N.d.S.; Costa, S.S.; Tavares, E.S. Increased antioxidant activity and changes in phenolic profile of Kalanchoe pinnata (Lamarck) Persoon (Crassulaceae) specimens grown under supplemental blue light. Photochem. Photobiol. 2013, 89, 391–399. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.K.; Chen, Y.Y.; Hu, T.T.; Zhang, S.J.; Zhang, Y.H.; Zhao, T.Y.; Yu, H.E.; Kang, Y.F. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Cioc, M.; Szewczyk, A.; Zupnik, M.; Kalisz, A.; Pawlowska, B. LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tissue Organ Cult. 2018, 132, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Seo, J.M.; Lee, M.K.; Chun, J.H.; Antonisamy, P.; Arasu, M.V.; Suzuki, T.; Al-Dhabi, N.A.; Kim, S.J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crops Prod. 2014, 54, 320–326. [Google Scholar] [CrossRef]
- Lal, N.; Sachan, P. Effect of different visible light wavelengths on seed germination and photosynthetic pigment contents in Vigna unguiculata (L.) Walp. Indian J. Biol. 2017, 4, 132–136. [Google Scholar]
- Azad, M.; Kim, W.; Park, C.; Cho, D. Effect of artificial LED light and far infrared irradiation on phenolic compound, isoflavones and antioxidant capacity in soybean (Glycine max L.) sprout. Foods 2018, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Lu, Y.; Wu, Q.; Liu, Y.; Xia, Y.; Xia, K.; Cui, J. UV-B-induced anthocyanin accumulation in hypocotyls of radish sprouts continues in the dark after irradiation. J. Sci. Food Agric. 2016, 96, 886–892. [Google Scholar] [CrossRef]
- Papoutsis, K.; Vuong, Q.; Pristijono, P.; Golding, J.; Bowyer, M.; Scarlett, C.; Stathopoulos, C. Enhancing the total phenolic content and antioxidants of lemon pomace aqueous extracts by applying UV-C irradiation to the dried powder. Foods 2016, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
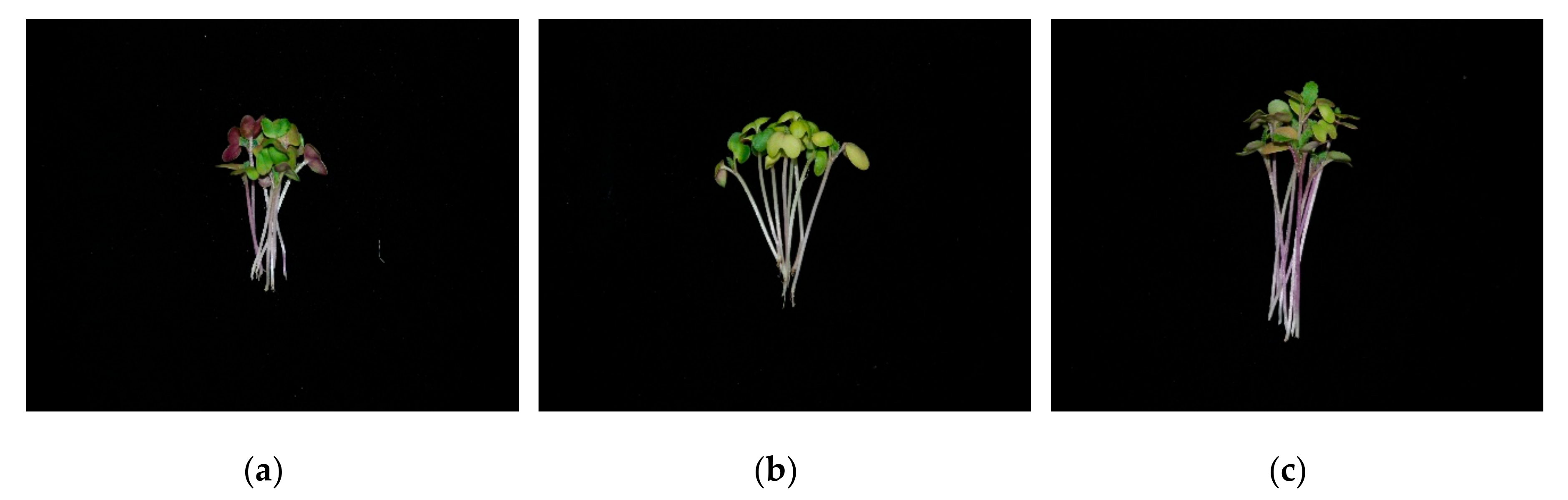


| LED Color | Dry Weight (mg) | ||
|---|---|---|---|
| 1 Week | 2 Weeks | 3 Weeks | |
| White | 0.22 ± 0.02 b Z | 0.56 ± 0.04 a | 0.90 ± 0.08 a |
| Blue | 0.21 ± 0.01 b | 0.39 ± 0.07 b | 0.64 ± 0.03 b |
| Red | 0.28 ± 0.03 a | 0.63 ± 0.05 a | 1.00 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica juncea Sprouts. Horticulturae 2020, 6, 77. https://doi.org/10.3390/horticulturae6040077
Park CH, Park YE, Yeo HJ, Kim JK, Park SU. Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica juncea Sprouts. Horticulturae. 2020; 6(4):77. https://doi.org/10.3390/horticulturae6040077
Chicago/Turabian StylePark, Chang Ha, Ye Eun Park, Hyeon Ji Yeo, Jae Kwang Kim, and Sang Un Park. 2020. "Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica juncea Sprouts" Horticulturae 6, no. 4: 77. https://doi.org/10.3390/horticulturae6040077
APA StylePark, C. H., Park, Y. E., Yeo, H. J., Kim, J. K., & Park, S. U. (2020). Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica juncea Sprouts. Horticulturae, 6(4), 77. https://doi.org/10.3390/horticulturae6040077





