Effects of Metamitron under Different Relative Humidity Conditions on the Fruit Abscission of Malus domestica Borkh. Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Plant Material
2.1.1. Plant Material
2.1.2. Treatment Implementation and Experimental Design
2.2. Metamitron Concentration
2.3. Leaf Net Photosynthesis
2.4. Leaf Soluble Sugars
2.5. Shoot Growth
2.6. Yield Parameters
2.7. Statistical Analysis
3. Results
3.1. Environmental Conditions
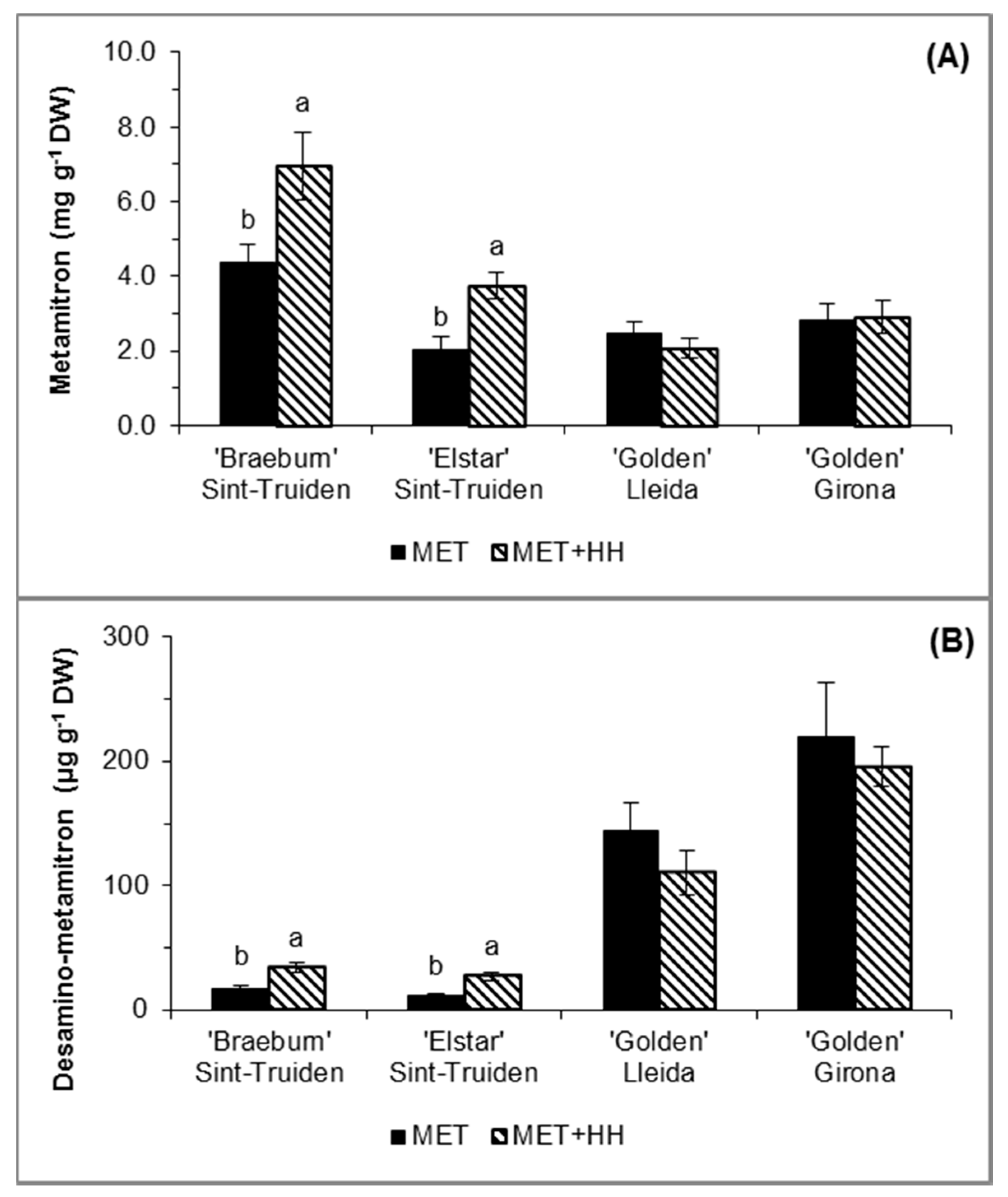
3.2. Metamitron Concentration in Leaves
3.3. Leaf Net Photosynthesis
3.4. Leaf Soluble Sugars
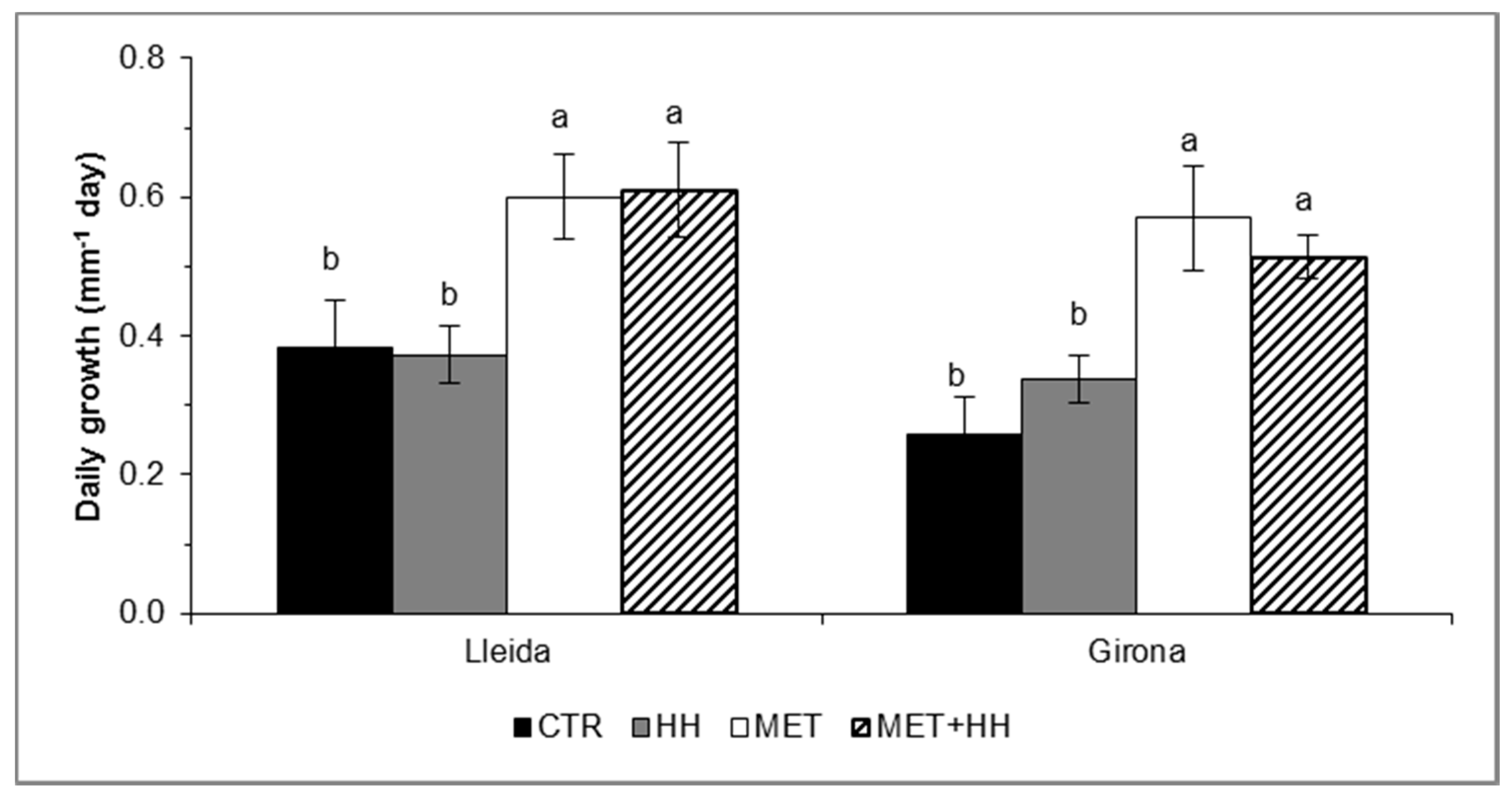
3.5. Shoot Growth and Yield Parameters
4. Discussion
4.1. Metamitron Absorption
4.2. Leaf Net Photosynthesis
4.3. Leaf Soluble Sugars
4.4. Shoot Growth and Thinning Efficacy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lakso, A.N.; Robinson, T.L.; Goffinet, M.C.; White, M.D. Apple Fruit Growth Responses to Varying Thinning Methods and Timing. Acta Hortic. 2001, 557, 407–412. [Google Scholar] [CrossRef]
- Robinson, T.L.; Lakso, A.N. Between Year and Within Year Variation in Chemical Fruit Thinning Efficacy of Apple During Cool Springs. Acta Hortic. 2004, 636, 283–294. [Google Scholar] [CrossRef]
- Abbaspoor, M.; Teicher, H.B.; Streibig, J.C. The Effect of Root-Absorbed PSII Inhibitors on Kautsky Curve Parameters in Sugar Beet. Weed Res. 2006, 46, 226–235. [Google Scholar] [CrossRef]
- Guidi, L.; Degl’innocenti, E. Imaging of Chlorophyll a Fluorescence: A Tool to Study Abiotic Stress in Plants. In Abiotic Stress in Plants—Mechanisms and Adaptations 2011; Shanker, A., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-394-1. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Byers, R.; Carbaugh, D.; Presley, C.; Wolf, T. The Influence of Low Light Levels on Apple Fruit Abscission. J. Hortic. Sci. 1991, 66, 1–17. [Google Scholar] [CrossRef]
- Lakso, A.; Grapadelli, L.C. Implications of Pruning and Training Practices to Carbon Portioning and Fruit Development in Apple Fruit. J. Hortic. Sci. 1992, 70, 389–394. [Google Scholar] [CrossRef]
- Zibordi, M.; Domingos, S.; Corelli Grappadelli, L. Thinning Apples Via Shading: An Appraisal Under Field Conditions. J. Hortic. Sci. Biotech. 2009, 84, 138–144. [Google Scholar] [CrossRef]
- Elsysy, M.; Serra, S.; Schwallier, P.; Musacchi, S.; Einhorn, T. Net Enclosure of ‘Honeycrisp’ and ‘Gala’ Apple Trees at Different Bloom Stages Affects Fruit Set and Alters Seed Production. Agronomy 2019, 9, 478. [Google Scholar] [CrossRef]
- Miller, S.; Hott, C.; Tworkoski, T. Shade Effects on Growth, Flowering and Fruit of Apple. J. Appl. Hortic. 2015, 17, 101–105. [Google Scholar] [CrossRef]
- Forshey, G.; Elfving, D. The Relationship between Vegetative Growth and Fruiting in Apple Trees. In Horticultural Reviews; Janick, J., Ed.; Timber Press: Portland, OR, USA, 1989; Volume 11. [Google Scholar] [CrossRef]
- Bepete, M.; Lakso, A. Differential Effects of Shade on Early-season Fruit and Shoot Growth Rates in ‘Empire’ Apple. HortScience 1998, 33, 823–825. [Google Scholar] [CrossRef]
- Jones, K.M.; Bound, S.A.; Oakford, M.J.; Gillard, P. Modeling Thinning of Pome Fruits. Plant Growth Regul. 2000, 31, 75–84. [Google Scholar] [CrossRef]
- Doerflinger, F.; Lakso, A.; Braun, P. Adapting Malusim Apple Tree Model for the ‘Gala’ Cultivar. Proc. Ixth IS on Modelling in Fruit Research and Orchard Management. Acta Hortic. 2015, 1068, 267–271. [Google Scholar] [CrossRef]
- Rosa, N.; Verjans, W.; Oliveira, C.; Bylemans, D.; Remy, S. Comparison between 6-Benzyladenine and Metamitron as Thinning Agents in ‘Royal Gala’, ‘Cripps Pink’ and ‘Red Delicious’ Apple Cultivars. Proc. Proceedings of the EUFRIN Thinning working Group Symposia. Acta Hortic. 2018, 1221, 52–58. [Google Scholar] [CrossRef]
- Washington State University. Crop Protection Guide for Tree Fruits in Washington—Apple Chemical Thinning. Available online: http://cpg.treefruit.wsu.edu/bioregulator-sprays/apple-chemical-thinning/ (accessed on 4 August 2020).
- Robinson, T.L.; Hoying, S.; Sazom, M.M.; Rufato, A. Precision Crop Load Management Part 2. N. Y. Fruit Q. 2013, 22, 9–13. [Google Scholar]
- Lakso, A.; Robinson, T.L. Decision Support for Apple Thinning Based on Carbon Balance Modeling. Proc. IXth IS on Modelling in Fruit Research and Orchard Management. Acta Hortic. 2015, 1068, 235–242. [Google Scholar] [CrossRef]
- New England—Tree Fruit Management Guide. Available online: https://netreefruit.org/apples/plant-growth-regulators/apple-fruit-thinning (accessed on 5 August 2020).
- Orbovic, V.; Achor, D.; Petacek, P.; Syvertsen, J. Air Temperature, RELATIVE HUMIDITY, and Leaf Age Affect Penetration of Urea through Grapefruit Leaf Cuticles. J. Am. Hortic. Sci. 2001, 126, 44–50. [Google Scholar] [CrossRef]
- Hernandez, M.; Montes, F.; Ruiz, F.; Lopez, G.; Pita, P. The Effect of Vapour Pressure Deficit on Stomatal Conductance, Sap pH and Leaf-specific Hydraulic Conductance in Eucalyptus Globulus Clones Grown Under Two Watering Regimes. Ann. Bot. 2016, 117, 1063–1071. [Google Scholar] [CrossRef]
- Merilo, E.; Yarmolinsky, D.; Jalakas, P.; Parik, H.; Tulva, I.; Rasulov, B.; Kilk, K.; Kollist, H. Stomatal VPD Response: There is More to the Story Than ABA. Plant Physiol. 2018, 176, 851–864. [Google Scholar] [CrossRef]
- Schulze, E.D.; Lange, O.L.; Buschbom, U.; Kappen, L.; Evenari, M. Stomatal Responses Changes in RELATIVE HUMIDITY in Plants Growing in the Desert. Planta 1972, 108, 259–270. [Google Scholar] [CrossRef]
- Patin, F.; Blatt, M. Stomatal Response to RELATIVE HUMIDITY: Blurring the Boundary between Active and Passive Movement. Plant Physiol. 2018, 176, 485–488. [Google Scholar] [CrossRef]
- Lafer, G. Effects Of Chemical Thinning With Metamitron on Fruit Set, Yield and Fruit Quality of ‘Elstar’. Proceedings of XIth International Symposium on Plant Bioregulators in Fruit Production. Acta Hortic. 2010, 884, 531–536. [Google Scholar] [CrossRef]
- Robinson., T.L. Managing Fruit Abscission in Apple. XXIX IHC—Proc. Int. Symposia on Abscission Processes in Horticulture and Non-Destructive Assessment of Fruit Attributes. Acta Hortic. 2016, 1119. [Google Scholar] [CrossRef]
- Lordan, J.; Reginato, G.H.; Lakso, A.N.; Francescatto, P.; Robinson, T.L. Natural Fruitlet Abscission as Related To Apple Tree Carbon Balance Estimated With the Malusim Mode. Sci. Hortic. 2019, 247, 296–309. [Google Scholar] [CrossRef]
- Lesueur, C.; Knittl, P.; Gartner, M.; Mentler, A.; Furelative Humidityacker, M. Analysis of 140 pesticides from conventional farming foodstuff samples after extractions with the modified QuECHERS method. Food Control 2008, 19, 906–914. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rodrigues, A.P.; Semedo, J.N.; Pais, I.P.; Martins, L.D.; Simões-Costa, M.C.; Leitão, A.E.; Fortunato, A.S.; Batista-Santos, P.; Palos, I.M.; et al. Sustained Photosynthetic Performance of Coffea Spp. Under Long-Term Enhanced [CO2]. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Nemeskéry, E.; Sardi, É.; Kovács-Nagy, E.; Stefanavits Bányai, É.; Nagy, J.; Nyéki, J.; Szabó, T. Studies on the Drough Responses of Apple Trees (Malus domestica Borkh.) Grafted on Different Rootstocks. Int. J. Hortic. Sci. 2009, 15, 29–36. [Google Scholar] [CrossRef]
- Locatelli, G.; Pio, R.; Rayane, B.B.; Souza, F.B.M.; Castro, E.M.; Zambon, C.R. Leaf Anatomy of Apple Trees during Seasonal Periods under Subtropical Conditions. HortScience 2019, 54, 1887–1895. [Google Scholar] [CrossRef]
- Byers, R. Influence of Temperature and Darkness on Apple Fruit Abscission and Chemical Thinning. J. Tree Prod. 2002, 3, 41–53. [Google Scholar] [CrossRef]
- Brunner, P. Impact of Metamitron as a Thinning Compound on Apple Plants. Proc. XIIth IS on Plant Bioregulators in Fruit Production. Acta Hortic. 2014, 1042, 173–182. [Google Scholar] [CrossRef]
- Gabardo, G.; Kretzschmar, A.; Petri, J.; Couto, M.; Hawerooth, F.; Silva, C. Taxa Fotossintética em Macieiras Tratadas com Metamitron. Rev. Elet. Cient. UERGS 2017, 3, 617–633. [Google Scholar] [CrossRef]
- Stander, O.P.J.; Botes, J.; Krogscheepers, C. The Potential Use of Metamitron as a Chemical Fruit-Thinning Agent in Mandarin. HortTechnology 2018, 28, 28–34. [Google Scholar] [CrossRef]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a souce, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion with plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Breen, K.; Tustin, S.; Palmer, J.; Boldingh, H.; Close, D. Revisiting the role of carbohydrate reserves in fruit set and early-season growth of apple. Sci. Hortic. 2020, 261. [Google Scholar] [CrossRef]
- Petri, J.P.; Couto, M.; Gabardo, G.C.; Francescatto, P.; Hawerroth, F.J. Metamitron Replacing Carbaryl in Post Bloom Thinning of Apple Trees. Rev. Bras. Frutic. 2016, 38. [Google Scholar] [CrossRef]
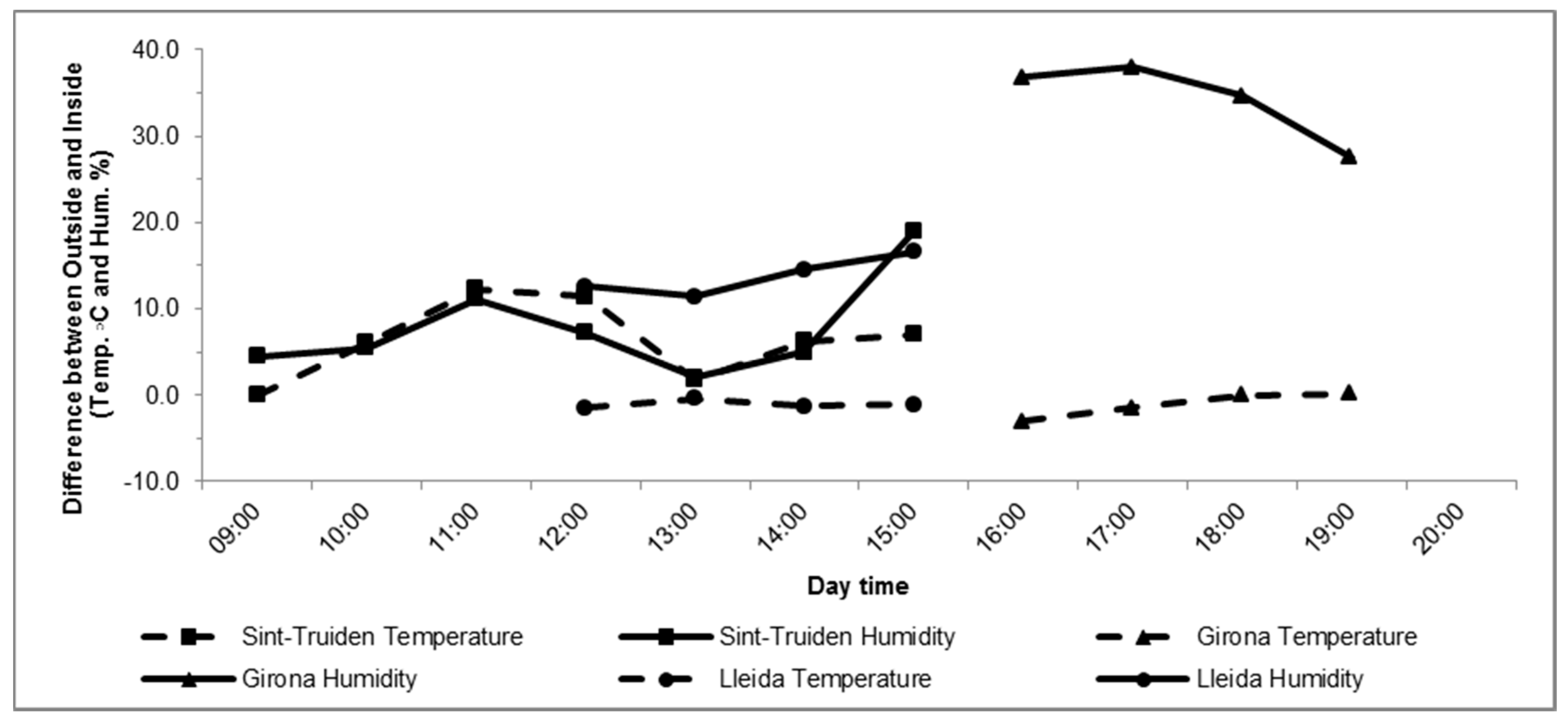

| Location/ Cultivar | Fruit Diameter (mm) | Global Irradiance MJ/m2—5 Days After | Night Temperature °C—5 Nights Before | Night Temperature °C—5 Nights After | Diurnal Temperature °C—Application Day | Relative Humidity % | |
|---|---|---|---|---|---|---|---|
| Control | Control | Control | Control | Control | HH | ||
| 2018 | |||||||
| Sint-Truiden/ ‘Braeburn’ | 14 ± 0.1 | 21.7 ± 0.6 | 12.1 ± 0.6 | 9.7 ± 0.9 | 17.2 ± 0.9 | 65.9 ± 3.6 | 73.9 ± 2.7 |
| Sint-Truiden/ ‘Elstar’ | 13 ± 0.1 | 21.7 ± 0.6 | 12.1 ± 0.6 | 9.7 ± 0.9 | 17.2 ± 0.9 | 65.9 ± 3.6 | 73.9 ± 2.7 |
| 2019 | |||||||
| Girona/ ‘Golden Reinders’ | 14 ± 0.1 | 21.4 ± 2.4 | 12.0 ± 0.9 | 13.4 ± 0.4 | 16.9 ± 0.8 | 40.8 ± 1.0 | 76.1 ± 3.7 |
| Lleida/ ‘Golden Reinders’ | 12 ± 0.2 | 21.4 ± 1.9 | 11.1 ± 1.1 | 10.3 ± 1.1 | 18.5 ± 1.0 | 44.3 ± 1.4 | 58.0 ± 3.3 |
| Treatment z | 5 DAS | |||||||||
| Sucrose | Glucose | Fructose | Sorbitol | Total | ||||||
| ‘Braeburn’ | ||||||||||
| CTR | 32.8 ± 2.3 | aA y | 28.7 ± 2.4 | NS | 3.2 ± 0.5 | aA | 85.7 ± 5.4 | bB | 150.6 ± 10.1 | aA |
| HH | 32.0 ± 1.6 | aA | 31.9 ± 1.8 | 3.1 ± 0.3 | aA | 98.4 ± 3.5 | aB | 166.2 ± 6.7 | aA | |
| MET | 23.4 ± 2.2 | bA | 28.2 ± 0.9 | 2.3 ± 0.4 | bA | 75.8 ± 5.2 | cB | 131.7 ± 7.6 | bA | |
| MET + HH | 17.7 ± 1.8 | cA | 31.4 ± 2.4 | 3.0 ± 0.6 | aA | 70.0 ± 3.6 | cA | 122.0 ± 6.2 | bA | |
| ‘Elstar’ | ||||||||||
| CTR | 36.6 ± 1.8 | aA | 39.6 ± 2.4 | aA | 7.8 ± 0.5 | aA | 100.7 ± 2.8 | aA | 184.7 ± 4.6 | aA |
| HH | 34.0 ± 1.5 | aA | 40.9 ± 2.3 | aA | 6.8 ± 0.7 | aA | 99.6 ± 3.3 | aA | 181.2 ± 6.1 | aA |
| MET | 30.2 ± 2.5 | bA | 39.8 ± 2.6 | aA | 6.4 ± 0.5 | aA | 82.6 ± 3.4 | bA | 159.0 ± 8.2 | bA |
| MET + HH | 22.6 ± 1.2 | cA | 39.4 ± 1.9 | aA | 3.4 ± 0.5 | bA | 82.2 ± 3.6 | bA | 147.6 ± 4.8 | bA |
| 7 DAS | ||||||||||
| Sucrose | Glucose | Fructose | Sorbitol | Total | ||||||
| ‘Braeburn’ | ||||||||||
| CTR | 15.7 ± 0.6 | abB | 30.9 ± 1.3 | NS | 2.4 ± 0.2 | aA | 98.0 ± 2.1 | bA | 147.1 ± 3.4 | bA |
| HH | 19.7 ± 2.3 | aB | 34.2 ± 2.5 | 2.6 ± 0.7 | aA | 122.5 ± 6.5 | aA | 179.0 ± 9.3 | aA | |
| MET | 13.1 ± 0.8 | bB | 28.6 ± 2.8 | 1.9 ± 0.4 | bB | 86.0 ± 5.0 | cA | 129.7 ± 6.7 | cA | |
| MET + HH | 10.6 ± 1.6 | bB | 28.0 ± 3.5 | 1.6 ± 0.4 | bB | 77.3±7.3 | cA | 117.5 ± 11.8 | cA | |
| ‘Elstar’ | ||||||||||
| CTR | 29.4 ± 4.1 | aA | 39.4 ± 2.4 | aA | 9.3 ± 1.9 | aA | 102.6 ± 3.2 | aA | 191.0 ± 4.9 | aA |
| HH | 31.0 ± 1.2 | aA | 38.3 ± 2.2 | aA | 6.1 ± 0.4 | abA | 98.4 ± 3.7 | aA | 173.9 ± 7.0 | bA |
| MET | 25.9 ± 1.1 | aA | 34.1 ± 0.8 | aA | 5.2 ± 0.5 | bA | 88.3 ± 4.0 | bA | 153.5 ± 5.2 | cA |
| MET + HH | 16.5 ± 2.9 | bB | 26.7 ± 4.1 | bB | 4.1 ± 0.6 | bA | 64.9 ± 10.2 | cB | 112.3 ± 17.6 | dB |
| Treatment z | 2 DAS | |||||||||
| Sucrose | Glucose | Fructose | Sorbitol | Total | ||||||
| Leida | ||||||||||
| CTR | 19.0 ± 1.3 | aB y | 27.5 ± 1.2 | aA | 7.2 ± 0.5 | bA | 62.1 ± 3.7 | aB | 122.3 ± 2.5 | aA |
| HH | 21.5 ± 1.4 | aB | 26.6 ± 1.4 | aA | 8.0 ± 0.7 | bA | 66.2 ± 2.3 | aB | 115.8 ± 3.9 | abA |
| MET | 14.8 ± 1.6 | bA | 25.2 ± 1.4 | aA | 9.8 ± 1.0 | aA | 56.3 ± 3.6 | aB | 106.1 ± 3.7 | bB |
| MET+HH | 15.2 ± 2.6 | bA | 25.0 ± 1.3 | aA | 8.7 ± 0.8 | abA | 60.4 ± 3.1 | aB | 110.5 ± 2.6 | bB |
| Girona | ||||||||||
| CTR | 16.6 ± 0.9 | aA | 21.7 ± 1.0 | aA | 7.2 ± 0.4 | bA | 53.8 ± 2.0 | NS | 99.3 ± 2.1 | aA |
| HH | 13.7 ± 1.0 | aA | 20.6 ± 1.2 | aA | 7.2 ± 0.4 | bA | 60.0 ± 2.7 | 101.6 ± 2.5 | aA | |
| MET | 9.7 ± 0.7 | bA | 19.7 ± 0.7 | aA | 9.2 ± 0.6 | aA | 55.1 ± 1.2 | 93.8 ± 1.7 | bA | |
| MET+HH | 10.0 ± 1.0 | bA | 18.1 ± 0.8 | aA | 10.3 ± 1.0 | aA | 60.9 ± 1.8 | 99.2 ± 2.0 | aA | |
| 5 DAS | ||||||||||
| Sucrose | Glucose | Fructose | Sorbitol | Total | ||||||
| Leida | ||||||||||
| CTR | 29.3 ± 1.9 | aA | 21.8 ± 0.5 | abB | 6.2 ± 0.6 | bA | 76.3 ± 3.2 | aA | 129.7 ± 5.8 | aA |
| HH | 31.7 ± 2.2 | aA | 23.8 ± 0.9 | aA | 5.9 ± 0.2 | bB | 75.2 ± 3.7 | aA | 133.2 ± 5.8 | aA |
| MET | 16.5 ± 2.5 | bA | 19.3 ± 0.9 | bB | 9.6 ± 1.2 | aA | 68.2 ± 6.6 | bA | 120.7 ± 4.7 | abA |
| MET+HH | 15.3 ± 1.8 | bA | 19.2 ± 0.7 | bB | 8.7 ± 0.8 | aA | 62.6 ± 3.6 | bB | 105.8 ± 4.5 | bB |
| Girona | ||||||||||
| CTR | 13.4 ± 0.9 | aA | 24.3 ± 0.6 | aA | 7.2 ± 0.4 | bA | 58.6 ± 1.9 | NS | 103.7 ± 2.5 | aA |
| HH | 13.5 ± 0.6 | aA | 21.6 ± 0.7 | abA | 5.9 ± 0.4 | bA | 55.3 ± 2.0 | 96.3 ± 1.9 | abA | |
| MET | 8.8 ± 1.0 | bA | 19.8 ± 1.0 | bA | 8.4 ± 0.7 | aA | 56.7 ± 2.7 | 93.6 ± 3.2 | bA | |
| MET+HH | 9.4 ± 0.8 | bA | 19.5 ± 0.9 | bA | 7.1 ± 0.8 | bB | 54.4 ± 2.5 | 88.7 ± 1.9 | bB | |
| 10 DAS | ||||||||||
| Sucrose | Glucose | Fructose | Sorbitol | Total | ||||||
| CTR | 16.8 ± 0.7 | aB | 23.0 ± 1.1 | aB | 6.6 ± 0.6 | aA | 80.7 ± 2.9 | aA | 126.0 ± 2.0 | aA |
| HH | 17.5 ± 0.8 | aB | 24.0 ± 0.9 | aA | 7.3 ± 0.4 | aA | 81.1 ± 3.2 | aA | 132.0 ± 2.7 | aA |
| MET | 15.8 ± 0.8 | aA | 24.3 ± 1.0 | aA | 7.5 ± 0.5 | aB | 76.6 ± 2.8 | aA | 124.1 ± 3.0 | aA |
| MET+HH | 16.8 ± 0.9 | aA | 24.2 ± 0.9 | aA | 6.8 ± 0.5 | aB | 78.6 ± 3.1 | aA | 126.3 ± 3.0 | aA |
| Location | Cultivar Year | Treatment z | Fruits/100 Flower Clusters | Fruit Weight (g) | Yield/Tree (kg) | % Fruits >70 mm | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sint-Truiden | ‘Braeburn’ 2018 | CTR | 62.0 ± 5.4 | a y | 166.0 ± 4.4 | b | 14.7 ± 0.9 | a | 75.9 ± 3.3 | b |
| HH | 58.0 ± 5.5 | a | 170.1 ± 4.5 | b | 14.4 ± 1.3 | a | 79.9 ± 4.6 | b | ||
| MET | 27.1 ± 5.7 | b | 214.8 ± 7.8 | a | 7.4 ± 0.9 | b | 94.4 ± 4.6 | a | ||
| MET+HH | 20.6 ± 4.4 | b | 241.8 ± 5.4 | a | 7.8 ± 1.3 | b | 98.9 ± 1.1 | a | ||
| ‘Elstar’ 2018 | CTR | 104.3 ± 12.2 | a | 121.0 ± 6.5 | b | 9.8 ± 0.6 | a | 67.9 ± 9.1 | b | |
| HH | 101.0 ± 14.2 | a | 125.3 ± 6.7 | b | 8.8 ± 1.2 | a | 60.3 ± 5.4 | b | ||
| MET | 62.4 ± 12.3 | ab | 151.0 ± 5.1 | a | 6.9 ± 1.0 | ab | 83.9 ± 2.6 | a | ||
| MET+HH | 42.5 ± 4.2 | b | 157.1 ± 4.5 | a | 4.7 ± 0.6 | b | 87.7 ± 3.0 | a | ||
| Girona | ‘Golden Reinders’ 2019 | CTR | 141.6 ± 8.5 | NS | 126.7 ± 5.0 | NS | 48.2 ± 3.5 | NS | 22.6 ± 6.3 | b |
| HH | 143.2 ± 7.9 | 129.4 ± 4.8 | 41.1 ± 4.1 | 28.4 ± 4.5 | b | |||||
| MET | 126.7 ± 9.0 | 141.6 ± 8.5 | 38.1 ± 4.0 | 43.0 ± 2.6 | a | |||||
| MET+HH | 129.4 ± 4.8 | 143.2 ± 2.9 | 34.7 ± 2.6 | 42.6 ± 3.4 | a | |||||
| Lleida | ‘Golden Reinders’ 2019 | CTR | 137.8 ± 6.9 | NS | 113.1 ± 4.7 | NS | 45.1 ± 3.5 | NS | 44.1 ± 7.3 | NS |
| HH | 127.0 ± 9.5 | 118.8 ± 3.2 | 45.2 ± 4.1 | 46.2 ± 3.4 | ||||||
| MET | 106.4 ± 6.2 | 129.4 ± 2.8 | 39.1 ± 0.8 | 61.3 ± 2.0 | ||||||
| MET+HH | 104.0 ± 14.6 | 127.6 ± 4.9 | 38.1 ± 3.3 | 58.3 ± 4.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, N.; Verjans, W.; Àvila, G.; Carbó, J.; Bonany, J.; Ramalho, J.C.; Asín, L.; Oliveira, C.M. Effects of Metamitron under Different Relative Humidity Conditions on the Fruit Abscission of Malus domestica Borkh. Cultivars. Horticulturae 2020, 6, 89. https://doi.org/10.3390/horticulturae6040089
Rosa N, Verjans W, Àvila G, Carbó J, Bonany J, Ramalho JC, Asín L, Oliveira CM. Effects of Metamitron under Different Relative Humidity Conditions on the Fruit Abscission of Malus domestica Borkh. Cultivars. Horticulturae. 2020; 6(4):89. https://doi.org/10.3390/horticulturae6040089
Chicago/Turabian StyleRosa, Nídia, Wim Verjans, Glória Àvila, Joaquim Carbó, Joan Bonany, José Cochicho Ramalho, Luís Asín, and Cristina Moniz Oliveira. 2020. "Effects of Metamitron under Different Relative Humidity Conditions on the Fruit Abscission of Malus domestica Borkh. Cultivars" Horticulturae 6, no. 4: 89. https://doi.org/10.3390/horticulturae6040089
APA StyleRosa, N., Verjans, W., Àvila, G., Carbó, J., Bonany, J., Ramalho, J. C., Asín, L., & Oliveira, C. M. (2020). Effects of Metamitron under Different Relative Humidity Conditions on the Fruit Abscission of Malus domestica Borkh. Cultivars. Horticulturae, 6(4), 89. https://doi.org/10.3390/horticulturae6040089






