Augmenting Nutrient Acquisition Ranges of Greenhouse Grown CBD (Cannabidiol) Hemp (Cannabis sativa) Cultivars
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Macronutrient Leaf Concentration
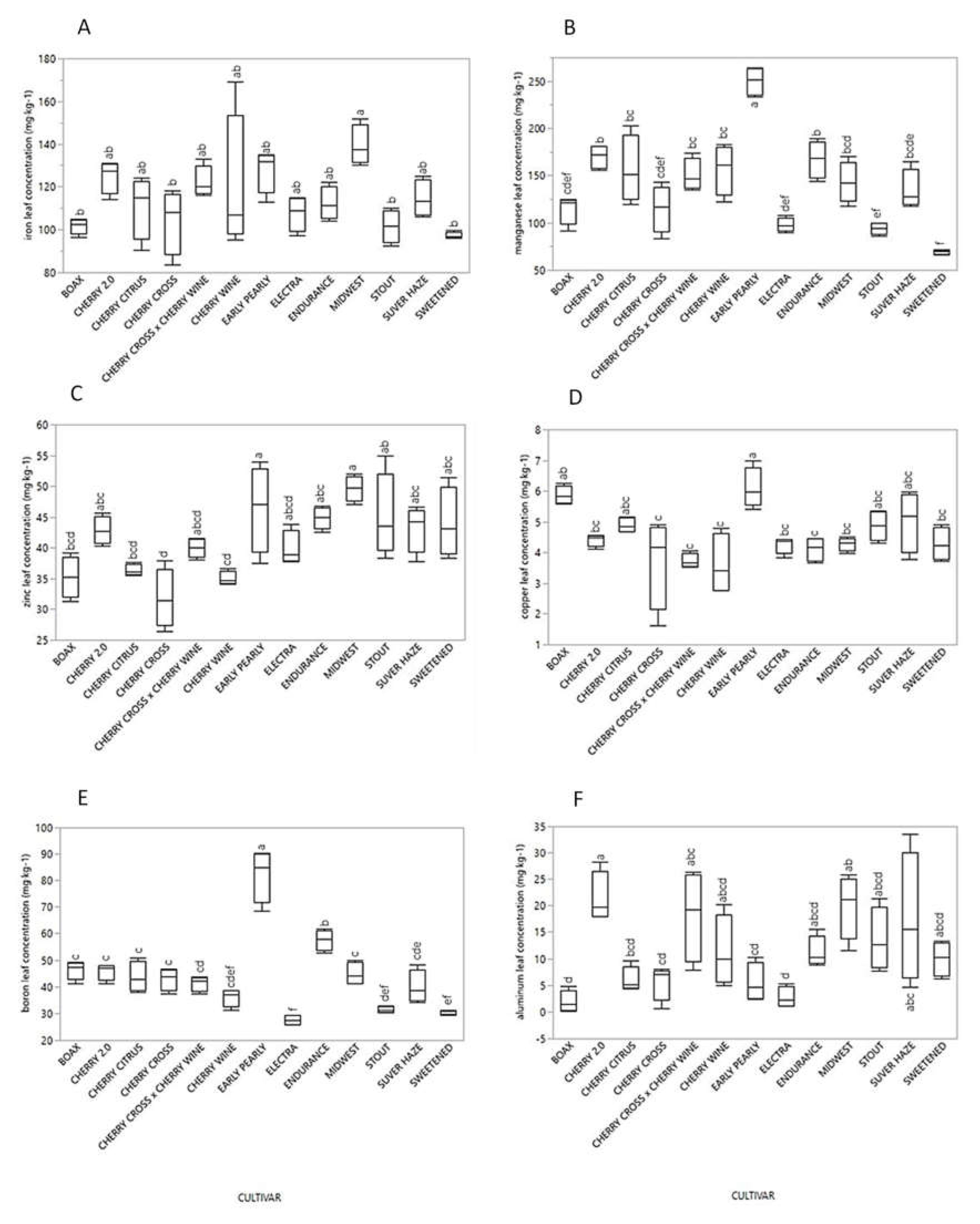
3.2. Micronutrients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farag, S.; Kayser, O. The cannabis plant: Botanical aspects. In Handbook of Cannabis and Related Pathologies; Academic Press: Cambridge, MA, USA, 2017; pp. 3–12. [Google Scholar]
- Small, E. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Spitzer-Rimon, B.; Duchin, S.; Bernstein, N.; Kamenetsky, R. Architecture and florogenesis in female Cannabis sativa plants. Front. Plant Sci. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Cherney, J.H.; Small, E. Industrial hemp in North America: Production, politics and potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- White, C.M. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Hemp as an Agricultural Commodity: Congressional Research Service. 2018. Available online: https://crsreports.congress.gov/product/pdf/R/R45525. (accessed on 23 September 2020).
- Dingha, B.; Sandler, L.; Bhowmik, A.; Akotsen-Mensah, C.; Jackai, L.; Gibson, K.; Turco, R. Industrial hemp knowledge and interest among North Carolina organic farmers in the United States. Sustainability 2019, 11, 2691. [Google Scholar] [CrossRef]
- Mark, T.; Shepherd, J.; Olson, D.; Snell, W.; Proper, S.; Thornsbury, S. Economic Viability of Industrial Hemp in the United States: A Review of State Pilot Programs. Available online: https://www.ers.usda.gov/webdocs/publications/95930/eib-217.pdf?v=4149.6 (accessed on 23 September 2020).
- Bryson, G.M.; Mills, H.A.; Sasseville, D.N.; Jones, J.B., Jr.; Barker, A.V. Plant Analysis Handbook III: A Guide to Sampling, Preparation, Analysis and Interpretation for Agronomic and Horticultural Crops; Micro-Macro Publishing, Inc.: Athens, GA, USA, 2014. [Google Scholar]
- Landis, H.; Hicks, K.; Cockson, P.; Henry, J.B.; Smith, J.T.; Whipker, B.E. Expanding Leaf Tissue Nutrient Survey Ranges for Greenhouse Cannabidiol-Hemp. Crop Forage Turfgrass Manag. 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Cockson, P.; Landis, H.; Smith, T.; Hicks, K.; Whipker, B.E. Characterization of nutrient disorders of Cannabis sativa. Appl. Sci. 2019, 9, 4432. [Google Scholar] [CrossRef]
- NCDA&CS. Plant, Waste, Solution, and Media Analytical Procedures; North Carolina Department of Agricultural and Consumer Services Agronomic Division: Raleigh, NC, USA, 2015. Available online: www.ncagr.gov/agronomi/documents/PWSMMethodology.pdf (accessed on 23 September 2020).
- Small, E.; Cronquist, A. A practical and natural taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]

| Cultivar | Macronutrient (%) | |||||
|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | |
| BaOx | 4.25–4.52 (4.36) | 0.33–0.38 (0.36) | 2.23–2.43 (2.37) | 1.66–2.37 (2.09) | 0.34–0.44 (0.41) | 0.28–0.32 (0.30) |
| Cherry 2.0 | 4.03–4.35 (4.22) | 0.32–0.35 (0.34) | 2.83–3.18 (2.98) | 2.38–3.14 (2.60) | 0.48–0.55 (0.51) | 0.28–0.30 (0.29) |
| Cherry Citrus | 3.87–4.24 (4.09) | 0.31–0.37 (0.34) | 2.30–2.75 (2.50) | 3.01–5.16 (4.04) | 0.38–0.61 (0.50) | 0.31–0.37 (0.34) |
| Cherry Cross | 4.41–4.58 (4.50) | 0.33–0.37 (0.35) | 2.13–2.37 (2.25) | 2.00–2.70 (2.37) | 0.48–0.56 (0.53) | 0.28–0.33 (0.30) |
| Cherry Wine | 3.75–4.68 (4.27) | 0.29–0.42 (0.38) | 2.08–2.62 (2.40) | 2.94–4.95 (4.05) | 0.48–0.69 (0.60) | 0.27–0.35 (0.31) |
| Cherry Cross × Cherry Wine | 3.72–4.19 (3.91) | 0.26–0.34 (0.30) | 2.13–2.65 (2.46) | 2.79–3.61 (3.18) | 0.58–0.61 (0.59) | 0.27–0.32 (0.29) |
| Early Pearly | 4.57–4.98 (4.76) | 0.33–0.40 (0.37) | 2.62–2.89 (2.79) | 3.83–5.34 (4.76) | 0.38–0.50 (0.45) | 0.30–0.34 (0.32) |
| Electra | 4.56–4.70 (4.65) | 0.33–0.36 (0.34) | 2.09–2.23 (2.16) | 1.50–1.96 (1.77) | 0.37–0.44 (0.41) | 0.31–0.35 (0.33) |
| Endurance | 3.77–4.44 (4.13) | 0.30–0.38 (0.34) | 2.87–3.41 (3.06) | 3.68–4.93 (4.21) | 0.42–0.54 (0.47) | 0.29–0.33 (0.32) |
| Midwest | 4.76–4.93 (4.82) | 0.37–0.41 (0.40) | 2.74–3.06 (2.88) | 2.33–2.96 (2.58) | 0.47–0.55 (0.50) | 0.31–0.34 (0.32) |
| Stout | 4.37–4.64 (4.49) | 0.38–0.43 (0.40) | 2.45–2.64 (2.55) | 1.63–2.05 (1.80) | 0.42–0.51 (0.46) | 0.30–0.33 (0.32) |
| Suver Haze | 3.29–4.36 (3.75) | 0.27–0.39 (0.33) | 2.65–2.76 (2.72) | 2.23–3.56 (2.80) | 0.40–0.58 (0.50) | 0.25–0.32 (0.28) |
| Sweetened | 3.84–4.09 (3.96) | 0.36–0.39 (0.38) | 2.20–2.33 (2.28) | 1.51–1.68 (1.62) | 0.34–0.38 (0.36) | 0.27–0.29 (0.28) |
| 1Significance | *** | ** | *** | *** | *** | ** |
| 2MSD | 0.58 | 0.08 | 0.39 | 1.29 | 0.14 | 0.05 |
| 3 Survey Range | 3.29–4.98 (4.30) | 0.26–0.43 (0.36) | 2.08–3.41 (2.57) | 1.50–5.34 (2.91) | 0.34–0.69 (0.48) | 0.25–0.37 (0.31) |
| Reference Range | Macronutrient (%) | |||||
|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | |
| 1 Survey Range | 3.29–4.98 (4.30) | 0.26–0.43 (0.36) | 2.08–3.41 (2.57) | 1.50–5.34 (2.91) | 0.34–0.69 (0.48) | 0.25–0.37 (0.31) |
| 2 Reference Survey Range | 2.65–4.47 (3.75) | 0.31–0.44 (0.35) | 1.54–2.98 (2.42) | 0.53–2.14 (1.15) | 0.25–0.46 (0.32) | 0.19–0.29 (0.24) |
| 3 Reference Survey Range | 3.30–4.76 | 0.24–0.49 | 1.83–2.35 | 1.47–4.42 | 0.40–0.81 | 0.17–0.26 |
| 4 Deficiency Threshold | 1.62 | 0.09 | 0.41 | 0.39 | 0.12 | 0.11 |
| 5Nutrient Acquisition Ranges | 2.65–4.98 | 0.24–0.49 | 1.54–3.41 | 0.53–5.34 | 0.25–0.81 | 0.17–0.41 * |
| Cultivar | Micronutrient (mg·kg−1) | |||||
|---|---|---|---|---|---|---|
| Fe | Mn | Zn | Cu | B | Al | |
| BaOx | 96.5–105.0 (101.6) | 91.4–125.0 (114.6) | 31.3–39.2 (35.2) | 5.6–6.3 (5.9) | 41.3–49.2 (46.3) | 0.1–4.8 (1.9) |
| Cherry 2.0 | 114.0–131.0 (125.0) | 156.0–181.0 (170.3) | 40.3–45.7 (42.9) | 4.1–4.6 (4.4) | 41.2–47.9 (45.8) | 17.9–28.2 (21.4) |
| Cherry Citrus | 90.6–124.0 (111.2) | 120.0–203.0 (156.5) | 35.5–37.7 (36.4) | 4.7–5.2 (4.9) | 38.0–51.0 (43.7) | 4.3–9.6 (6.0) |
| Cherry Cross | 83.5–118.0 (104.4) | 83.6–143.0 (115.2) | 26.4–38.0 (31.8) | 1.6–4.9 (3.7) | 37.4–46.6 (42.9) | 0.6–8.0 (5.6) |
| Cherry Wine | 95.1–169.0 (119.5) | 122.0–183.0 (157.0) | 34.2–36.6 (35.1) | 2.8–4.8 (3.6) | 31.4–38.7 (36.1) | 5.0–20.2 (11.3) |
| Cherry Cross × Cherry Wine | 116.0–133.0 (122.3) | 135.0–174.0 (150.5) | 38.1–41.6 (40.0) | 3.5–4.1 (3.7) | 37.3–43.7 (41.3) | 7.8–26.4 (18.2) |
| Early Pearly | 113.0–135.0 (128.0) | 233.0–264.0 (250.0) | 37.5–53.9 (46.4) | 5.4–7.0 (6.1) | 68.6–90.5 (82.2) | 2.4–10.3 (5.5) |
| Electra | 97.2–115.0 (107.6) | 89.9–108.0 (98.0) | 37.8–43.9 (39.8) | 3.9–4.4 (4.3) | 25.8–29.3 (27.6) | 1.1–5.3 (2.7) |
| Endurance | 104.0–122.0 (112.3) | 144.0–189.0 (167.3) | 42.6–46.8 (44.9) | 3.6–4.5 (4.1) | 52.9–61.7 (57.7) | 8.9–15.6 (11.2) |
| Midwest | 130.0–152.0 (139.3) | 118.0–170.0 (143.0) | 47.0–52.0 (49.6) | 4.0–4.5 (4.3) | 41.1–50.0 (44.8) | 11.6–25.9 (20.0) |
| Stout | 92.6–110.0 (101.4) | 86.4–100.0 (93.9) | 38.3–54.9 (45.1) | 4.3–5.4 (4.9) | 30.4–33.0 (31.5) | 7.7–21.3 (13.5) |
| Suver Haze | 106.0–125.0 (114.5) | 118.0–165.0 (134.5) | 37.8–46.7 (43.2) | 3.8–6.0 (5.0) | 34.0–48.3 (39.9) | 4.6–33.6 (17.3) |
| Sweetened | 96.1–99.7 (97.4) | 66.5–71.3 (69.3) | 38.4–51.4 (44.0) | 3.7–4.9 (4.3) | 29.4–31.2 (30.0) | 6.3–13.3 (10.0) |
| 1Significance | ** | *** | *** | *** | *** | *** |
| 2MSD | 32.0 | 48.4 | 9.9 | 1.6 | 11.3 | 14.3 |
| 3Survey Range | 83.5–169.0 (114.2) | 66.5–264.0 (140.0) | 26.4–54.9 (41.1) | 1.6–7.0 (4.6) | 25.8–90.5 (43.8) | 0.1–33.6 (11.1) |
| Reference Range | Micronutrient (mg·kg−1) | |||||
|---|---|---|---|---|---|---|
| Fe | Mn | Zn | Cu | B | Al | |
| 1 Survey Range | 83.5–169.0 (114.2) | 66.5–264.0 (140.0) | 26.4–54.9 (41.1) | 1.6–7.0 (4.6) | 25.8–90.5 (43.8) | 0.1–33.6 (11.1) |
| 2 Reference Survey Range | 59.0–132.0 (82.2) | 24.3–71.9 (37.1) | 23.2–46.2 (31.0) | 1.8–11.4 (3.5) | 22.6–57.3 (35.9) | 0.68–71.0 (5.58) |
| 3 Reference Survey Range | 100.0–150.0 | 41.0–93.0 | 24.0–52.0 | 5.0–7.1 | 56.0–105.0 | † NR |
| 4 Deficiency Threshold | 60.1 | 7.56 | 10.7 | 1.41 | 2.46 | NR |
| 4 Toxicity Threshold | NR | 47.9 | NR | NR | 671.8 | NR |
| 5Nutrient Acquisition Ranges | 59.0–169.0 | 24.3–264.0 | 23.2–54.9 | 1.6–11.4 | 22.6–105.0 | 0.1–71.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowski, J.; Edmisten, K.; Davis, J.; McGinnis, M.; Hicks, K.; Cockson, P.; Veazie, P.; Whipker, B.E. Augmenting Nutrient Acquisition Ranges of Greenhouse Grown CBD (Cannabidiol) Hemp (Cannabis sativa) Cultivars. Horticulturae 2020, 6, 98. https://doi.org/10.3390/horticulturae6040098
Kalinowski J, Edmisten K, Davis J, McGinnis M, Hicks K, Cockson P, Veazie P, Whipker BE. Augmenting Nutrient Acquisition Ranges of Greenhouse Grown CBD (Cannabidiol) Hemp (Cannabis sativa) Cultivars. Horticulturae. 2020; 6(4):98. https://doi.org/10.3390/horticulturae6040098
Chicago/Turabian StyleKalinowski, Jennifer, Keith Edmisten, Jeanine Davis, Michelle McGinnis, Kristin Hicks, Paul Cockson, Patrick Veazie, and Brian E. Whipker. 2020. "Augmenting Nutrient Acquisition Ranges of Greenhouse Grown CBD (Cannabidiol) Hemp (Cannabis sativa) Cultivars" Horticulturae 6, no. 4: 98. https://doi.org/10.3390/horticulturae6040098
APA StyleKalinowski, J., Edmisten, K., Davis, J., McGinnis, M., Hicks, K., Cockson, P., Veazie, P., & Whipker, B. E. (2020). Augmenting Nutrient Acquisition Ranges of Greenhouse Grown CBD (Cannabidiol) Hemp (Cannabis sativa) Cultivars. Horticulturae, 6(4), 98. https://doi.org/10.3390/horticulturae6040098






