Effects of Interval Flooding Stress on Physiological Characteristics of Apple Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
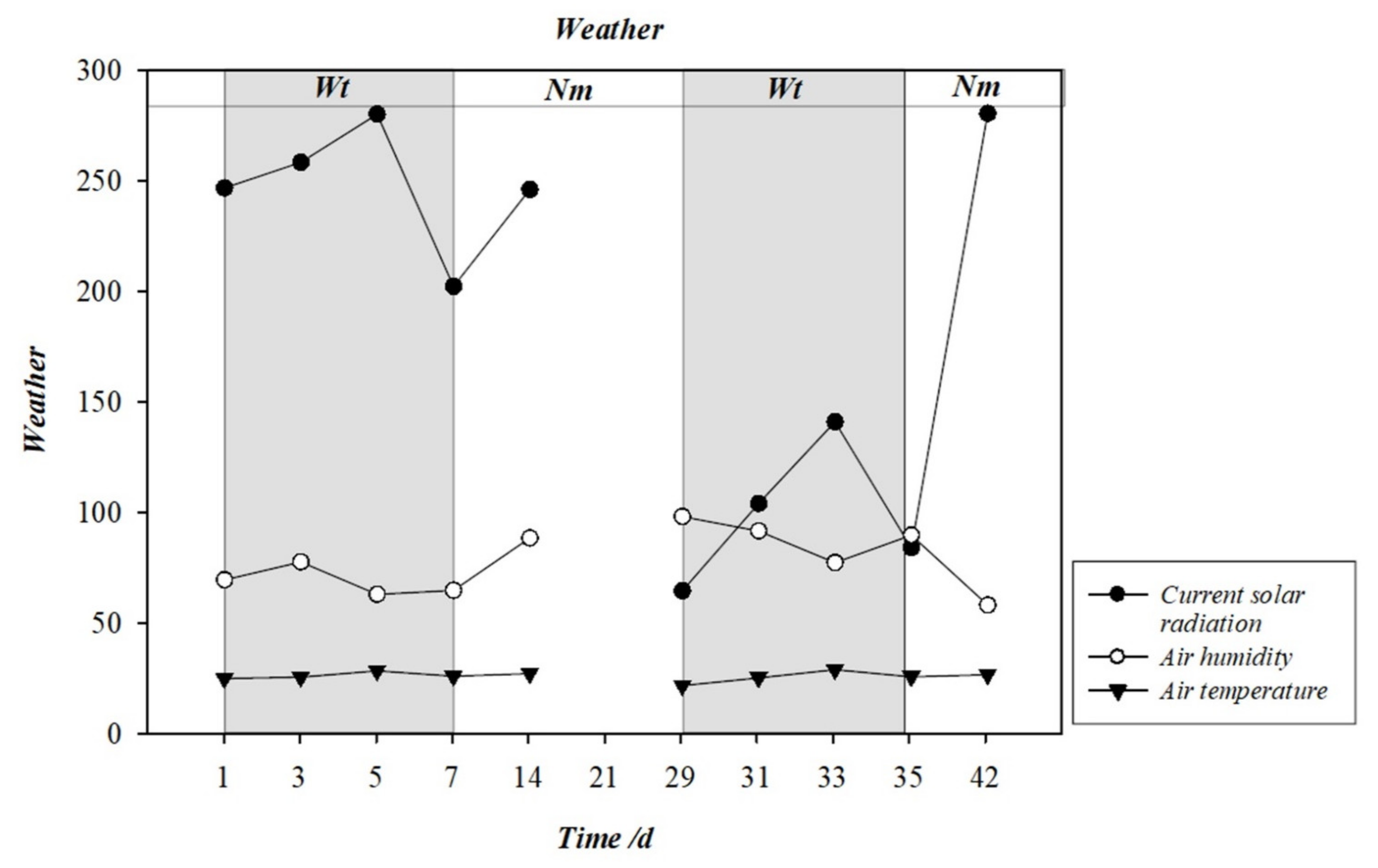
2.3. Measurement and Methods of Indicators
2.3.1. Plant Morphology Observation
2.3.2. Determination of Leaf Water Content (LWC)
2.3.3. Determination of the Electrical Impedance Spectroscopy (EIS) of Leaves
2.3.4. Determination of the Rate of Electrolyte Leakage (REL)
2.3.5. Determination of Leaf Starch and Soluble Sugar Content
2.3.6. Determination of Maximum Photochemical Efficiency (Fv/Fm)
2.3.7. Determination of Relative Chlorophyll Content (SPAD)
2.4. Data Processing and Analysis
3. Results
3.1. Plant Leaf Morphology
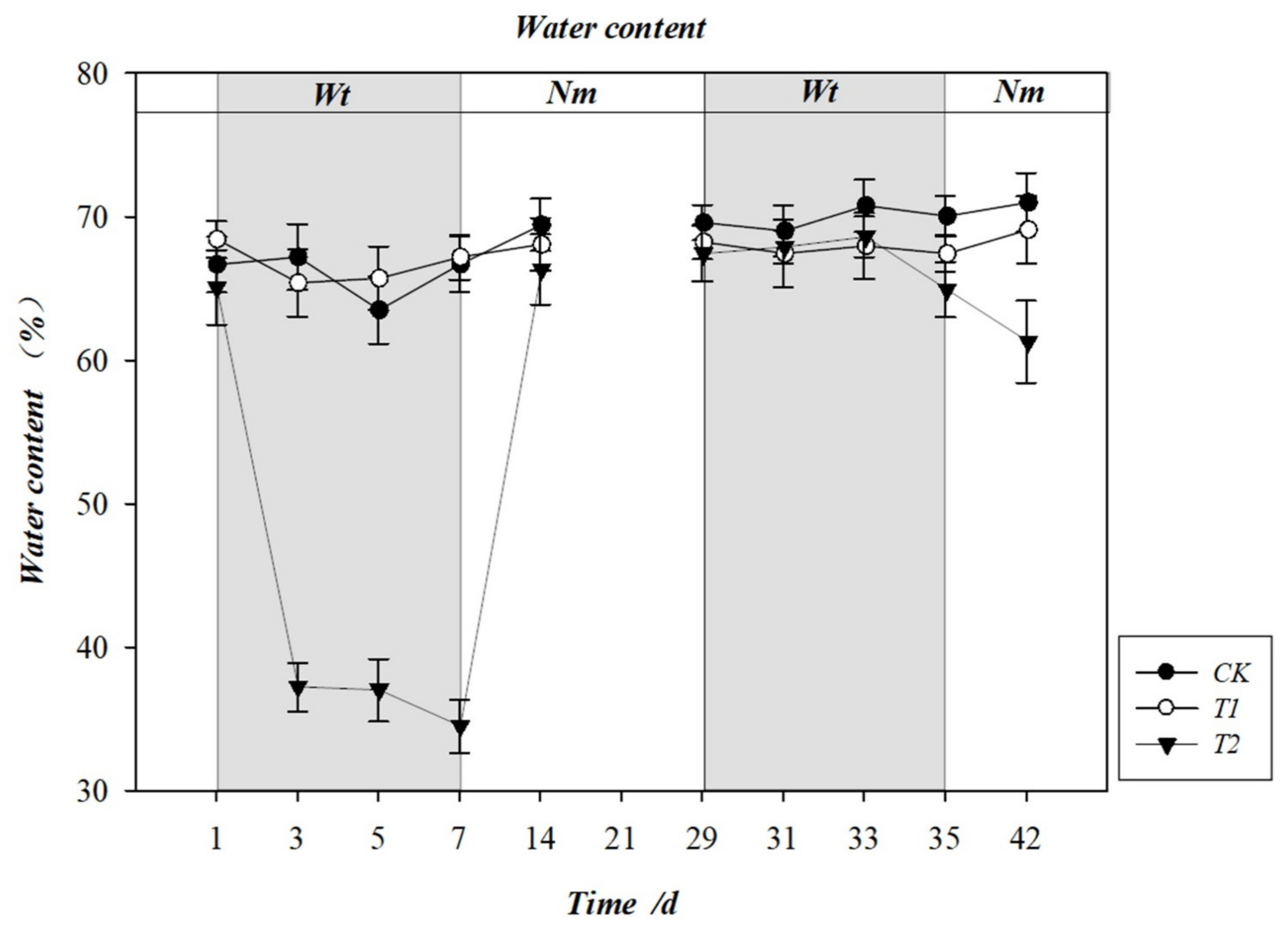
3.2. Effect of Water Stress on Leaf Water Content (LWC)
3.3. Effect of Water Stress on High Frequency Resistance r and Low Frequency Resistance r1 of Leaves
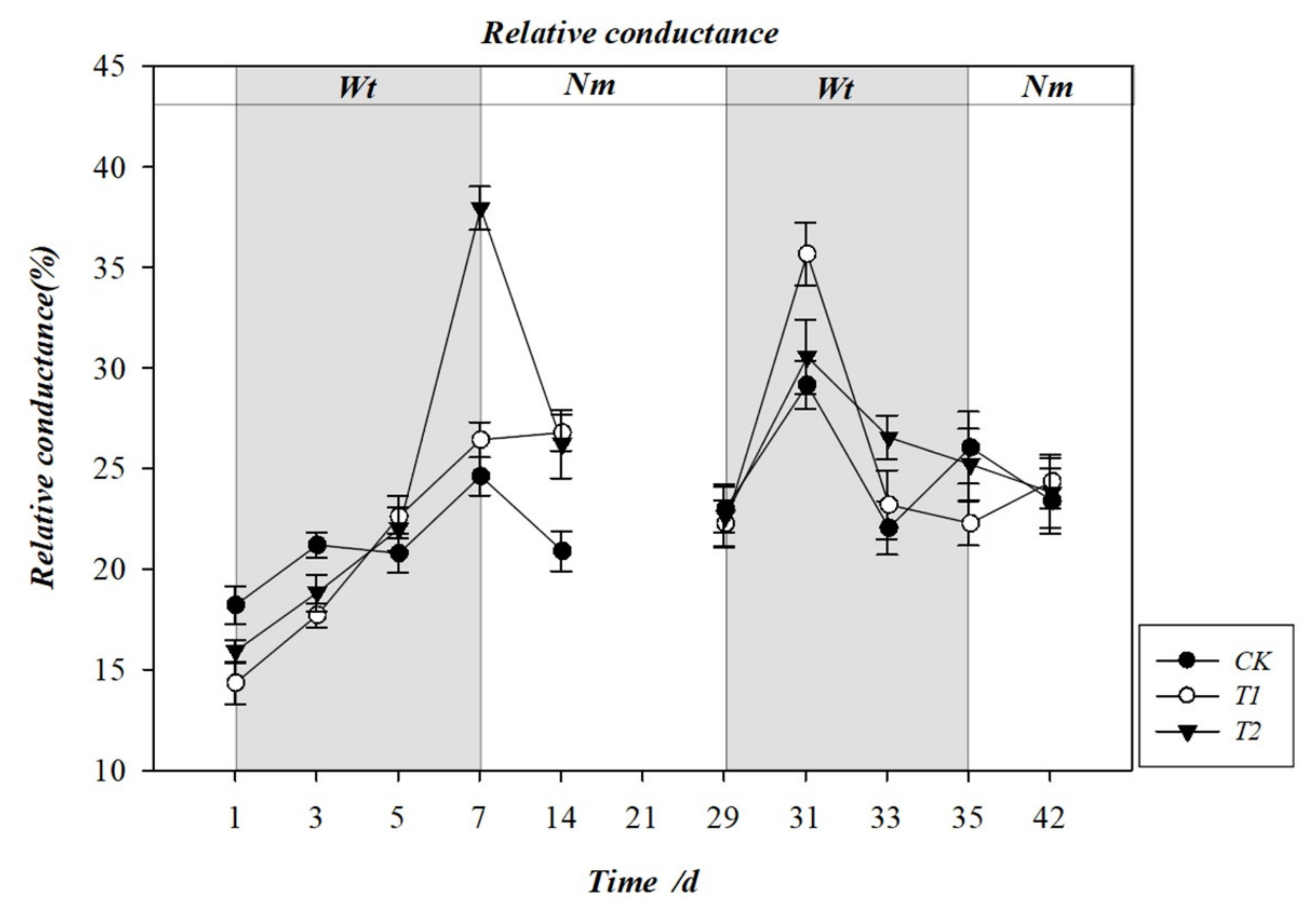
3.4. Effect of Water Stress on the Rate of Electrolyte Leakage (REL)
3.5. Effect of Water Stress on Leaf Starch and Soluble Sugar Content
3.6. Effect of Water Stress on the Maximum Photochemical Efficiency Fv/Fm
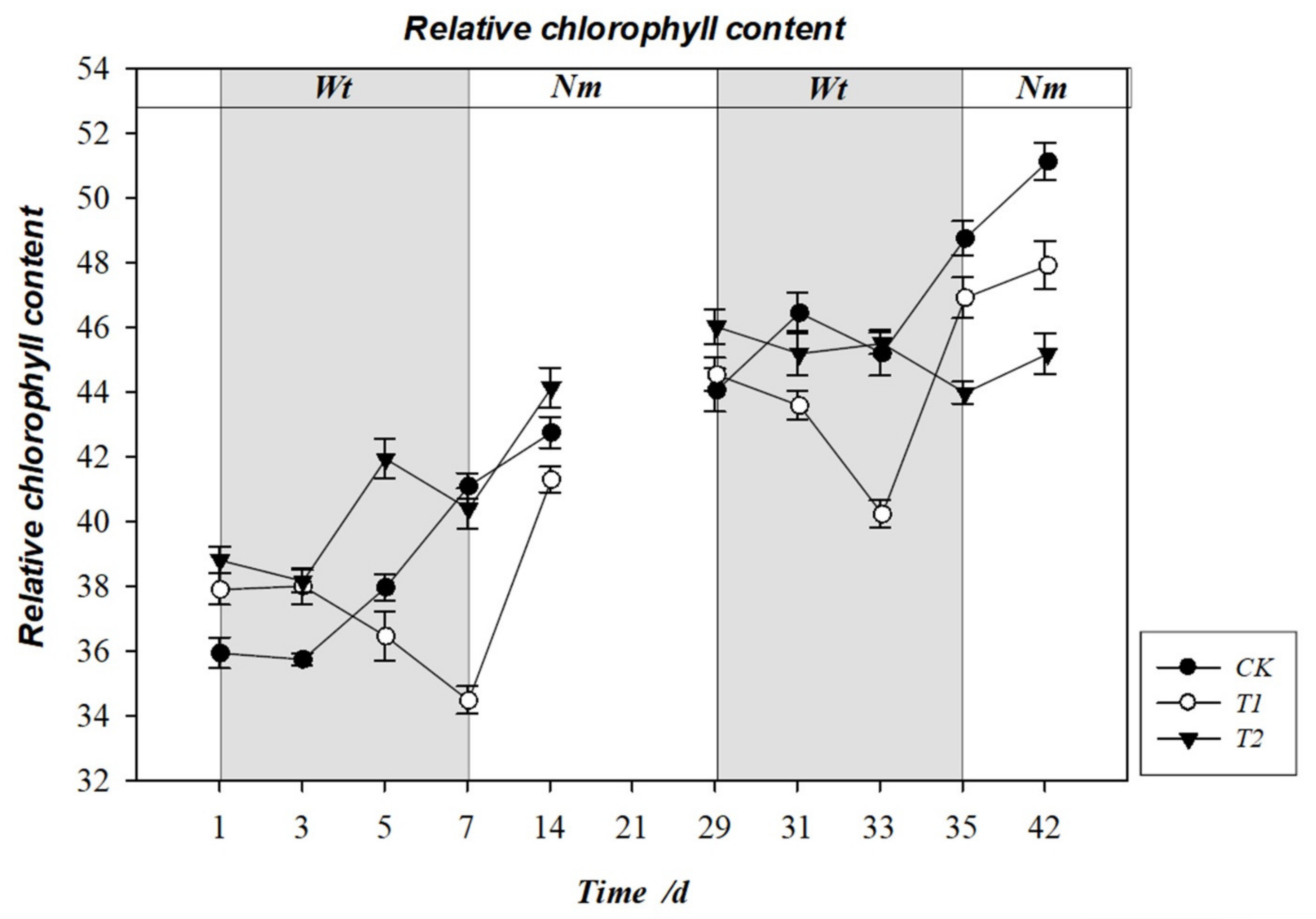
3.7. Effect of Water Stress on Relative Chlorophyll Content (SPAD)
3.8. Principal Component Analysis of Leaf Physiological and Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Qiang, X.M.; Li, X.H.; Li, J. Under the New Situation, China’s Apple Industry Develops with the High Quality; Northwest Horticulture: Harbin, China; Mabton, WA, USA, 2021; pp. 6–8. [Google Scholar]
- Chen, X.S.; Han, M.Y.; Su, G.L.; Liu, F.Z.; Guo, G.N.; Jiang, Y.M.; Mao, Z.Q.; Peng, F.T.; Shu, H.R. Discussion on today’s world apple industry trends and the suggestions on sustainable and efficient development of apple industry in China. J. Fruit Sci. 2010, 27, 598–604. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, H.K.; Zhang, T.R.; Yang, J.F.; Zang, R.Q.; Zhang, Y.Y.; Wang, L.C. Effects of Black Ground Fabric Micro-ridge Mulching on Root Zone Soil Moisture of Dwarf Apple. Acta Agric. Boreali-Occident. Sin. 2019, 8, 631–640. [Google Scholar] [CrossRef]
- Jackson, R.B.; Sperry, J.S.; Dawson, T.E. Root water uptake and transport: Using physiological processes in global predictions. Trends Plant Sci. 2000, 5, 482–488. [Google Scholar] [CrossRef]
- Zheng, F.; Sun, C.; Li, J.P. Climate Change: New Dimensions in Disaster Risk, Exposure, Vulner ability, and Resilience. Clim. Chang. Res. 2012, 8, 79–83. [Google Scholar] [CrossRef]
- Gao, L.Y.; Li, H.Q.; Chen, T.; Shen, Y.X.; Ren, C.M. Advances in plant response to waterlogging. J. Grassl. Forage Sci. 2010, 2, 1–5. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The physiological ecology of woody plants. Tree Physiol. 1991, 8, 213. [Google Scholar] [CrossRef]
- Gu, X.B.; Xue, L.; Lu, L.H.; Song, G.H.; Xie, M.; Zhang, H.Q. Characteristics of the response of Actinidia polygama to long-term waterlogging stress. J. Fruit Sci. 2019, 36, 327–337. [Google Scholar] [CrossRef]
- Yan, F.F. Study on the Response of Apple Dwarf Rootstock to Water Stress; Baoding Agricultural University of Hebei: Baoding, China, 2010. [Google Scholar]
- Guo, A.X.; Shi, X.J.; Wang, Y.X.; Hu, Y.; Zhu, Y.F. Effect of drought stress on the photosynthesis, chloroplast ultrastructure and antioxidant system in leaves of three apple rootstocks. Agric. Res. Arid Areas 2019, 37, 178–186. [Google Scholar] [CrossRef]
- Bai, D.F.; Li, Z.; Qi, X.J.; Chen, J.Y.; Gu, H.; Huang, W.Q.; Ren, J.J.; Zhong, Y.P.; Fang, J.B. Physiological responses and tolerance evaluation of four species of Actinidia to waterlogging stress. J. Fruit Sci. 2019, 36, 163–173. [Google Scholar] [CrossRef]
- Du, X.M.; Yang, Y.Z.; Gao, J.D.; Wang, Q.; Cai, H.C.; Li, C.Y. Advances of Effect of Apple Rootstocks on Grafted Varieties. Acta Agric. Boreali-Occident. Sin. 2020, 29, 1–8. [Google Scholar]
- Mao, K.; Dong, Q.; Li, C.; Liu, C.; Ma, F. Genome Wide Identification and Characterization of Apple bHLH Transcription Factors and Expression Analysis in Response to Drought and Salt Stress. Front. Plant Sci. 2017, 8, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.A.N.J.E.E.V.; Sharma, N. Effect of rootstocks on leaf water potential, water relations, antioxidant activities and drought tolerant in flemish beauty pear under water stress conditions. Indian J. Plant Physiol. 2008, 13, 266–271. [Google Scholar] [CrossRef]
- Xu, Q.H.; Li, Z.; Xu, K.; Wang, K.; Yang, F.; Cheng, C.G. Effects of continuous drought stress and rehydration on osmotic regulation of three apple rootstocks. China Fruits 2010, 3, 17–22. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.P.; Fu, Y.D.; Heng, D. Physiological Responses of Waterlogging on Different Rootstock Combinations of Cabernet Sauvignon Grape. Acta Hortic. Sin. 2013, 40, 2105–2114. [Google Scholar] [CrossRef]
- Zhao, S.J. Plant Physiology Experiment Guidance M; China Agricultural Science and Technology Press: Beijing, China, 1998; Volume 10. [Google Scholar]
- Li, H.S. Modern Plant Physiology, 3rd ed.; M. Life World, Higher Education Press: Beijing, China, 2012; Volume 11, p. 2. [Google Scholar]
- Zhang, X.Z. Experimental Techniques in Plant Physiology; Liaoning Science and Technology Press: Shenyang, China, 1994. [Google Scholar]
- Zhao, J.; Chen, S. Identification of the Ore-Forming Anomaly Component by MSVD Combined with PCA from Element Concentrations in Fracture Zones of the Laochang Ore Field, Gejiu, SW China. J. Earth Sci. 2021, 32, 427–438. [Google Scholar] [CrossRef]
- Jia, L.; Qin, X.; Lyu, D.; Qin, S.; Zhang, P. ROS production and scavenging in three cherry rootstocks under short-term waterlogging conditions. Sci. Hortic. 2019, 257. [Google Scholar] [CrossRef]
- Man Cuso, S.; Rinaldelli, E. Response of young mycorrhizal and non-mycorrhizal Plants of olive tree (Olea European L.) to saline conditions. Dynamics of electrical impedance Parameters of shoots and leaves. Adv. Hortic. Sci. 1996, 10, 126–134. [Google Scholar]
- Deng, X.; Chen, X.B.; Long, S.H.; Qiu, C.S.; Guo, Y.; Hao, D.M.; Wang, Y.F. Effects of water stress on physiology and dry matter accumulation of flax (Linum usitatissimum L.) seedling. Chin. J. Oil Crop. Sci. 2015, 37, 846–851. [Google Scholar]
- Wang, H.Z.; Liang, Z.S.; Han, X.L.; Han, L. Effect of soil drought on seedling growth and dry matter allocation of four native tree species on Loess Plateau. J. Plant Resour. Environ. 2005, 14, 10–15. [Google Scholar] [CrossRef]
- Ziegler, V.H.; Ploschuk, E.; Weibel, A.; Insausti, P. Short-term responses to flooding stress of three Prunus rootstocks. Sci. Hortic. 2017, 224, 135–141. [Google Scholar] [CrossRef]
- Lilley, J.M.; Ludlow, M.M. Expression of osmotic adjustment and dehydration tolerance in diverse rice lines. Field Crop. Res. 1996, 48, 185–197. [Google Scholar] [CrossRef]
- Qiu, L.I.U.; Zhihui, L.I.; Jiyou, W.U. Research Progress on Leaf Anatomical Structures of Plants under Drought Stress. Agric. Basic Sci. Technol. 2016, 17, 4–7. [Google Scholar] [CrossRef]
- Yin, D.M.; Chen, S.M.; Chen, F.D.; Guan, Z.Y.; Fang, W.M. Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ. Exp. Bot. 2010, 68, 122–130. [Google Scholar] [CrossRef]
- Masoumi, A.; Kafi, M.; Nabati, J.; Khazaei, H.R.; Davari, K.; Zare Mehrgerdi, M. Effect of Drought Stress on Leaf Water Status, Electrolyte Leakage, Photosynthesis Parameters and Chlorophyll Fluorescence of Two Kochia Ecotypes (Kochia scoparia) Irrigated with Saline Water. Pizhūhishhā-yi zirā̒ī-i Īrān. Iran. J. Field Crop. Res. 2012, 10, 476–484. [Google Scholar] [CrossRef]
- Wang, H.B.; Miao, L.F.; Fan, Y. Effects of Prolonged Waterlogging Stress on Growth and Physiological Characteristics of Mango Seedlings. Nat. Sci. J. Hainan Univ. 2021, 39, 141–145. [Google Scholar] [CrossRef]
- Liu, Y.L.; Tang, X.B.; Li, L.Z. Effects of waterlogging stress on solute and Starch contents in Ginkgo biloba leaves. J. Hubei Agric. Mech. 2009, 5, 60–61. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, B.; Zhang, G. Correlation of contents of soluble sugar and starch in leaves and stems of white birch under waterlogging and flooding stresses. J. For. Ecol. Sci. 2018, 33, 50–55. [Google Scholar]
- Desotgiu, R.; Pollastrini, M.; Cascio, C.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Chlorophyll a fluorescence analysis along a vertical gradient of the crown in a poplar (Oxford clone) subjected to ozone and water stress. Tree Physiol. 2012, 32, 976–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunch, C.K.; LaFountain, A.M.; Thomas, S.; Frank, H.A.; Lewis, L.A.; Cardon, Z.G. The xanthophyll cycle and NPQ in diverse desert and aquatic green algae. Photosynth. Res. 2013, 115, 139–151. [Google Scholar] [CrossRef]
- Rappaport, F.; Béal, D.; Joliot, A.; Joliot, P. On the advantages of using green light to study fluorescence yield changes in leaves. Biochim. Biophys Acta 2007, 1767, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazár, D. Chlorophyll a fluorescence induction. Biochim. Biophys. Acta 1999, 1412, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Turan, M.A.; Katkat, V.; Taban, S. Variations in Proline, Chlorophyll and Mineral Elements Content of Wheat Plants Grown under Salinity Stress. J. Agron. 2007, 6, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.I.; Reigosa, M.J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching, and heat energy dissipation in three C3 perennial species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, S.B.; Cao, K.F. Stimulation of Cyclic Electron Flow during Recovery After Chilling-Induced Photoinhibition of PSII. Plant Cell Physiol. 2010, 51, 1922–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.Q.; Ren, P.; Xu, Z.S. Effects of Water Stress on Photosynthetic Characteristics and Yield Formation in Oats (Avena sativa L.) with Different Drought Resistance. J. Triticeae Crop. 2020, 40, 1399–1407. [Google Scholar]
- Ueki, K.; Iwamori, H. Geochemical differentiation processes for arc magma of the Sengan volcanic cluster, Northeastern Japan, constrained from principal component analysis. Lithos 2017, 290–291. [Google Scholar] [CrossRef]
- Seymen, M. How does the flooding stress occurring in different harvest times affect the morpho-physiological and biochemical characteristics of spinach? Sci. Hortic. 2021, 275. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; do Amarante, L. Time-course biochemical analyses of soybean plants during waterlogging and reoxygenation. Environ. Exp. Bot. 2020, 180. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, T.; Tan, G.F.; Huang, Y.; Wang, F.; Xiong, A.S. Effects of dark treatment and regular light recovery on the growth characteristics and regulation of chlorophyll in water dropwort. Plant Growth Regul. Vol. 2018, 85, 293–303. [Google Scholar] [CrossRef]
- Bruce, T.J.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory ingresses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef] [PubMed]





| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.260 | 0.122 | 0.111 | 0.678 | 0.159 | 0.215 | 0.233 | 0.018 * | 0.027 * | 0.078 |
| T2–CK | 0.303 | 0.000 ** | 0.000 ** | 0.000 ** | 0.003 ** | 0.051 | 0.392 | 0.063 | 0.000 ** | 0.011 * |
| T1–T2 | 0.056 | 0.000 ** | 0.000 ** | 0.000 ** | 0.076 | 0.452 | 0.730 | 0.575 | 0.034 * | 0.381 |
| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.727 | 0.025 * | 0.992 | 0.620 | 0.015 * | 0.526 | 0.033 * | 0.472 | 0.039 * | 0.310 |
| T2–CK | 0.791 | 0.655 | 0.325 | 0.010 ** | 0.462 | 0.702 | 0.110 | 0.675 | 0.209 | 0.971 |
| T1–T2 | 0.539 | 0.066 | 0.330 | 0.033 * | 0.079 | 0.312 | 0.560 | 0.258 | 0.393 | 0.293 |
| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.649 | 0.049 * | 0.593 | 0.731 | 0.092 | 0.051 | 0.176 | 0.148 | 0.099 | 0.385 |
| T2–CK | 0.493 | 0.658 | 0.308 | 0.117 | 0.518 | 0.448 | 0.074 | 0.838 | 0.019 * | 0.486 |
| T1–T2 | 0.816 | 0.022 * | 0.622 | 0.216 | 0.287 | 0.205 | 0.645 | 0.211 | 0.447 | 0.122 |
| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.346 | 0.006 ** | 0.403 | 0.596 | 0.171 | 0.808 | 0.324 | 0.842 | 0.517 | 0.759 |
| T2–CK | 0.574 | 0.046 * | 0.600 | 0.002 ** | 0.205 | 0.884 | 0.823 | 0.368 | 0.890 | 0.597 |
| T1–T2 | 0.700 | 0.401 | 0.753 | 0.007 ** | 0.915 | 0.922 | 0.444 | 0.481 | 0.609 | 0.862 |
| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.989 | 0.036 * | 0.989 | 0.818 | 0.005 ** | 1.000 | 0.396 | 0.314 | 0.206 | 0.281 |
| T2–CK | 0.841 | 0.023 * | 0.373 | 0.499 | 0.064 | 0.884 | 0.763 | 0.841 | 0.687 | 0.009 ** |
| T1–T2 | 0.829 | 0.863 | 0.365 | 0.656 | 0.336 | 0.922 | 0.585 | 0.421 | 0.389 | 0.128 |
| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.445 | 0.958 | 0.005 ** | 0.771 | 0.774 | 0.578 | 0.122 | 0.204 | 0.113 | 0.045 * |
| T2–CK | 0.952 | 0.327 | 0.232 | 0.125 | 0.084 | 0.120 | 0.024 * | 0.002 ** | 0.000 ** | 0.003 ** |
| T1–T2 | 0.410 | 0.302 | 0.000 ** | 0.068 | 0.150 | 0.318 | 0.475 | 0.075 | 0.014 * | 0.350 |
| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.411 | 0.839 | 0.020 | 0.156 | 0.345 | 0.084 | 0.532 | 0.988 | 0.155 | 0.908 |
| T2–CK | 0.403 | 0.369 | 0.210 | 0.020 * | 0.362 | 0.907 | 0.105 | 0.936 | 0.043 * | 0.820 |
| T1–T2 | 0.103 | 0.591 | 0.062 | 0.026 * | 0.974 | 0.062 | 0.482 | 0.948 | 0.518 | 0.911 |
| Pairwise Comparison | Process Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 29 d | 31 d | 33 d | 35 d | 42 d | |
| T1–CK | 0.342 | 0.048 * | 0.074 | 0.251 | 0.835 | 0.841 | 0.266 | 0.984 | 0.383 | 0.213 |
| T2–CK | 0.137 | 0.137 | 0.675 | 0.149 | 0.282 | 0.415 | 0.624 | 0.893 | 0.028 * | 0.025 * |
| T1–T2 | 0.662 | 0.597 | 0.164 | 0.013 * | 0.202 | 0.537 | 0.530 | 0.878 | 0.166 | 0.288 |
| Items | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalue Percentage of variance Cumulative variance | 2.154 26.92 26.92 | 1.397 17.46 44.38 | 1.043 13.03 57.42 |
| Eigenvectors | |||
| LWC r r1 REL Starch Soluble sugar Fv/Fm SPAD | 0.22 0.85 0.87 −0.30 −0.58 0.29 0.08 0.13 | 0.31 0.10 −0.06 0.68 −0.12 0.45 0.08 0.80 | −0.41 0.08 0.01 0.05 0.30 −0.05 0.91 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Qian, J.; Yuan, W.; Yang, X.; Di, B.; Meng, Y.; Shao, J. Effects of Interval Flooding Stress on Physiological Characteristics of Apple Leaves. Horticulturae 2021, 7, 331. https://doi.org/10.3390/horticulturae7100331
Zhou P, Qian J, Yuan W, Yang X, Di B, Meng Y, Shao J. Effects of Interval Flooding Stress on Physiological Characteristics of Apple Leaves. Horticulturae. 2021; 7(10):331. https://doi.org/10.3390/horticulturae7100331
Chicago/Turabian StyleZhou, Pengyu, Ji Qian, Weidong Yuan, Xin Yang, Bao Di, Yu Meng, and Jianzhu Shao. 2021. "Effects of Interval Flooding Stress on Physiological Characteristics of Apple Leaves" Horticulturae 7, no. 10: 331. https://doi.org/10.3390/horticulturae7100331
APA StyleZhou, P., Qian, J., Yuan, W., Yang, X., Di, B., Meng, Y., & Shao, J. (2021). Effects of Interval Flooding Stress on Physiological Characteristics of Apple Leaves. Horticulturae, 7(10), 331. https://doi.org/10.3390/horticulturae7100331





