Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges
Abstract
:1. Introduction
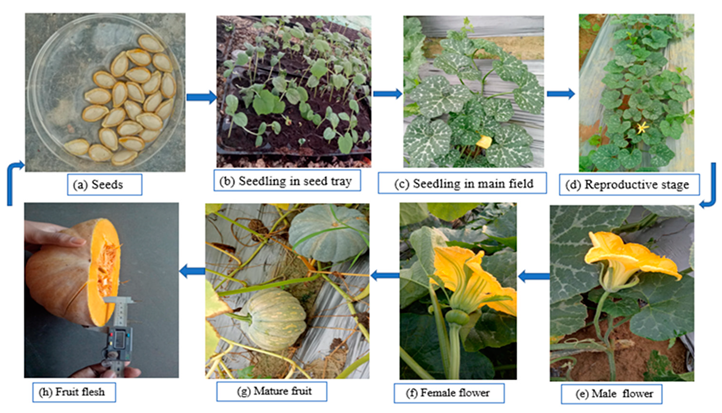
2. Botanical Description of Pumpkin
3. Origin and Distribution of Pumpkin
4. World Scenario of Pumpkin Production and Area Harvested
5. Nutritional Aspects of Pumpkin Parts (Leaves, Pulp, Seeds, Seeds Oil, Powder)
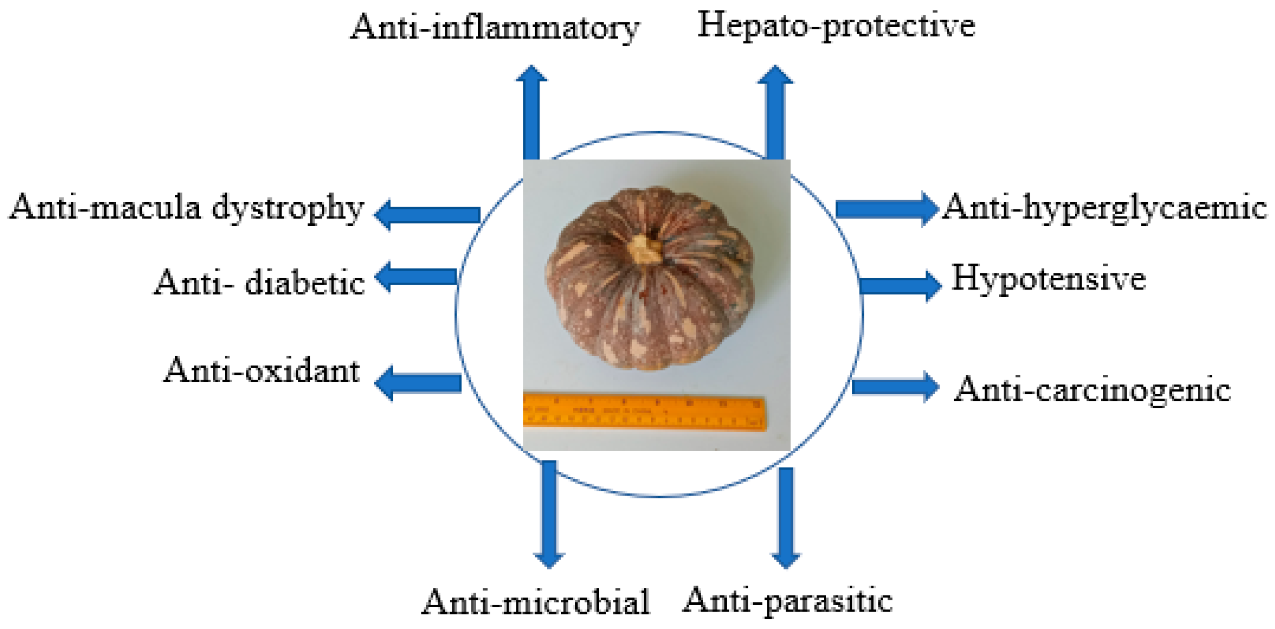
6. Medication and Pharmacological Values of Pumpkin
7. Potential Uses of Pumpkin (Ready-to-Eat Meals)
8. Study of Genetic Diversity in Pumpkin (Cucurbita spp.) Genotypes
8.1. Qualitative and Quantitative Morphological Diversity of Pumpkin Genotypes
8.2. Molecular Diversity Study in Pumpkin Genotypes
9. Hybridization Breeding for Crop Improvement
9.1. Diverse Mating Design Used in Hybridization
- (i)
- the mode of fertilization (self- or out-pollinated);
- (ii)
- the mode of crossing that can be used (non-natural or natural),
- (iii)
- the mode of pollen transmission (air or insect);
- (iv)
- the existence of the male sterility structure;
- (v)
- the objective of the plan (breeding or hereditary analysis),
- (vi)
- the magnitude of the required population [124].
- (1)
- they deliver info on the inherited control of the traits under examination;
- (2)
- they create a breeding progeny that can be used as the foundation for choice and develop probable hybrid varieties;
- (3)
- they offer estimations of heritable gain; and
- (4)
- they offer info for assessing the parental materials used in a breeding program [124].
9.2. Why Choose Diallel Mating Approach in Hybridization Breeding?
- (1)
- in terms of population coverage, and it stated that Biparental mating > North Carolina I > poly cross > North Carolina III > North Carolina II > diallel mating, in that directive of declining effectiveness;
- (2)
- in terms of volume of info, diallel mating > North Carolina II > North Carolina III > North Carolina I > Biparental mating, [124].
- -
- general combining ability (GCA),
- -
- specific combining ability (SCA),
- -
- genetic parameters,
- -
- heritability in broad and narrow senses,
- -
- and gene action (additive and non-additive) [141]
9.3. Research on Development of High Yield and Quality Pumpkin Hybrid Using Diallel Mating Design
9.3.1. Evaluation of Combining Ability
9.3.2. Evaluation of Heterosis or Hybrid Vigor
9.3.3. Evaluation of Heritability of the Desired Traits for Pumpkin Breeding
9.3.4. Evaluation of Gene Action of the Desired Traits for Pumpkin Breeding
10. Future Prospects
- More awareness needs to be spread to tropical populations about the need of eating fruits and vegetables, with pumpkin being one of them, as one of the measures to promote human health and combat hunger.
- Youths as well as women must be encouraged and informed about pumpkin production as well as the various value-added options for pumpkin that can provide potential sources of revenue and contribute to overall socioeconomic prosperity. Farmers should be motivated to keep track of their production in order to monitor their agribusiness’s success.
- In case of surplus pumpkin production, this expertise of pumpkin value addition should be disseminated with local people to reduce postharvest wastage, which would benefit the overall socio-economic well-being of the growers concerned.
- The tropics’ pumpkin landraces require the establishment of a GenBank for maintenance and future crop enhancement to address yield as well as pests and illnesses. Landraces collecting and in situ preservation aid to combat the threat of endangerment and loss of crop genetic variation.
- Local species’ genetic variability can be exploited to generate superior pumpkin cultivars that can withstand changing and limited environmental conditions. The introduction of foreign cultivars with a confined genetic background has put the genetic variability and preservation of landrace pumpkins at risk.
- A cost-benefit economic analysis of value-added options should be conducted to identify which option will provide farmers with the highest profit margin for their pumpkin harvest.
- Governments, community, entrepreneurs, and other stakeholders must work together to encourage the popularization of native landraces by generating high-quality seeds, creating new disease-resistant, high-yielding varieties, and supporting traditional food festivals to encourage indigenous cuisines.
- Nutrient analysis and therapeutic applications of naturalized pumpkins are needed. Traditional food manufacturing techniques must be investigated in order to help local people in terms of medicine, nutrition, and economics.
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galluzzi, G.; Van Duijvendijk, C.; Collette, L.; Azzu, N.; Hodgkin, T. Biodiversity for food and agriculture: Contributing to food security and sustainability in a changing world. In Proceedings of the Outcomes of an Expert Workshop Held by FAO and the Platform on Agrobiodiversity Research, Rome, Italy, 14–16 April 2010. [Google Scholar]
- Prescott-Allen, R.; Prescott-Allen, C. How many plants feed the world? Conserv. Biol. 1990, 4, 365–374. [Google Scholar] [CrossRef]
- FAO. Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture; UK Distributor, Stationery Office; FAO, Viale delle Terme di Caracalla: Rome, Italy, 2010; ISBN 925-106-5-349. [Google Scholar]
- Rehm, S.; Espig, G. The Cultivated Plants of the Tropics and Subtropics; Margraf New Media GmbH: Weikersheim, Germany, 1991. [Google Scholar]
- Wilson, E.O. The Diversity of Life Penguin; Penguin Books Ltd.: London, UK, 1992. [Google Scholar]
- Mokyr, J. The Oxford Encyclopedia of Economic History; Oxford University Press: Oxford, UK, 2003; Volume 5, ISBN 019-510-5-079. [Google Scholar]
- Galluzzi, G.; López Noriega, I. Conservation and use of genetic resources of underutilized crops in the Americas—A continental analysis. Sustainability 2014, 6, 980–1017. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, L.; Hussain, A.; Rasul, G. Tapping the potential of neglected and underutilized food crops for sustainable nutrition security in the mountains of Pakistan and Nepal. Sustainability 2017, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Nyabera, L.A.; Nzuki, I.W.; Runo, S.M.; Amwayi, P.W. Assessment of genetic diversity of pumpkins (Cucurbita spp.) from western Kenya using SSR molecular markers. Mol. Biol. Rep. 2021, 48, 2253–2260. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Noreen, S.; Rehman, H.; Shabbir, H.; Ramzan, M.A. Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima). J. Food Process. Preserv. 2021, 45, e15542. [Google Scholar] [CrossRef]
- Kumar, V.; Mishra, D.P.; Yadav, G.C.; Yadav, S. Exploitation of heterobeltiosis and economic heterosis for horticultural yield, and its attributes and biochemical traits in pumpkin (Cucurbita moschata Duch. ex. Poir) under salt affected soil. Curr. Sci. 2018, 115, 1550–1556. [Google Scholar] [CrossRef]
- Buzigi, E.; Pillay, K.; Siwela, M. Potential of pumpkin to combat vitamin A deficiency during complementary feeding in low- and middle-income countries: Variety, provitamin A carotenoid content and retention, and dietary reference intakes. Crit. Rev. Food Sci. Nutr. 2021, 1–10. [Google Scholar] [CrossRef]
- Clinton, C.S.; Erik, B.G.; Feibert, L.D. Can Pumpkins Be Grown for Snack Seed Purposes in The Treasure Valley? In Bill Buhrig, Malheur County Extension Service; Oregon State University: Corvallis, OR, USA, 2015. [Google Scholar]
- Ndinya, C.A. The genetic diversity of popular African leafy vegetables in western Kenya. In Genetic Diversity in Horticultural Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 127–159. [Google Scholar]
- Chelang’a, P.K.; Obare, G.A.; Kimenju, S.C. Analysis of urban consumers’ willingness to pay a premium for African Leafy Vegetables (ALVs) in Kenya: A case of Eldoret Town. Food Secur. 2013, 5, 591–595. [Google Scholar] [CrossRef]
- Zelaya, I.A.; Owen, M.D.K.; Van Gessel, M.J. Transfer of glyphosate resistance: Evidence of hybridization in Conyza (Asteraceae). Am. J. Bot. 2007, 94, 660–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandel, B.P. Status, prospect and problems of hybrid maize (Zea mays L.) in Nepal: A brief review. Genet. Resour. Crop Evol. 2020, 68, 1–10. [Google Scholar] [CrossRef]
- Virmani, S.S. Heterosis in rice. In Monographs on Theoretical and Applied Genetics; Virmani, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Ma’arup, R.; Trethowan, R.M.; Ahmed, N.U.; Bramley, H.; Sharp, P.J. Emmer wheat (Triticum dicoccon Schrank) improves water use efficiency and yield of hexaploid bread wheat. Plant Sci. 2020, 295, 110212. [Google Scholar] [CrossRef]
- Shafiin, W.N.S.S.; Ablah, N.L.; Nur Fatihah, H.N.; Alam, M.A.; Ma’Arup, R.; Jahan, M.S.; Mustafa, K.A.; Alias, N. Breeding strategies for enhancing nutrient content and quality in Cucurbitaceae: A review. Int. J. Veg. Sci. 2020, 27, 1–24. [Google Scholar] [CrossRef]
- Paris, H.S. Genetic Resources of Pumpkins and Squash, Cucurbita spp. In Genetics and Genomics of Cucurbitaceae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 111–154. [Google Scholar]
- Seymen, M.; Uslu, N.; Türkmen, Ö.; Al Juhaimi, F.; Özcan, M.M. Chemical compositions and mineral contents of some hull-less pumpkin seed and oils. J. Am. Oil Chem. Soc. 2016, 93, 1095–1099. [Google Scholar] [CrossRef]
- Mahfouze, S.A.; Ottai, M.E.S. Assessment of genetic variability for some Hyoscymus species using biochemical and molecular markers. J. Appl. Sci. Res. 2011, 7, 1752–1759. [Google Scholar]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genet. Res. Int. 2015, 2015, 431487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano, M.R.; Santalla, M.; Casquero, P.A.; De Ron, A.M. Patterns of genetic diversity in landraces of common bean (Phaseolus vulgaris L.) from Galicia. Plant Breed. 1998, 117, 49–56. [Google Scholar] [CrossRef]
- Sundharaiya, K.; Rajangam, J.; Suresh, V.; Subramaniyan, P.; Sathish, G. Genetic divergence in landraces of pumpkin (Cucurbita moschata Duch. ex. Poir.). In Proceedings of the III International Symposium on Underutilized Plant Species 1241, Tamil Nadu, India, 5–8 August 2015; pp. 181–186. [Google Scholar]
- Hayman, B.I. The theory and analysis of diallel crosses. II. Genetics 1958, 43, 63. [Google Scholar] [CrossRef]
- Jinks, J.L. The F2 and backcross generations from a diallel set of crosses. Heredity 1956, 10, 1–30. [Google Scholar] [CrossRef]
- Pandey, S.; Jha, A.; Kumar, S.; Rai, M. Genetics and heterosis of quality and yield of pumpkin. Indian J. Hortic. 2010, 67, 333–338. [Google Scholar]
- Hussien, A.H.; Hamed, A.A. Diallel analysis for studying heterosis and combining ability of some economical yield traits in pumpkin. J. Plant Prod. 2015, 6, 261–270. [Google Scholar] [CrossRef]
- Rakcejeva, T.; Galoburda, R.; Cude, L.; Strautniece, E. Use of dried pumpkins in wheat bread production. Procedia Food Sci. 2011, 1, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, C. Systemetics of the Cucurbitaceae: An overview. In Biology and Utilization of the Cucurbitaceae; Bates, D.M., Robinson, R.W., Eds.; Cornel University Press: Ithaca, NY, USA, 1990; pp. 3–9. [Google Scholar]
- Aruah, C.B.; Uguru, M.I.; Oyiga, B.C. Variations among some Nigerian Cucurbita landraces. Afr. J. Plant Sci. 2010, 4, 374–386. [Google Scholar]
- Maynard, D.N.; Elmstrom, G.W.; Talcott, S.T.; Carle, R.B. “El Dorado’and’La Estrella”: Compact plant tropical pumpkin hybrids. HortScience 2002, 37, 831–833. [Google Scholar] [CrossRef]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Grubben, G.J.H.; Denton, O.A. Plant Resources of Tropical Africa 2. Vegetables; PROTA Foundation: Wageningen, The Netherlands, 2004; pp. 261–268. [Google Scholar]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2020, 226, 1240–1255. [Google Scholar] [CrossRef] [Green Version]
- Manjunathagowda, D.C.; Bommesh, J.C. Sex manipulation in cucurbitaceous vegetables. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1839–1851. [Google Scholar]
- Paris, H.S. Summer squash: History, diversity, and distribution. Horttechnology 1996, 6, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Tindall, H.D. Vegetables in the Tropics; Macmillan International Higher Education: London, UK, 1983; ISBN 134-917-2-235. [Google Scholar]
- Lee, H.-Y.; Jang, S.; Yu, C.-R.; Kang, B.-C.; Chin, J.-H.; Song, K. Population Structure and Genetic Diversity of Cucurbita moschata Based on Genome-Wide High-Quality SNPs. Plants 2021, 10, 56. [Google Scholar] [CrossRef]
- FAOSTAT Statistic Database. 2017. Available online: http://faostat.fao.orge (accessed on 27 July 2021).
- Mashitoa, F.M.; Shoko, T.; Shai, J.L.; Slabbert, R.M.; Sivakumar, D. Changes in phenolic metabolites and biological activities of pumpkin leaves (Cucurbita moschata Duchesne ex Poir.) during blanching. Front. Nutr. 2021, 8, 86. [Google Scholar]
- Lyimo, M.H.; Nyagwegwe, S.; Mnkeni, A.P. Investigations on the effect of traditional food processing, preservation and storage methods on vegetable nutrients: A case study in Tanzania. Plant Foods Hum. Nutr. 1991, 41, 53–57. [Google Scholar] [CrossRef]
- Adubofuor, J.; Anomah, J.W.; Amoah, I. Anti-nutritional factors and mineral composition of pumpkin pulp and functional properties of pumpkin-wheat composite flour for bread preparation. Int. J. Innov. Food Sci. Technol. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Hashash, M.M.; El-Sayed, M.M.; Abdel-Hady, A.A.; Hady, H.A.; Morsi, E.A. Nutritional potential, mineral composition and antioxidant activity squash (Curcurbita pepo L.) fruits grown in Egypt. Inflammation 2017, 9, 11–12. [Google Scholar]
- Seymen, M. Seed Yield and Characteristics in a Half-Diallel Pumpkin Population. Selcuk J. Agric. Food Sci. 2020, 34, 200–206. [Google Scholar]
- Rani, R.; Kumar, S.; Yadav, S. Pumpkin and chia seed as dietary fibre source in meat products: A review. Pharma Innov. J. 2021, 10, 477–485. [Google Scholar]
- Murkovic, M.; Hillebrand, A.; Winkler, J.; Leitner, E.; Pfannhauser, W. Variability of fatty acid content in pumpkin seeds (Cucurbita pepo L.). Z. Lebensm. -Unters. Forsch. 1996, 203, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Boujemaa, I.; El Bernoussi, S.; Harhar, H.; Tabyaoui, M. The influence of the species on the quality, chemical composition and antioxidant activity of pumpkin seed oil. OCL 2020, 27, 7. [Google Scholar] [CrossRef]
- Kundu, H.; Grewal, R.B.; Goyal, A.; Upadhyay, N.; Prakash, S. Effect of incorporation of pumpkin (Cucurbita moshchata) powder and guar gum on the rheological properties of wheat flour. J. Food Sci. Technol. 2014, 51, 2600–2607. [Google Scholar] [CrossRef] [Green Version]
- Kumari, N.; Sindhu, S.C.; Rani, V.; Kumari, V. Shelf Life Evaluation of Biscuits and Cookies Incorporating Germinated Pumpkin Seed Flour. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1436–1443. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, S.; Gray, D.A.; Channell, G.A.; Morris, G.A.; Harding, S.E. The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Food Res. Int. 2011, 44, 862–867. [Google Scholar] [CrossRef]
- Xia, T.; Wang, Q. D-chiro-inositol found in Cucurbita ficifolia (Cucurbitaceae) fruit extracts plays the hypoglycaemic role in streptozocin-diabetic rats. J. Pharm. Pharmacol. 2006, 58, 1527–1532. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Effect of preparation and storage conditions on physical and chemical properties of puree, puree juices and cloudy juices obtained from pumpkin with added Japanese quince and strawberries. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.M. Extrusion of Foods; CRC Press: Boca Raton, FL, USA, 1990; Volume 1. [Google Scholar]
- FSA. E. coli O157 Control of Cross-Contamination Guidance for Food Business Operators and Enforcement Authorities; The Food Standards Agency (FSA): London, UK, 2011. [Google Scholar]
- Assous, M.T.M.; Saad, E.M.S.; Dyab, A.S. Enhancement of quality attributes of canned pumpkin and pineapple. Ann. Agric. Sci. 2014, 59, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Ready-to-eat snack products: The role of extrusion technology in developing consumer acceptable and nutritious snacks. Int. J. Food Sci. Technol. 2013, 48, 893–902. [Google Scholar] [CrossRef]
- Norfezah, M.N.; Hardacre, A.; Brennan, C.S. Comparison of waste pumpkin material and its potential use in extruded snack foods. Food Sci. Technol. Int. 2011, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Available online: https://www.google.com/search?q=image+of+processed+food+from+pumpkin&tbm (accessed on 28 July 2021).
- Kumar, J.; Singh, D.K.; Har Ram, H. Genetic diversity in indigenous germplasm of pumpkin. Indian J. Hortic. 2006, 63, 101–102. [Google Scholar]
- Suma, A.; Elsy, C.R.; Sivaraj, N.; Padua, S.; Yadav, S.K.; John, K.J.; Krishnan, S. Genetic diversity and distribution of cucumber (Cucumis sativus L.) landraces in India: A study using DIVA-GIS approach. Electron. J. Plant Breed. 2019, 10, 1532–1540. [Google Scholar] [CrossRef]
- Swarup, V. Vegetable Science and Technology in India; Kalayani Publishers: Ludhiana, India, 2014; ISBN 812-722-6-955. [Google Scholar]
- Shivananda, M.M.; Madalageri, M.B.; Chikkur, S.S.; Mohankumar, A.B.; Yathiraj, K. Genetic divergence studies in pumpkin (Cucurbita spp.). Int. J. Plant Sci. 2013, 8, 29–34. [Google Scholar]
- Barzegar, R.; Peyvast, G.; Ahadi, A.M.; Rabiei, B.; Ebadi, A.A.; Babagolzadeh, A. Biochemical systematic, population structure and genetic variability studies among Iranian Cucurbita (Cucurbita pepo L.) accessions, using genomic SSRs and implications for their breeding potential. Biochem. Syst. Ecol. 2013, 50, 187–198. [Google Scholar] [CrossRef]
- Soltani, F.; Karimi, R.; Kashi, A. Estimation of genetic diversity in Cucurbita species using morphological and phytochemical analysis. Int. J. Veg. Sci. 2017, 23, 42–53. [Google Scholar] [CrossRef]
- Montes-Hernandez, S.; Merrick, L.C.; Eguiarte, L.E. Maintenance of squash (Cucurbita spp.) landrace diversity by farmers’ activities in Mexico. Genet. Resour. Crop Evol. 2005, 52, 697–707. [Google Scholar] [CrossRef]
- Akter, S.; Rasul, M.G.; Islam, A.K.M.A.; Rahman, M.M. Genetic variability, correlation and path coefficient analysis of yield and quality traits in pumpkin (Cucurbita moschata Duch ex Poir.). Bangladesh J. Plant Breed. Genet. 2013, 26, 25–33. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Behera, T.K.; Kole, C. Genetics, Genomics and Breeding of Cucurbits; CRC Press: Boca Raton, FL, USA, 2011; ISBN 157-808-7-66X. [Google Scholar]
- Masud, M.A.T.; Chowdhury, M.A.; Hossain, M.A.; Hossain, S.M.M. Multivariate analysis of pumpkin. Bangladesh J. Plant Breed. Genet 1995, 8, 45–50. [Google Scholar]
- Mohsin, G.M.; Islam, M.S.; Rahman, M.S.; Ali, L.; Hasanuzzaman, M. Genetic variability, correlation and path coefficients of yield and its components analysis in pumpkin (Cucurbita moschata Duch ex Poir). Int. J. Agric. Res. Innov. Technol. 2017, 7, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Dhatt, A.S.; Sharma, M.; Kaur, B. Advances in Improvement of Pumpkin and Squashes. In Accelerated Plant Breeding; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2, pp. 301–335. [Google Scholar]
- Sali, A.; Haziri, A.; Fetahu, S.; Aliaga, N.; Rusinovci, I.; Haziri, I.; Arapi, V. Morphological and nutritive variation in a collection of Cucurbita pepo L. growing in Kosova. Not. Sci. Biol. 2011, 3, 119–122. [Google Scholar]
- Dudley, J.W.; Moll, R.H. Interpretation and use of estimates of heritability and genetic variances in plant breeding 1. Crop Sci. 1969, 9, 257–262. [Google Scholar] [CrossRef]
- Bello, O.B.; Ige, S.A.; Azeez, M.A.; Afolabi, M.S.; Abdulmaliq, S.Y.; Mahamood, J. Heritability and genetic advance for grain yield and its component characters in maize Zea mays L.). Int. J. Plant Res. 2012, 2, 138–145. [Google Scholar]
- Rutkoski, J.; Singh, R.P.; Huerta-Espino, J.; Bhavani, S.; Poland, J.; Jannink, J.L.; Sorrells, M.E. Genetic Gain from Phenotypic and Genomic Selection for Quantitative Resistance to STEM Rust of Wheat; Crop Society of America: Madison, WI, USA, 2015. [Google Scholar]
- Esquinas-Alcazar, J.T.; Gulick, P.J. Genetic Resources of Cucurbitaceae. A Global Report; IBPGR Secretariat: Rome, Italy, 1983. [Google Scholar]
- Kiramana, J.K.; Isutsa, D.K. First detailed morphological characterisation of qualitative traits of extensive naturalized pumpkin germplasm in Kenya. Int. J. Dev. Sustain. 2015, 6, 500–525. [Google Scholar]
- Du, X.; Sun, Y.; Li, X.; Zhou, J.; Li, X. Genetic divergence among inbred lines in Cucurbita moschata from China. Sci. Hortic. 2011, 127, 207–213. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; Gonçalves, L.S.A.; Rodrigues, R.; Baba, V.Y.; Sudré, C.P.; Dos Santos, M.H.; Aranha, F.M. Genetic divergence among pumpkin landraces. Semin. Ciên. Agrár. 2016, 37, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Kiramana, J.K.; Isutsa, D.K. Genetic diversity of pumpkin accessions in Kenya revealed using morphological characters, diversity index, CATPCA and factor analysis. Int. J. Sci. Res. 2018, 3, 57–79. [Google Scholar]
- Kumar, V.; Mishra, D.P.; Yadav, G.C.; Dwivedi, D.K. Genetic diversity assessment for morphological, yield and biochemical traits in genotypes of pumpkin. J. Pharmacogn. Phytochem. 2017, 6, 14–18. [Google Scholar]
- Sampath, S.; Krishnamoorthy, V. Genetic Variability, Correlation and Path Analysis in Pumpkin (Cucurbita moschata Duch. ex. Poir). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- Gomes, R.S.; Machado Júnior, R.; De Almeida, C.F.; Chagas, R.R.; De Oliveira, R.L.; Delazari, F.T.; Da Silva, D.J.H. Brazilian germplasm of winter squash (Cucurbita moschata D.) displays vast genetic variability, allowing identification of promising genotypes for agro-morphological traits. PLoS ONE 2020, 15, e0230546. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.J.D.E.; De Carvalho, J.L.V.; Borges, R.M.E.; Kaser, I.M.; Lima, V.G.; Sousa, D.S.F.D.E. Variability of total carotenoids in C. Moschata genotypes. Embrapa Semiárido-Artig. Periódico Indexado 2015, 44, 247–252. [Google Scholar]
- Srikanth, M.; Bharad, S.G.; Thulasiram, L.B.; Potdukhe, N.R. Studies on genetic variability, heritability and genetic advance in pumpkin (Cucurbita moschata Duch ex Poir.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Naik, M.L. Studies on Genetic divergence in Pumpkin (Cucurbita moschata Duch. ex Poir.). Electron. J. Plant Breed 2015, 6, 1088–1095. [Google Scholar]
- Brdar-Jokanović, M.; Koren, A.; Ljevnaić-Mašić, B.; Kiprovski, B.; Sikora, V. Yield and quality parameters of Hokkaido type pumpkins grown in Serbia. Genetika 2019, 51, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Masud, M.A.T.; Zakaria, M.; Hossain, M.M.; Mian, M.A.K. Evaluation of pumpkin (Cucurbita moschata Duch. Ex Poir.) for yield and other characters. Bangladesh J. Agric. Res. 2017, 42, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Blank, A.F.; Silva, T.B.; Matos, M.L.; Carvalho Filho, J.L.S.; Silva-Mann, R. Genotypic, phenotypic and environmental parameters for morphological and agronomic characters in pumpkin. Hortic. Bras. 2013, 31, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Thirumdasu, R.K.; Chatterjee, R. Search for Superior Fruit Characters of Pumpkin Genotypes from Various Parts of India and Evaluation under Eastern Himalayan Region. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1643–1650. [Google Scholar] [CrossRef]
- Nagar, A.; Sureja, A.K.; Munshi, A.D.; Bhardwaj, R.; Kumar, S.; Tomar, B.S. Heritability, correlation and genetic divergence for different seed traits in pumpkin (Cucurbita moschata). Indian J. Agric. Sci 2017, 87, 1519–1523. [Google Scholar]
- Nagar, A.; Sureja, A.K.; Kumar, S.; Munshi, A.D.; Gopalakrishnan, S.; Bhardwaj, R. Genetic Variability and Principal Component Analysis for Yield and its Attributing Traits in Pumpkin (Cucurbita moschata Duchesne Ex Poir.). Soc. Plant Res. 2017, 133, 81. [Google Scholar] [CrossRef]
- Chaudhari, D.; Acharya, R.; Gohil, S.; Patel, N. Genetic divergence study in pumpkin (Cucurbita moschata Duch. Ex. Poir). J. Pharmacogn. Phytochem. 2017, 6, 744–747. [Google Scholar]
- Ferriol, M.; Picó, M.B.; Nuez, F. Genetic diversity of some accessions of Cucurbita maxima from Spain using RAPD and SBAP markers. Genet. Resour. Crop Evol. 2003, 50, 227–238. [Google Scholar] [CrossRef]
- Adhikari, S.; Saha, S.; Biswas, A.; Rana, T.S.; Bandyopadhyay, T.K.; Ghosh, P. Application of molecular markers in plant genome analysis: A review. Nucleus 2017, 60, 283–297. [Google Scholar] [CrossRef]
- Liu, C.; Ge, Y.; Wang, D.J.; Li, X.; Yang, X.X.; Cui, C.S.; Qu, S.P. Morphological and molecular diversity in a germplasm collection of seed pumpkin. Sci. Hortic. 2013, 154, 8–16. [Google Scholar] [CrossRef]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; De Munhoz, C.F. Microsatellite markers: What they mean and why they are so useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef]
- Chang-ping, C.H.U.P.X.; Cheng-ping, Z.C.L.I.U. Genetic diversity of Cucurbita moschata genotypes revealed by RAPD markers and agronomic traits. J. Nucl. Agric. Sci. 2007, 21, 441. [Google Scholar]
- Ntuli, N.R.; Tongoona, P.B.; Zobolo, A.M. Genetic diversity in Cucurbita pepo landraces revealed by RAPD and SSR markers. Sci. Hortic. 2015, 189, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Paris, H.S.; Yonash, N.; Portnoy, V.; Mozes-Daube, N.; Tzuri, G.; Katzir, N. Assessment of genetic relationships in Cucurbita pepo (Cucurbitaceae) using DNA markers. Theor. Appl. Genet. 2003, 106, 971–978. [Google Scholar] [CrossRef]
- Wu, J.; Chang, Z.; Wu, Q.; Zhan, H.; Xie, S. Molecular diversity of Chinese Cucurbita moschata germplasm collections detected by AFLP markers. Sci. Hortic. 2011, 128, 7–13. [Google Scholar] [CrossRef]
- Ferriol, M.; Pico, B.; De Cordova, P.F.; Nuez, F. Molecular diversity of a germplasm collection of squash (Cucurbita moschata) determined by SRAP and AFLP markers. Crop Sci. 2004, 44, 653–664. [Google Scholar] [CrossRef]
- Gong, L.; Paris, H.S.; Nee, M.H.; Stift, G.; Pachner, M.; Vollmann, J.; Lelley, T. Genetic relationships and evolution in Cucurbita pepo (pumpkin, squash, gourd) as revealed by simple sequence repeat polymorphisms. Theor. Appl. Genet. 2012, 124, 875–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, S.-C.; Hong, J.-H.; Kwon, Y.-S. DNA profiling of commercial pumpkin cultivars using simple sequence repeat polymorphisms. Hortic. Environ. Biotechnol. 2015, 56, 811–820. [Google Scholar] [CrossRef]
- Kong, Q.; Liu, Y.; Xie, J.; Bie, Z. Development of simple sequence repeat markers from de novo assembled transcriptomes of pumpkins. Plant Mol. Biol. Rep. 2020, 38, 130–136. [Google Scholar] [CrossRef]
- Katzir, N.; Tadmor, Y.; Tzuri, G.; Leshzeshen, E.; Mozes-Daube, N.; Danin-Poleg, Y.; Paris, H.S. Further ISSR and preliminary SSR analysis of relationships among accessions of Cucurbita pepo. In Proceedings of the VII Eucarpia Meeting on Cucurbit Genetics and Breeding 510, Ma’ale ha Hamisha, Israel, 19–23 March 2000; pp. 433–440. [Google Scholar]
- Katzir, N.; Portnoy, V.; Yonash, N.; Mozes-Daube, N.; Tzuri, G.; Paris, H.S. Use of AFLP, ISSR, and SSR marker systems to assess genetic diversity in Cucurbita pepo. In Proceedings of the Plant, Animal and Microbe Genomes Xth Conference, San Diego, CA, USA, 5–10 April 2002; Volume 10, p. 121. [Google Scholar]
- Blanca, J.; Cañizares, J.; Roig, C.; Ziarsolo, P.; Nuez, F.; Picó, B. Transcriptome characterization and high throughput SSRs and SNPs discovery in Cucurbita pepo (Cucurbitaceae). BMC Genom. 2011, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.N.; Kim, M.; Jung, J.-K.; Shim, E.-J.; Chung, S.-M.; Park, Y.; Lee, G.P.; Sim, S.-C. Genome-wide SNP discovery and core marker sets for assessment of genetic variations in cultivated pumpkin (Cucurbita spp.). Hortic. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, J.; Luo, M.; Li, S.; Tao, M.; Ye, X.; Duan, W.; Zhang, C.; Qin, Q.; Xiao, J.; Liu, S. A comparative study of distant hybridization in plants and animals. Sci. China Life Sci. 2018, 61, 285–309. [Google Scholar] [CrossRef]
- Norman, A.; Taylor, J.; Edwards, J.; Kuchel, H. Optimising genomic selection in wheat: Effect of marker density, population size and population structure on prediction accuracy. G3 Genes Genomes Genet. 2018, 8, 2889–2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education India, Benjamin-Cummings Pub Co.: San Francisco, CA, USA, 1996; ISBN 813-172-7-408. [Google Scholar]
- Fonseca, S.; Patterson, F.L. Hybrid Vigor in a Seven-Parent Diallel Cross in Common Winter Wheat (Triticum aestivum L.) 1. Crop Sci. 1968, 8, 85–88. [Google Scholar] [CrossRef]
- Fasahat, P.; Rajabi, A.; Rad, J.M.; Derera, J. Principles and utilization of combining ability in plant breeding. Biom. Biostat. Int. J. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Ferreira, M.G.; De Almeida, G.Q.; Pessoa, H.P.; Dariva, F.D.; De Dias, F.O.; Nick, C. Selection of squash “Menina Brasileira” carrying the allele “Bush” with high yield potential. Hortic. Bras. 2019, 37, 35–39. [Google Scholar] [CrossRef]
- Goulet, B.E.; Roda, F.; Hopkins, R. Hybridization in plants: Old ideas, new techniques. Plant Physiol. 2017, 173, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoodi, S.; Olfati, J.A.; Hamidoghli, Y.; Sabouri, A. Standard heterosis in Cucurbita moschata and Cucurbita pepo interspecific hybrids. Int. J. Veg. Sci. 2016, 22, 383–388. [Google Scholar] [CrossRef]
- Govindaraju, D.R. An elucidation of over a century old enigma in genetics—Heterosis. PLoS Biol. 2019, 17, e3000215. [Google Scholar] [CrossRef] [PubMed]
- Nduwumuremyi, A.; Tongoona, P.; Habimana, S. Mating designs: Helpful tool for quantitative plant breeding analysis. J. Plant Breed. Genet. 2013, 1, 117–129. [Google Scholar]
- Kearsey, M.J.; Pooni, H. Genetical Analysis of Quantitative Traits; Garland Science: New York, NY, USA, 2020; ISBN 100-014-4-178. [Google Scholar]
- Acquaah, G. Principles of Plant Genetics and Breeding; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 111-962-6-692. [Google Scholar]
- Mather, K.; Jinks, J.L. Introduction to Biometrical Genetics; Chapman and Hall Ltd.: London, UK, 1982. [Google Scholar]
- Sharma, J.R. Statistical and Biometrical Techniques in Plant Breeding; New Age International: New Delhi, India, 2006; ISBN 812-240-8-885. [Google Scholar]
- Tysdal, H.M.; Kiesselbach, T.A.; Westover, H.M. Alfalfa Breeding; University of Nebraska Lincoln: Lincoln, NE, USA, 1942. [Google Scholar]
- Wricke, G.; Weber, E. Quantitative Genetics and Selection in Plant Breeding; Walter de Gruyter: Berlin, Germany, 2010; ISBN 311-083-7-528. [Google Scholar]
- Jenkins, M.T.; Brunson, A.M. Methods of Testing Inbred Lines of Maize in Crossbred Combinations 1. Agron. J. 1932, 24, 523–530. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Testers and combining ability. In Quantitative Genetics in Maize Breeding; Springer: Berlin/Heidelberg, Germany, 2010; pp. 383–423. [Google Scholar]
- Le Clerg, E.L. Significance of Experimental Design in Plant Breeding; Iowa State University Press: Ames, IA, USA, 1966. [Google Scholar]
- Saleem, M.Y.; Atta, B.M.; Cheema, A.A.; Haq, M.M. Genetics of panicle-related traits of agronomic importance in rice through triple test cross analysis. Spanish J. Agric. Res. 2005, 3, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Comstock, R.E.; Robinson, H.F. Estimation of average dominance of genes. Heterosis 1952, 2, 494–516. [Google Scholar]
- Kearsey, M.J.; Pooni, H.S.; Bulmer, M. The Genetical Analysis of Quantitative Traits. Genet. Res. 1996, 68, 183. [Google Scholar]
- Kempthorne, O. An Introduction to Genetic Statistics; John Wiley and Sons, Inc.: New York, NY, USA, 1957. [Google Scholar]
- Griffing, B. Concept of general and specific combining ability in relation to diallel crossing systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Ha, V. Genetic analysis of some yield components and kernel quality in sweet corn. Rom. Agric. Res. 1999, 11, 9–20. [Google Scholar]
- Nistor, T.; Sturzu, R.; Nistor, G.; Mîrlogeanu, S.; Anghel, J. Genetic control of the agronomically useful genetic traits in cotton. Rom. Agric. Res. 2005, 22, 27–32. [Google Scholar]
- Jinks, J.L.; Hayman, B.I. The analysis of diallel crosses in maize genetic. Crop News 1953, 27, 48–54. [Google Scholar]
- Hayman, B.I. The analysis of variance of diallel tables. Biometrics 1954, 10, 235–244. [Google Scholar] [CrossRef]
- Abdein, M.A.E.; Hassan, H.M.F.; Dalia, H.M. General performance, combining abilities and heritability of yield and yield component traits in pumpkin (Cucurbita moschata Poir.) at different conditions. Curr. Appl. Sci. Technol. 2017, 17, 121–129. [Google Scholar]
- Allard, R.W.; Wiley, J. Principles of Plant Breeding; John Wiley and Sons, Inc.: New York, NY, USA, 1960. [Google Scholar]
- Han, Y.; Wang, K.; Liu, Z.; Pan, S.; Zhao, X.; Zhang, Q.; Wang, S. Research on Hybrid Crop Breeding Information Management System Based on Combining Ability Analysis. Sustainability 2020, 12, 4938. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S.; Dixit, S.K. Correlation coefficient and path analysis in bottle gourd (Lagenaria siceraria (Molina) Standl.). Progress. Hortic. 2006, 38, 256. [Google Scholar]
- Sprague, G.F.; Tatum, L.A. General vs. specific combining ability in single crosses of corn 1. Agron. J. 1942, 34, 923–932. [Google Scholar] [CrossRef]
- El-Shoura, A.M.; Abed, M.Y. Heterosis and combining ability for development of squash hybrids (Cucurbita pepo L.). J. Plant Prod. 2018, 9, 1181–1187. [Google Scholar] [CrossRef]
- Nishan, S.; Sidhu, M.K.; Dhatt, A.S. Heterosis and combining ability for bushy and butternut traits in pumpkin (Cucurbita moschata). Indian J. Agric. Sci. 2018, 88, 877–883. [Google Scholar]
- El-Hadi, A.; El-Aziz, A.; Abd Alla, M.A.; Ashak, M.G. Genetic Evalution of some Economical Traits in Summer Squash. J. Agric. Chem. Biotechnol. 2020, 11, 147–153. [Google Scholar] [CrossRef]
- El-Gazzar, T.M.; Nada, M.M.; Hussein, A.H.; Dawood, A.R. Evaluation of New Summer Squash Hybrids (Cucurbita pepo L.) Compare with some Commercial Cultivars. J. Plant Prod. 2020, 11, 841–845. [Google Scholar] [CrossRef]
- Singh, M.K.; Singh, V.B.; Yadav, G.C.; Kumar, P. To Study the Combining Ability and Gene Action Involve in Expression of Traits in Pumpkin (Cucurbita moschata Duch. ex. Poir). Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1535–1549. [Google Scholar] [CrossRef]
- Hatwa, P.K.; Yadav, V.S.; Thakur, R.; Mahawar, A.K. Estimation of heterosis in relation to combining ability for earliness, yield and quality attributes in pumpkin (Cucurbita moschata Duch. ex Poir). Indian J. Agric. Res. 2018, 52, 548–553. [Google Scholar]
- Mohsin, G.M.; Doullah, M.A.U.; Hasanuzzaman, M.; Biswas, B.K.; Islam, M.S.; Rahman, S.; Islam, A.; Dinajpur, B. Combining ability analysis in pumpkin (Cucurbita moschata Duch Ex Poir). Contemp. Res. India 2017, 7, 176–181. [Google Scholar]
- Am, E.-A.; El-Hadi, A.; Fathy, H.M.; Abdein, M. Heterosis, Heritability and Combining Abilities for some Earliness Traits in Squash (Cucurbita pepo, L.). Alex. Sci. Exch. J. 2014, 35, 203–214. [Google Scholar]
- Tamilselvi, N.A.; Jansirani, P.; Pugalendhi, L. Estimation of heterosis and combining ability for earliness and yield characters in pumpkin (Cucurbita moschata Duch. Ex. Poir). Afr. J. Agric. Res. 2015, 10, 1904–1912. [Google Scholar]
- Ahmed, B.; Masud, M.A.T.; Zakaria, M.; Hossain, M.M.; Mian, M.A.K. Heterosis and combining ability of pumpkin (Cucurbita moschata Duch. Ex Poir.). J. Agric. Stud. 2017, 5, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Darrudi, R.; Nazeri, V.; Soltani, F.; Shokrpour, M.; Ercolano, M.R. Evaluation of combining ability in Cucurbita pepo L. and Cucurbita moschata Duchesne accessions for fruit and seed quantitative traits. J. Appl. Res. Med. Aromat. Plants 2018, 9, 70–77. [Google Scholar] [CrossRef]
- Hayes, H.K.; Immer, F.R.; Smith, D.C. Methods of Plant Breeding; McGraw-Hill 1955 Edition: New York, NY, USA, 1955; p. 551. [Google Scholar]
- Singh, A.K.; Pan, R.S.; Bhavana, P. Heterosis and combining ability analysis in bittergourd (Momordica charantia L.). Bioscan 2013, 8, 1533–1536. [Google Scholar]
- Hayes, J.D.; Foster, C.A. Heterosis in self-pollinated crops with particular reference to barley, Heterosis in plant breeding. In Proceedings of the 7th Congress EUCARPIA, Roskilde, Denmark, 24–29 June 1976; pp. 239–256. [Google Scholar]
- Moll, R.H.; Stuber, C.W. Quantitative genetics—empirical results relevant to plant breeding. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1974; Volume 26, pp. 277–313. ISBN 0065-2113. [Google Scholar]
- Khattak, G.S.S.; Ashraf, M.; Zamir, R. Gene action for synchrony in pod maturity and indeterminate growth habit in mungbean (Vigna radiata (L.) Wilczek). Pak. J. Bot. 2004, 36, 589–594. [Google Scholar]
- Abd El-Hadi, A.H.; El-Adl, A.M.; Fathy, H.M.; Abdein, M.A. Heterosis and genetic behavior of some yield and yield component traits in squash (Cucurbita pepo, L.). Alex. Sci. Exch. J. 2014, 35, 178–189. [Google Scholar]
- Sherpa, P.; Seth, T.; Shende, V.D.; Pandiarana, N.; Mukherjee, S.; Chattopadhyay, A. Heterosis, dominance estimate and genetic control of yield and post-harvest quality traits of tomato. J. Appl. Nat. Sci. 2014, 6, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Cyril, N.C.; Ayinde, D.L.; Olatunji, O. Genetic variability and heritability of vegetative, fruit and seed yield traits in fluted pumpkin (Telfairia occidentalis Hook F). Afr. J. Biotechnol. 2014, 13, 3262–3270. [Google Scholar]
- Selvi, N.T.; Jansirani, P.; Pugalendhi, L. Studies on heterosis in pumpkin (Cucurbita moschata Duch. ex Poir). J. Hortic. Sci. 2014, 9, 131–140. [Google Scholar]
- Karipçin, M.Z.; İnal, B. Determination of heterosis and heterobeltiosis values of salt-tolerant summer squash (Cucurbita pepo l.) genotypes and genetic relationships of parental genomes. Appl. Ecol. Environ. Res. 2017, 15, 779–796. [Google Scholar] [CrossRef]
- Schmidt, P.; Hartung, J.; Bennewitz, J.; Piepho, H.-P. Heritability in plant breeding on a genotype-difference basis. Genetics 2019, 212, 991–1008. [Google Scholar] [CrossRef]
- Johnson, H.W.; Robinson, H.F.; Comstock, R.E. Estimates of genetic and environmental variability in soybeans 1. Agron. J. 1955, 47, 314–318. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Oki, Y.; Adachi, T.; Khan, M.H.R. Analyses of the genetic parameters (variability, heritability, genetic advance, relationship of yield and yield contributing characters) for some plant traits among Brassica Cultivars under phosphorus starved environmental cues. J. Fac. Environ. Sci. Technol. 2007, 12, 91–98. [Google Scholar]
- Obiadalla-Ali, H.A. Heterosis and nature of gene action for earliness and yield components in summer squash (Cucurbita pepo L.). Assiut J. Agric. Sci. 2006, 37, 123–135. [Google Scholar]
- Doijode, S.D.; Sulladmath, U.V. Genetics of certain vegetative and flowering characters in pumpkin (Cucurbita moschata, Poir.). Agric. Sci. Deistic India 1988, 8, 203–206. [Google Scholar]
- El-Hadi, A.; Zaied, K.A.; El-Raziq, A.; Eman, A. Estimating the genetic parameters of yield and yield component traits in squash (Cucurbita pepo L.). J. Agric. Chem. Biotechnol. 2013, 4, 321–332. [Google Scholar] [CrossRef]
- Nisha, S.K.; Veeraragavathatham, D. Heterosis and combining ability for fruit yield and its component traits in pumpkin (Cucurbita moschata Duch. ex Poir.). Adv. Appl. Res. 2014, 6, 158–162. [Google Scholar] [CrossRef]
- Marxmathi, P.; Krishnamoorthy, V.; Thankaraj, P. Combining Ability Studies in Pumpkin (Cucurbita moschata Duch ex Poir). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3033–3039. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lu, A.; Zhang, L.; Shen, M.; Xu, T.; Zhan, W.; Jin, H.; Zhang, Y.; Wang, W. Extraction and purification of pumpkin polysaccharides and their hypoglycemic effect. Int. J. Biol. Macromol. 2017, 98, 182–187. [Google Scholar] [CrossRef]





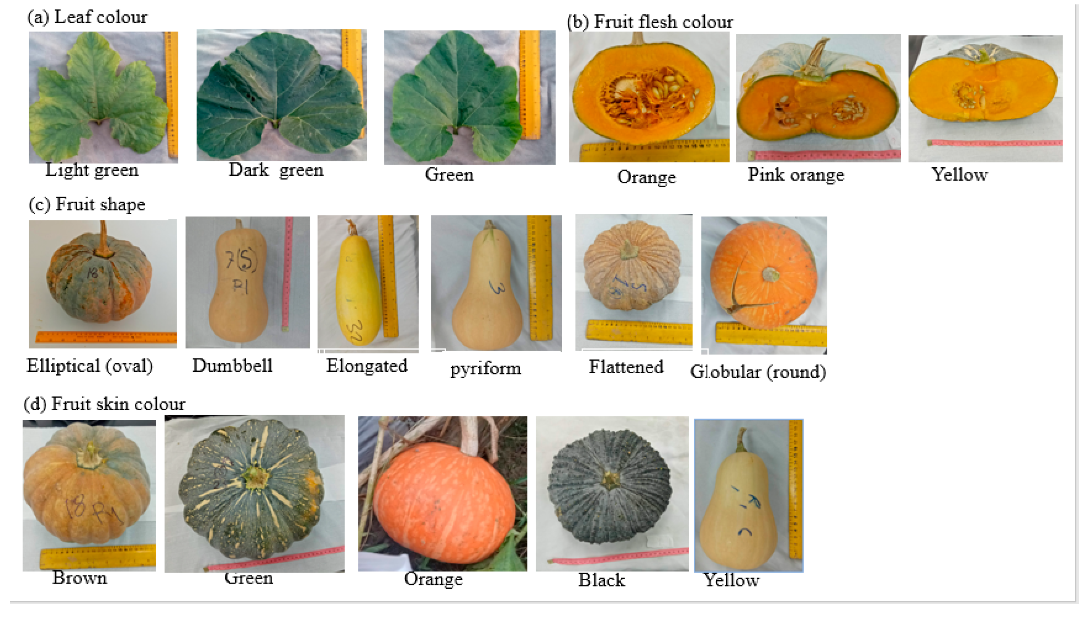


| Traits | Scale | Reporters |
|---|---|---|
| Leaf colour | Light green | [80] |
| Green | [80] | |
| Dark green | [80] | |
| Fruit shape | Globular (round) | [81,82,83] |
| Pyriform | [82,83] | |
| Flattened | [81,83] | |
| Dumbbell | [81,83] | |
| Elliptical (oval) | [82,83] | |
| Elongated | [80] | |
| Fruit ribs | Deep | [80,82] |
| Intermediate | [80,82] | |
| Absent | [80,82] | |
| Superficial | [80,82] | |
| Fruit skin colour | Yellow | [80,83] |
| Black | [80,83] | |
| Orange | [80,82,83] | |
| Brown | [80,82,83] | |
| Green | [80,82,83] | |
| Flesh colour | Yellow | [80,83] |
| Orange | [80,82,83] | |
| Pink orange | [80,82,83] | |
| Fruit skin texture | Smooth | [80,83] |
| Grainy | [80,83] | |
| Shallowly wavy | [80,83] | |
| Finely wrinkled | [80,83] |
| Research Materials | High GCV and High PCV Observed Variables | Researchers |
|---|---|---|
| Pumpkin-21 genotypes | Length of vine, fruits number per plant, plant yield, reducing sugars as well as β-carotene | [84] |
| Pumpkins (Cucurbita moschata Duch. ex. Poir)-32 genotypes | Plant yield, length of vine, fruits number per plant, weight of fruit, and weight of 100 seeds | [85] |
| Cucurbita moschata-91 genotypes | Seed number and seeds mass per fruit, days for blossoming, content of total carotenoid, and production of fruit | [86] |
| Pumpkin genotypes | Total carotenoid’s | [87] |
| Pumpkin genotypes-23 | length of vine, cavity of fruit, seed number per fruit, thickness of rind, and fruits number per vine | [88] |
| Pumpkin genotypes-25 | Weight of fruit | [89] |
| Pumpkins (Cucurbita maxima Duchesne)-40 genotypes | Fruit yield/plant and content of carotenoids | [90] |
| Pumpkins (Cucurbita moschata Duch. Ex Poir.)-19 genotypes | Average weight of fruit, thickness of flesh, fruits number per plant | [91] |
| Pumpkin accessions-7 genotypes | Flesh thickness in the peduncle | [92] |
| Pumpkin-21 genotypes | Length of fruit, weight of single fruit, TSS, and plant yield | [73] |
| Pumpkin-30 genotypes | Days to the first harvesting, ridge number per fruit, diameter of fruit length of fruit average weight of fruit, fruits number per plant, thickness of flesh, diameter of seed cavity, seed number per fruit, and fruit yield per plant | [93] |
| (Cucurbita moschata Duch. Ex Poir.)-76 genotypes | Seed yield per plant followed by weight of 100 seeds | [94] |
| Pumpkin-76 genotypes | Weight of plant weight of matured fruit, and yield of fruit per hectare | [95] |
| Pumpkin-40 genotypes | Weight of fruit, plant fruit yield, content of ß-carotene, thickness of flesh, node at the first male flowering, test weight of 100 seeds, fruit equatorial circumferences, and seed number per fruit. | [96] |
| Markers Name | Reporters |
|---|---|
| RAPD (Random Amplified Polymorphic DNA) | [97,101,102] |
| AFLP (Amplified Fragment length Polymorphism) | [103,104] |
| SRAP (Sequence Related Amplified Polymorphism) | [97,105] |
| SCAR (Sequence Characterized amplified region) | [106] |
| SSRs (Simple Sequence Repeats) | [102,106,107,108] |
| ISSRs (Inter-simple Sequence Repeats) | [103,109,110] |
| SBAPs (Sequence-Based Amplified Polymorphism) | [97] |
| SNPs (Single Nucleotide Polymorphisms) | [111,112] |
| Name of Mating Design | Proposed by | Source of Variance |
|---|---|---|
| Biparental mating design | [125] | Between families, Within Families, [124] |
| Poly cross | [126,127] | Progenies, Blocks [128] |
| Top cross | [129] | Progenies, Blocks [130] |
| Triple cross | [131] | Replication, Genotype [132] |
| North Carolina Mating design-I, (Hierarchical or Nested mating Design) | [133] | Males, Females, Within plots [124] |
| North Carolina Mating design-II, (Factorial mating Design) | [133] | Replications, Males, Females Males × Females. Within progenies [134] |
| North Carolina Mating design- III | [133] | Tester P, Males (F2), m, Tester × Parents Within FS Families [130] |
| Line × Tester | [135] | Replications, Lines, Testers Lines × Testers, Error [126] |
| Diallel mating design. Method-I | [136] | GCA, SCA, Reciprocal effects [136] |
| Diallel mating design, Method-II | [136] | GCA, SCA [136] |
| Diallel mating design, Method-III | [136] | GCA, SCA, Reciprocal effects [136] |
| Diallel mating design, Method-IV | [136] | GCA, SCA [136] |
| Sl.no | Research Materials | Traits for Dominance of GCA | Traits for Dominance of SCA | Researchers |
|---|---|---|---|---|
| 1 | 4 inbred lines | Diameter of fruit, flesh thickness, weigh of fruit, weight of seed, and TSS | Fruit yield per plant | [154] |
| 2 | 6 inbred lines | Fruit length, average fruit diameter, thickness of fruit flesh, Fruit weight, quantity of fruits per plant, and total soluble solids in fruits (TSS) | Days to the first female flowering | [155] |
| 3 | 6 inbred lines | Early maturities of fruit, average fruit diameter, individual fruit weight, fruit pulp thickness, number of fruits per plant, total fruit yield, number of seeds per fruit, and characters of seed | Early fruit maturities, average weight of fruit, total fruits number, total fruit yield per plant, characters of seed, and TSS (brix percentages) | [152] |
| 4 | 7 inbred lines and their F1 hybrids | Plant growth speed on the 50th day of transplanting, main plants stem lengths on the 50th day of transplanting, plants productivity, and shape of fruit | Yield per plant | [118] |
| 5 | 5 open pollinated lines | Total fruit output per plant, average fruit weight, and seed weight | Total fruit yield/plant, average fruit weight, 100 seed weight, and number of seeds per fruit are considered. | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosen, M.; Rafii, M.Y.; Mazlan, N.; Jusoh, M.; Oladosu, Y.; Chowdhury, M.F.N.; Muhammad, I.; Khan, M.M.H. Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges. Horticulturae 2021, 7, 352. https://doi.org/10.3390/horticulturae7100352
Hosen M, Rafii MY, Mazlan N, Jusoh M, Oladosu Y, Chowdhury MFN, Muhammad I, Khan MMH. Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges. Horticulturae. 2021; 7(10):352. https://doi.org/10.3390/horticulturae7100352
Chicago/Turabian StyleHosen, Monir, Mohd Y. Rafii, Norida Mazlan, Mashitah Jusoh, Yusuff Oladosu, Mst. Farhana Nazneen Chowdhury, Ismaila Muhammad, and Md Mahmudul Hasan Khan. 2021. "Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges" Horticulturae 7, no. 10: 352. https://doi.org/10.3390/horticulturae7100352
APA StyleHosen, M., Rafii, M. Y., Mazlan, N., Jusoh, M., Oladosu, Y., Chowdhury, M. F. N., Muhammad, I., & Khan, M. M. H. (2021). Pumpkin (Cucurbita spp.): A Crop to Mitigate Food and Nutritional Challenges. Horticulturae, 7(10), 352. https://doi.org/10.3390/horticulturae7100352






