Dehydrins and Soluble Sugars Involved in Cold Acclimation of Rosa wichurana and Rose Cultivar ‘Yesterday’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Field Condition
2.2. Controlled Freezing Test
2.3. Soluble Sugars
2.4. RNA Extraction and Reverse Transcription
2.5. Gene Isolation and Expression
2.6. Statistical Analysis
3. Results
3.1. Air Temperature and Day Length Condition
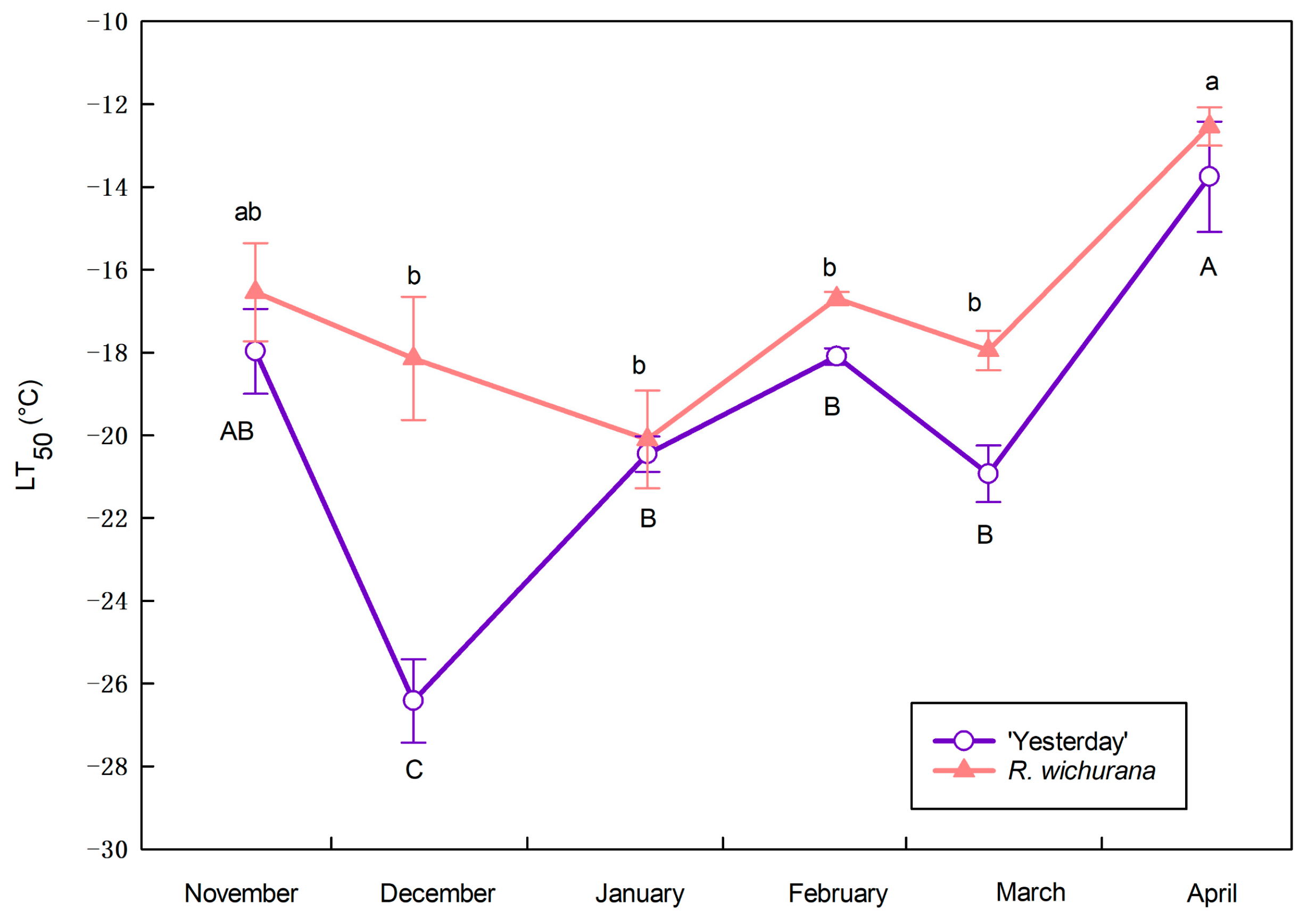
3.2. Cold Hardiness
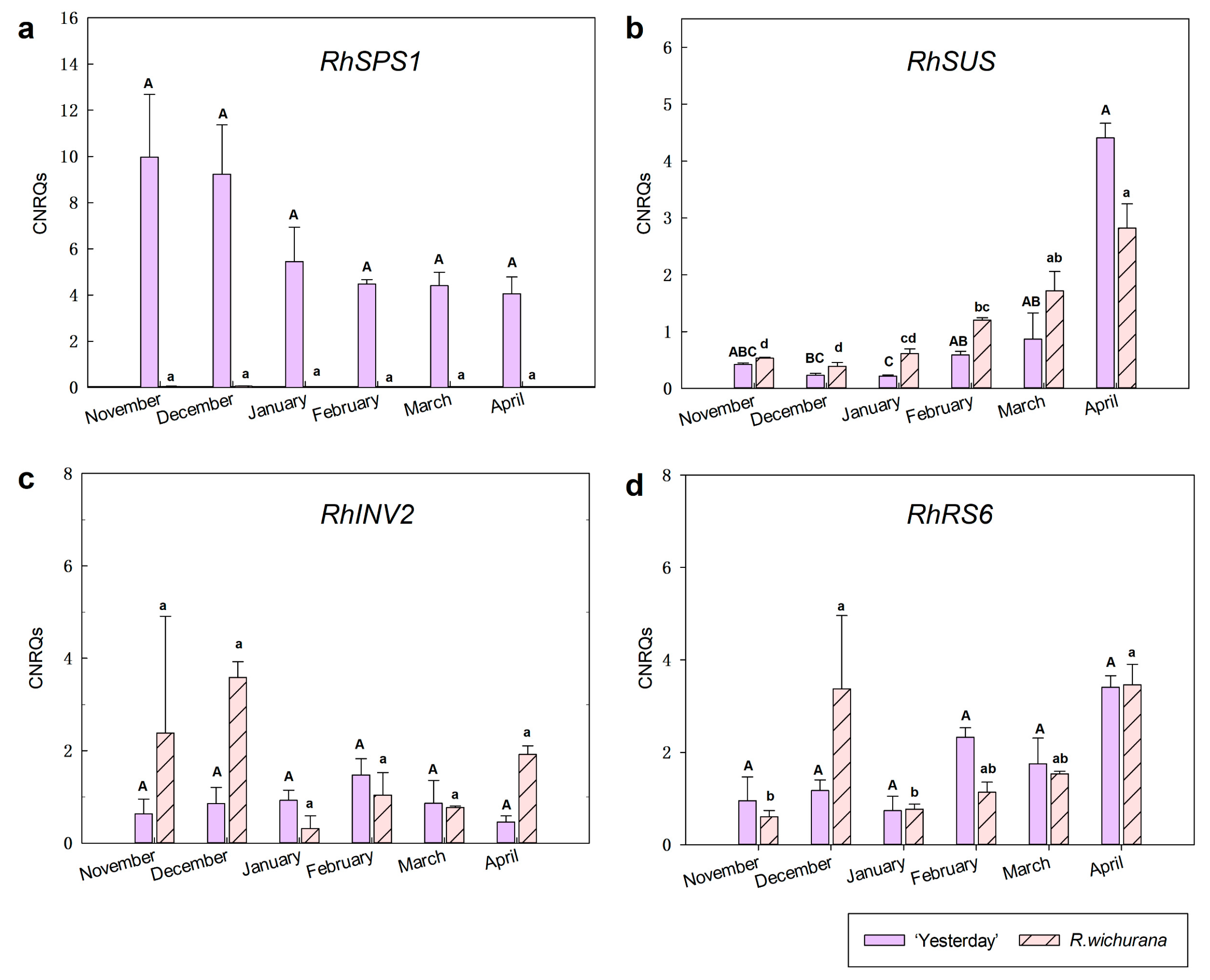
3.3. Expression Analysis of Dehydrins
3.4. Soluble Sugars
3.5. Expression Analysis of Sugar Metabolism-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jun, S.H.; Yu, D.J.; Hur, Y.Y.; Lee, H.J. Identifying reliable methods for evaluating cold hardiness in grapevine buds and canes. Hortic. Environ. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Kovaleski, A.P.; Grossman, J.J. Standardization of electrolyte leakage data and a novel liquid nitrogen control improve measurements of cold hardiness in woody tissue. Plant Methods 2021, 17, 1–20. [Google Scholar] [CrossRef]
- Huner, N.P.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Welling, A.; Moritz, T.; Palva, E.T.; Junttila, O. Independent activation of cold acclimation by low temperature and short photoperiod in hybrid aspen. Plant Physiol. 2002, 129, 1633–1641. [Google Scholar] [CrossRef] [Green Version]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef]
- Liu, B.; Xia, Y.-P.; Krebs, S.L.; Medeiros, J.; Arora, R. Seasonal responses to cold and light stresses by two elevational ecotypes of Rhododendron catawbiense: A comparative study of overwintering strategies. Environ. Exp. Bot. 2019, 163, 86–96. [Google Scholar] [CrossRef]
- Levitt, J. Chilling, Freezing, and High Temperature Stresses. In Responses of Plants to Environmental Stresses, 2nd ed.; Levitt, J., Ed.; Academic Press: New York, NY, USA, 1980; Volume 1, pp. 1–497. [Google Scholar]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold Acclimation of Arabidopsis thaliana (Effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Lin, S.-Z.; Zheng, H.-Q.; Lei, Y.; Zhang, Q.; Zhang, Z.-Y. The role of antioxidant system in freezing acclimation-induced freezing resistance of Populus suaveolens cuttings. For. Stud. China 2007, 9, 107–113. [Google Scholar] [CrossRef]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2012, 147, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Masocha, V.F.; Li, Q.; Zhu, Z.; Chai, F.; Sun, X.; Wang, Z.; Yang, L.; Wang, Q.; Xin, H. Proteomic variation in Vitis amurensis and V. vinifera buds during cold acclimation. Sci. Hortic. 2019, 263, 109143. [Google Scholar] [CrossRef]
- Wisniewski, M.; Nassuth, A.; Arora, R. Cold hardiness in trees: A mini-review. Front. Plant Sci. 2018, 9, 1394. [Google Scholar] [CrossRef]
- Horvath, D.P.; Zhang, J.; Chao, W.S.; Mandal, A.; Rahman, M.; Anderson, J.V. Genome-wide association studies and transcriptome changes during acclimation and deacclimation in divergent Brassica napus varieties. Int. J. Mol. Sci. 2020, 21, 9148. [Google Scholar] [CrossRef]
- Burchett, S.; Niven, S.; Fuller, M.P. The effect of cold acclimation on the water relations and freezing tolerance of Hordeum vulgare L. Cryo Lett. 2007, 27, 295–303. [Google Scholar]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef] [Green Version]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [Green Version]
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance. Plant Signal. Behav. 2011, 6, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Rekarte-Cowie, I.; Ebshish, O.S.; Mohamed, K.S.; Pearce, R.S. Sucrose helps regulate cold acclimation of Arabidopsis thaliana. J. Exp. Bot. 2008, 59, 4205–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolkers, W.F.; McCready, S.; Brandt, W.F.; Lindsey, G.G.; Hoekstra, F.A. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 2001, 1544, 196–206. [Google Scholar] [CrossRef]
- Trischuk, R.G.; Schilling, B.S.; Low, N.H.; Gray, G.R.; Gusta, L.V. Cold acclimation, de-acclimation and re-acclimation of spring canola, winter canola and winter wheat: The role of carbohydrates, cold-induced stress proteins and vernalization. Environ. Exp. Bot. 2014, 106, 156–163. [Google Scholar] [CrossRef]
- Wanner, L.A.; Junttila, O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 1999, 120, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.J.; Hwang, J.Y.; Chung, S.W.; Oh, H.D.; Yun, S.K.; Lee, H.J. Changes in cold hardiness and carbohydrate content in peach (Prunus persica) trunk bark and wood tissues during cold acclimation and deacclimation. Sci. Hortic. 2017, 219, 45–52. [Google Scholar] [CrossRef]
- Winde, J.; Sønderkær, M.; Nielsen, K.L.; Pagter, M. Is range expansion of introduced Scotch broom (Cytisus scoparius) in Denmark limited by winter cold tolerance? Plant Ecol. 2020, 221, 709–723. [Google Scholar] [CrossRef]
- Deslauriers, A.; Garcia, L.; Charrier, G.; Buttò, V.; Pichette, A.; Paré, M. Cold acclimation and deacclimation in wild blueberry: Direct and indirect influence of environmental factors and non-structural carbohydrates. Agric. For. Meteorol. 2021, 301–302, 108349. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Hand, S.C.; Crowe, L.M.; Crowe, J.H. Cryoprotection of phosphofructokinase with organic solutes: Characterization of enhanced protection in the presence of divalent cations. Arch. Biochem. Biophys. 1986, 250, 505–512. [Google Scholar] [CrossRef]
- Uemura, M.; Steponkus, P.L. Cold acclimation in plants: Relationship between the lipid composition and the cryostability of the plasma membrane. J. Plant Res. 1999, 112, 245–254. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zhao, M.-G.; Tian, Q.-Y.; Zhang, W.-H. Comparative studies on tolerance of Medicago truncatula and Medicago falcata to freezing. Planta 2011, 234, 445–457. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Zuther, E.; Buchel, K.; Hundertmark, M.; Stitt, M.; Hincha, D.K.; Heyer, A.G. The role of raffinose in the cold acclimation response of Arabidopsis thaliana. FEBS Lett. 2004, 576, 169–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unda, F.; Canam, T.; Preston, L.; Mansfield, S.D. Isolation and characterization of galactinol synthases from hybrid poplar. J. Exp. Bot. 2011, 63, 2059–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, Y.; Jiang, S.; Xu, F.; Wang, H.; Wei, Y.; Shao, X. PpINH1, an invertase inhibitor, interacts with vacuolar invertase PpVIN2 in regulating the chilling tolerance of peach fruit. Hortic. Res. 2020, 7, 168. [Google Scholar] [CrossRef]
- Ouyang, L.; Leus, L.; Van Labeke, M.-C. Three-year screening for cold hardiness of garden roses. Sci. Hortic. 2018, 245, 12–18. [Google Scholar] [CrossRef]
- Flint, H.L.; Boyce, B.R.; Beattie, D.J. Index of injury—A useful expression of freezing injury to plant tissues as determined by the electrolytic method. Can. J. Plant Sci. 1967, 47, 229–230. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.C.; Arora, R.; Townsend, E.C. Comparing Gompertz and Richards Functions to Estimate Freezing Injury in Rhododendron Using Electrolyte Leakage. J. Am. Soc. Hortic. Sci. 1998, 123, 246–252. [Google Scholar] [CrossRef]
- Luypaert, G.; Witters, J.; Van Huylenbroeck, J.; De Clercq, P.; De Riek, J.; De Keyser, E. Induced expression of selected plant defence related genes in pot azalea, Rhododendron simsii hybrid. Euphytica 2017, 213, 227. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [Green Version]
- Pipino, L. Improving Seed Production Efficiency for Hybrid Rose Breeding. Doctoral Dissertation, Ghent University, Ghent, Belgium, 2011. [Google Scholar]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; Van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [Green Version]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Artlip, T.S.; Callahan, A.M.; Bassett, C.L.; Wisniewski, M. Seasonal expression of a dehydrin gene in sibling deciduous and evergreen genotypes of peach (Prunus persica [L.] Batsch). Plant Mol. Biol. 1997, 33, 61–70. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.-L.; Wang, L.; Zhou, Y.-H.; Huang, Y.-T.; Hao, X.; Wang, Y.-C.; Wang, B.; Yang, Y.-J.; Wang, X.-C. Effects of cold acclimation on sugar metabolism and sugar-related gene expression in tea plant during the winter season. Plant Mol. Biol. 2015, 88, 591–608. [Google Scholar] [CrossRef]
- Kalberer, S.R.; Wisniewski, M.; Arora, R. Deacclimation and reacclimation of cold-hardy plants: Current understanding and emerging concepts. Plant Sci. 2006, 171, 3–16. [Google Scholar] [CrossRef]
- Kalberer, S.R.; Leyva-Estrada, N.; Krebs, S.L.; Arora, R. Frost dehardening and rehardening of floral buds of deciduous azaleas are influenced by genotypic biogeography. Environ. Exp. Bot. 2007, 59, 264–275. [Google Scholar] [CrossRef]
- Pagter, M.; Hausman, J.-F.; Arora, R. Deacclimation kinetics and carbohydrate changes in stem tissues of Hydrangea in response to an experimental warm spell. Plant Sci. 2011, 180, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Bassett, C.; Norelli, J.; Macarisin, D.; Artlip, T.; Gasic, K.; Korban, S. Expressed sequence tag analysis of the response of apple (Malus × domestica ‘Royal Gala’) to low temperature and water deficit. Physiol. Plant. 2008, 133, 298–317. [Google Scholar] [CrossRef] [PubMed]
- Kontunen-Soppela, S.; Taulavuori, K.; Taulavuori, E.; Lähdesmäki, P.; Laine, K. Soluble proteins and dehydrins in nitrogen-fertilized Scots pine seedlings during deacclimation and the onset of growth. Physiol. Plant. 2000, 109, 404–409. [Google Scholar] [CrossRef]
- Arora, R.; Rowland, L.J.; Ogden, E.L.; Dhanaraj, A.L.; Marian, C.O.; Ehlenfeldt, M.K.; Vinyard, B. Dehardening kinetics, bud development, and dehydrin metabolism in blueberry cultivars during deacclimation at constant, warm temperatures. J. Am. Soc. Hortic. Sci. 2004, 129, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Arora, R.; Wisniewski, M. Accumulation of a 60-kD dehydrin protein in peach xylem tissues and its relationship to cold acclimation. HortScience 1996, 31, 923–925. [Google Scholar] [CrossRef] [Green Version]
- Marian, C.O.; Eris, A.; Krebs, S.L.; Arora, R. Environmental regulation of a 25 kDa dehydrin in relation to Rhododendron cold Acclimation. J. Am. Soc. Hortic. Sci. 2004, 129, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Welling, A.; Rinne, P.; Viherä-Aarnio, A.; Kontunen-Soppela, S.; Heino, P.; Palva, E.T. Photoperiod and temperature differentially regulate the expression of two dehydrin genes during overwintering of birch (Betula pubescens Ehrh.). J. Exp. Bot. 2004, 55, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, N.; Xia, H.; Wu, S.; Ma, F. Genome-wide identification and expression profiling of dehydrin gene family in Malus domestica. Mol. Biol. Rep. 2012, 39, 10759–10768. [Google Scholar] [CrossRef]
- Shin, H.; Oh, S.-I.; Kim, M.-A.; Yun, S.K.; Oh, Y.; Son, I.-C.; Kim, H.-S.; Kim, D. Relationship between cold hardiness and dehydrin gene expression in peach shoot tissues under field conditions. Hortic. Environ. Biotechnol. 2015, 56, 280–287. [Google Scholar] [CrossRef]
- Joosen, R.V.L.; Lammers, M.; Balk, P.A.; Brønnum, P.; Konings, M.C.J.M.; Perks, M.; Stattin, E.; Van Wordragen, M.F.; Van Der Geest, A.H.M. Correlating gene expression to physiological parameters and environmental conditions during cold acclimation of Pinus sylvestris, identification of molecular markers using cDNA microarrays. Tree Physiol. 2006, 26, 1297–1313. [Google Scholar] [CrossRef] [Green Version]
- Koster, K.; Leopold, A.C. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988, 88, 829–832. [Google Scholar] [CrossRef] [Green Version]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M.; Anchordoguy, T.J. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology 1990, 27, 219–231. [Google Scholar] [CrossRef]
- Pagter, M.; Jensen, C.R.; Petersen, K.K.; Liu, F.; Arora, R. Changes in carbohydrates, ABA and bark proteins during seasonal cold acclimation and deacclimation in Hydrangea species differing in cold hardiness. Physiol. Plant. 2008, 134, 473–485. [Google Scholar] [CrossRef]
- Palonen, P.; Buszard, D.; Donnelly, D. Changes in carbohydrates and freezing tolerance during cold acclimation of red raspberry cultivars grown in vitro and in vivo. Physiol. Plant. 2008, 110, 393–401. [Google Scholar] [CrossRef]
- Van Labeke, M.-C.; Volckaert, E. Evaluation of electrolyte leakage for detecting cold acclimatization in six deciduous tree species. Acta Hortic. 2010, 885, 403–410. [Google Scholar] [CrossRef]
- Schrader, S.; Sauter, J.J. Seasonal changes of sucrose-phosphate synthase and sucrose synthase activities in poplar wood (Populus × canadensis Moench ‘robusta’) and their possible role in carbohydrate metabolism. J. Plant Physiol. 2002, 159, 833–843. [Google Scholar] [CrossRef]
- Goldner, W.; Thom, M.; Maretzki, A. Sucrose metabolism in sugarcane cell suspension cultures. Plant Sci. 1991, 73, 143–147. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]


| Genes in Roses | Functional Annotation | Species | Acc. No. |
|---|---|---|---|
| RhDHN5/6 * | Dehydrin | Prunus persica | U34809 |
| RhSPS1 | Sucrose-phosphate synthase | Camellia sinensis | KF696388 |
| RhSUS | Sucrose synthase | Camellia sinensis | KF921302 |
| RhINV2 | Invertase | Camellia sinensis | KP053402 |
| RhRS6 | Raffinose synthase | Camellia sinensis | KP162174 |
| Gene | Acc. No. | F or R | Primer Sequence 5′–3′ | Amplicon Size (bp) | PCR Efficiencies |
|---|---|---|---|---|---|
| RhDHN5 | MH249069 | F | GGTCACAAGGACGATCCCTA | 86 | 1.886 |
| R | CCCTTATGCTCTTGGTGCTC | ||||
| RhDHN6 | MH249070 | F | CCGTGAGAATAAGGGAGTGG | 106 | 1.914 |
| R | GCCGTAACCCGGTGTAGTAG | ||||
| RhSUS | MH249072 | F | AGACCCTTCTCACTGGGACA | 142 | 1.798 |
| R | GCGATCAAGGTTGGAGACA | ||||
| RhINV2 | MH249073 | F | TCTGTGGCAACTGATGTTGTT | 130 | 1.893 |
| R | TTGTTCGTCCACCTTGAGC | ||||
| RhRS6 | MH249076 | F | CATTAGTGGCGGACCTGTTT | 84 | 1.912 |
| R | CCGTCCGGCAATACTATCTT | ||||
| RhPGK * | EC586265.1 | F | GCCAAAGTCATCTTGGCTTC | 101 | 1.869 |
| R | CCACTCCAAGGAGCTCAGAC | ||||
| RhRPS18c * | BI977264.1 | F | ATCTCGAGCGGTTGAAGAAG | 97 | 1.890 |
| R | TGCGACCAGTAGTCTTGGTG | ||||
| Rh2-UBC9 * | EC586612.1 | F | GACCCAAATCCTGATGATCC | 104 | 1.903 |
| R | CGTACTTCTGGGTCCAGCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, L.; Leus, L.; De Keyser, E.; Van Labeke, M.-C. Dehydrins and Soluble Sugars Involved in Cold Acclimation of Rosa wichurana and Rose Cultivar ‘Yesterday’. Horticulturae 2021, 7, 379. https://doi.org/10.3390/horticulturae7100379
Ouyang L, Leus L, De Keyser E, Van Labeke M-C. Dehydrins and Soluble Sugars Involved in Cold Acclimation of Rosa wichurana and Rose Cultivar ‘Yesterday’. Horticulturae. 2021; 7(10):379. https://doi.org/10.3390/horticulturae7100379
Chicago/Turabian StyleOuyang, Lin, Leen Leus, Ellen De Keyser, and Marie-Christine Van Labeke. 2021. "Dehydrins and Soluble Sugars Involved in Cold Acclimation of Rosa wichurana and Rose Cultivar ‘Yesterday’" Horticulturae 7, no. 10: 379. https://doi.org/10.3390/horticulturae7100379
APA StyleOuyang, L., Leus, L., De Keyser, E., & Van Labeke, M.-C. (2021). Dehydrins and Soluble Sugars Involved in Cold Acclimation of Rosa wichurana and Rose Cultivar ‘Yesterday’. Horticulturae, 7(10), 379. https://doi.org/10.3390/horticulturae7100379






