Screening of ‘King’ Mandarin Hybrids as Tolerant Citrus Rootstocks to Flooding Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Growth Parameters
2.3. Chlorophyll Concentration
2.4. Gas Exchange Parameters
2.5. Fluorescence
2.6. Leaf Water Relations
2.7. ABA Analysis
2.8. RNA Extraction and Real-Time RT-PCR Analysis
2.9. Statistical Analyses
3. Results
3.1. Plant Growth
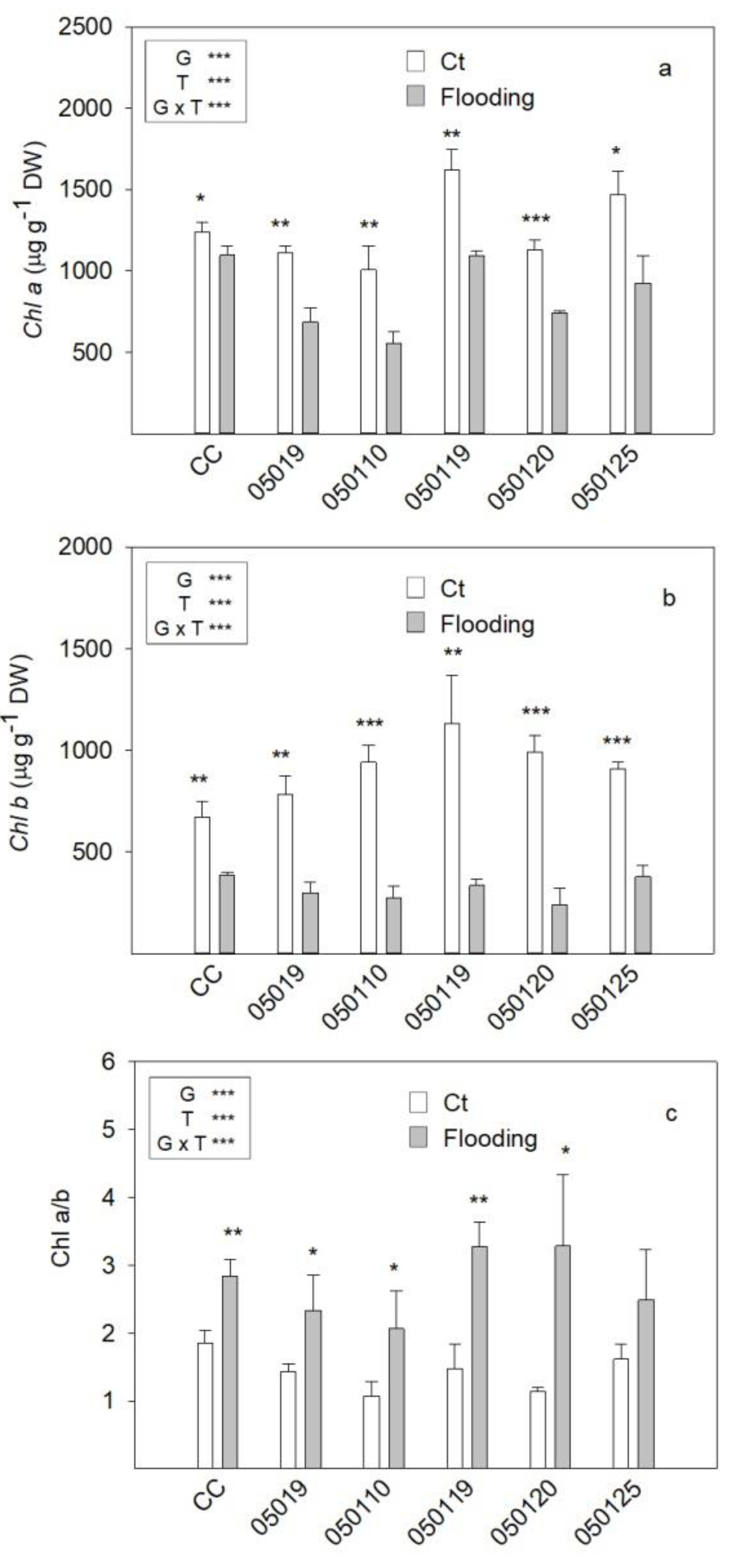
3.2. Chlorophyll Concentration
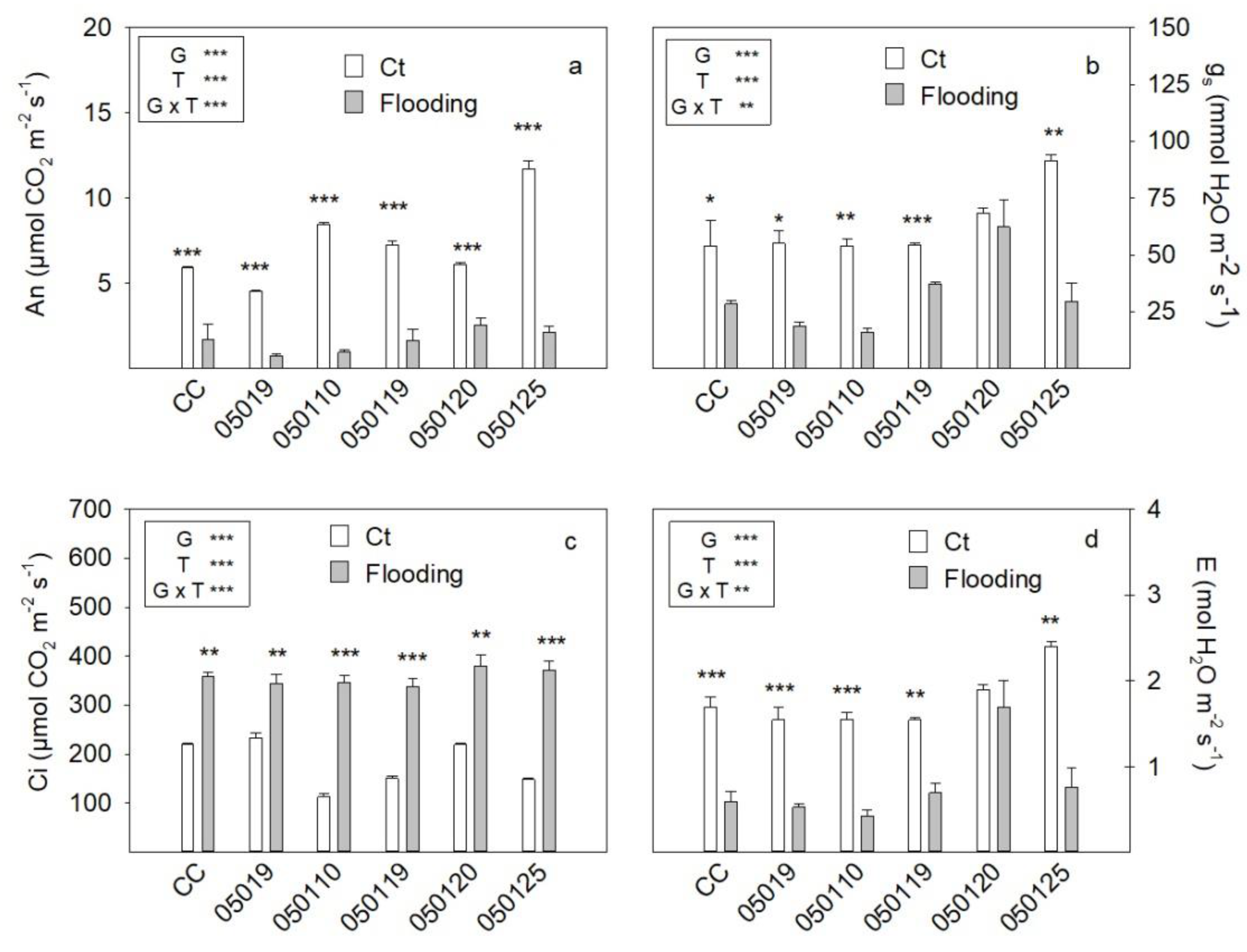
3.3. Gas Exchange Parameters
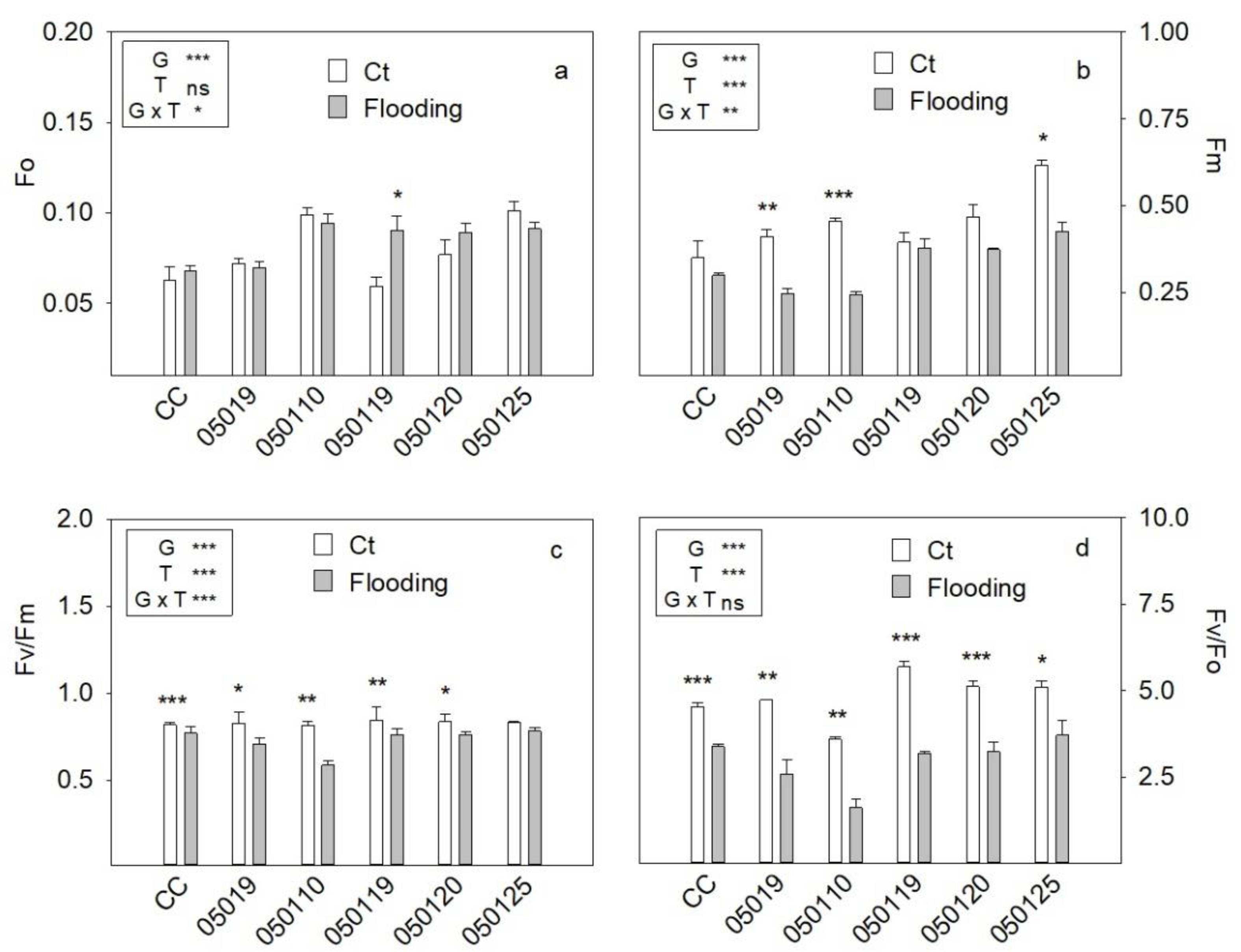
3.4. Fluorescence
3.5. Water Relation Parameters
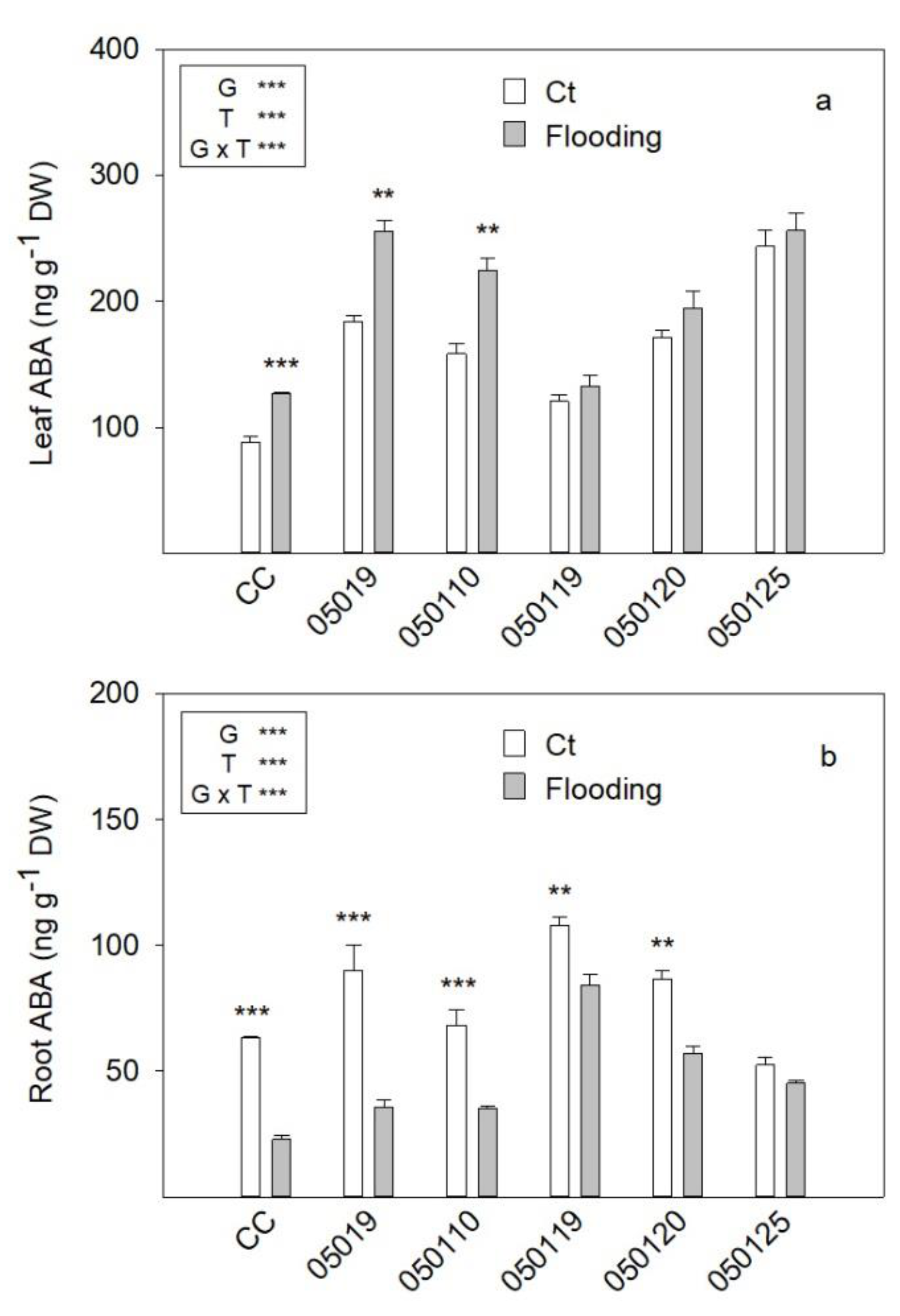
3.6. ABA Concentration
3.7. PIP Gene Expression
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sullivan, M.; Vantoai, T.; Fausey, N.R.; Beuerlein, J.; Parkinson, R.; Soboyejo, A. Evaluating On-Farm Flooding Impacts on Soybean. Crop Sci. 2001, 41, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, B.; Davies, F.S.; Crane, J.H. Responses of Subtropical and Tropical Fruit Trees to Flooding in Calcareous Soil. HortScience 2006, 41, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, R.; Mizutani, F.; Rutto, K. Selection of Rootstocks for Flooding and Drought Tolerance in Citrus Species. Pak. J. Biol. Sci. 2002, 5, 509–512. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Llosá, M.J.; Carrasco, J.L.; Perez-Amador, M.A.; Navarro, L.; Ancillo, G. Differential gene expression analysis provides new insights into the molecular basis of iron deficiency stress response in the citrus rootstock Poncirus trifoliata (L.) Raf. J. Exp. Bot. 2009, 61, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cuenca, M.-R.; Forner-Giner, M.Á.; Iglesias, D.J.; Primo-Millo, E.; Legaz, F. Strategy I responses to Fe-deficiency of two Citrus rootstocks differing in their tolerance to iron chlorosis. Sci. Hortic. 2013, 153, 56–63. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.-R.; Quiñones, A.; Forner-Giner, M.Á. Screening of ‘King’ mandarin (Citrus nobilis Lour) × Poncirus trifoliata ((L.) Raf.) hybrids as citrus rootstocks tolerants to iron chlorosis. Sci. Hortic. 2016, 198, 61–69. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.-R.; Primo-Capella, A.; Forner-Giner, M.Á. Screening of ‘King’ mandarin (Citrus nobilis Lour) × Poncirus trifoliata ((L.) Raf.) hybrids as salt stress-tolerant citrus rootstocks. Hortic. Environ. Biotechnol. 2021, 62, 337–351. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Syvertsen, J.P.; Gimeno, V.; Botía, P.; Perez-Perez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant. 2007, 130, 532–542. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.-R.; Quiñones, A.; Primo-Millo, E.; Forner-Giner, M.Á. Flooding Impairs Fe Uptake and Distribution in Citrus Due to the Strong Down-Regulation of Genes Involved in Strategy I Responses to Fe Deficiency in Roots. PLoS ONE 2015, 10, e0123644. [Google Scholar] [CrossRef] [Green Version]
- Partiya, R.; Fotouhi Ghazvini, R.; Fifaei, R.; Ghasemnezhad, M. Response of Different Citrus Genotypes to Continuous Flooding Conditions. Int. J. Hortic. Sci. Technol. 2018, 5, 253–263. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Zablotowicz, R.M.; Smith, M.L. Soil temperature and flooding effects on two species of citrus. Plant Soil 1983, 72, 3–12. [Google Scholar] [CrossRef]
- Forner, J.B.; Forner-Giner, M.A.; Alcaide, A. Forner-Alcaide 5 and Forner-Alcaide 13: Two New Citrus Rootstocks Released in Spain. HortScience 2003, 38, 629–630. [Google Scholar] [CrossRef] [Green Version]
- Forner-Giner, M.A.-M. Performance of Forner-Alcaide 5 and Forner-Alcaide 13, hybrids of Cleopatra mandarin × Poncirus trifoliate, as Salinity-Tolerant Citrus Rootstocks. J. Am. Pomol. Soc. 2009, 63, 72. [Google Scholar]
- González-Mas, M.C.; Llosa, M.J.; Quijano, A.; Forner-Giner, M.A. Rootstock Effects on Leaf Photosynthesis in ‘Navelina’ Trees Grown in Calcareous Soil. HortScience 2009, 44, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-N.; Ye, X.-R.; Molina, J.; Roose, M.L.; Mirkov, T.E. Sequence analysis of a 282-kilobase region surrounding the citrus Tristeza virus resistance gene (Ctv) locus in Poncirus trifoliata L. Raf. Plant Physiol. 2003, 131, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cuenca, M.-R.; Iglesias, D.J.; Forner-Giner, M.A.; Primo-Millo, E.; Legaz, F. The effect of sodium bicarbonate on plant performance and iron acquisition system of FA-5 (Forner-Alcaide 5) citrus seedlings. Acta Physiol. Plant. 2013, 35, 2833–2845. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Lloyd, J.; Kriedemann, P.E. Salinity and drought stress effects on foliar ion concentration, water relations, and photosynthetic characteristics of orchard citrus. Aust. J. Agric. Res. 1988, 39, 619–627. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Gamir, J.; Ancillo, G.; González-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner-Giner, M.A. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef]
- Arbona, V.; Gómez-Cadenas, A. Hormonal Modulation of Citrus Responses to Flooding. J. Plant Growth Regul. 2008, 27, 241. [Google Scholar] [CrossRef]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Yelenosky, G. Photosynthetic responses of citrus trees to soil flooding. Physiol. Plant. 1991, 81, 7–14. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Jover, S.; Quiñones, A.; Forner-Giner, M.Á.; Rodríguez-Gamir, J.; Legaz, F.; Primo-Millo, E.; Iglesias, D.J. Flooding affects uptake and distribution of carbon and nitrogen in citrus seedlings. J. Plant Physiol. 2012, 169, 1150–1157. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008, 132, 452–466. [Google Scholar] [CrossRef]
- Argamasilla, R.; Gómez-Cadenas, A.; Arbona, V. Metabolic and Regulatory Responses in Citrus Rootstocks in Response to Adverse Environmental Conditions. J. Plant Growth Regul. 2014, 33, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Sánchez, M.C.; Domingo, R.; Morales, D.; Torrecillas, A. Water relations of Fino lemon plants on two rootstocks under flooded conditions. Plant Sci. 1996, 120, 119–125. [Google Scholar] [CrossRef]
- Birner, T.P.; Steudle, E. Effects of anaerobic conditions on water and solute relations, and on active transport in roots of maize (Zea mays L.). Planta 1993, 190, 474–483. [Google Scholar] [CrossRef]
- Ehlert, C.; Maurel, C.; Tardieu, F.; Simonneau, T. Aquaporin-Mediated Reduction in Maize Root Hydraulic Conductivity Impacts Cell Turgor and Leaf Elongation Even without Changing Transpiration. Plant Physiol. 2009, 150, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Andersen, P.C.; Lombard, P.B.; Westwood, M.N. Effect of root anaerobiosis on the water relations of several Pyrus species. Physiol. Plant. 1984, 62, 245–252. [Google Scholar] [CrossRef]
- Davies, F.S.; Flore, J.A. Flooding, gas exchange and hydraulic root conductivity of highbush blueberry. Physiol. Plant. 1986, 67, 545–551. [Google Scholar] [CrossRef]
- Else, M.A.; Davies, W.J.; Malone, M.; Jackson, M.B. A Negative Hydraulic Message from Oxygen-Deficient Roots of Tomato Plants? (Influence of Soil Flooding on Leaf Water Potential, Leaf Expansion, and Synchrony between Stomatal Conductance and Root Hydraulic Conductivity). Plant Physiol. 1995, 109, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Forrest, K.L.; Bhave, M. Major intrinsic proteins (MIPs) in plants: A complex gene family with major impacts on plant phenotype. Funct. Integr. Genom. 2007, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant Aquaporins: Membrane Channels with Multiple Integrated Functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [Green Version]
- Javot, H.; Maurel, C. The Role of Aquaporins in Root Water Uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.-T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll Determination in Intact Tissues Using N,N-Dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [Green Version]
- Gaskin, P.; MacMillan, J. Discussion of spectra of TMSI ester TMSI ethers. In GCeMS of the Gibberellins and Related Compounds: Methodology and a Library of Spectra; University of Bristol: Bristol, UK, 1991. [Google Scholar]
- Bustin, S.A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Agüero, J.; del Carmen Vives, M.; Velázquez, K.; Pina, J.A.; Navarro, L.; Moreno, P.; Guerri, J. Effectiveness of gene silencing induced by viral vectors based on Citrus leaf blotch virus is different in Nicotiana benthamiana and citrus plants. Virology 2014, 460–461, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Estornell, L.H.; Gómez, M.D.; Pérez-Amador, M.A.; Talón, M.; Tadeo, F.R. Secondary abscission zones: Understanding the molecular mechanisms triggering stylar abscission in citrus. Acta Hortic. 2016, 1119, 65–72. [Google Scholar] [CrossRef]
- Brunner, A.M.; Yakovlev, I.A.; Strauss, S.H. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.-S.; Zou, Y.-N.; Huang, Y.-M. The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of waterlogging on growth, root system architecture and antioxidant enzyme activities of citrus seedlings. Fungal Ecol. 2013, 6, 37–43. [Google Scholar] [CrossRef]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Jackson, W.T. The Role of Adventitious Roots in Recovery of Shoots Following Flooding of the Original Root Systems. Am. J. Bot. 1955, 42, 816–819. [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Timbre, K.; Luqman, S.; Shukla, R.K. Mentha arvensis exhibit better adaptive characters in contrast to Mentha piperita when subjugated to sustained waterlogging stress. Protoplasma 2014, 251, 603–614. [Google Scholar] [CrossRef]
- Gibberd, M.R.; Gray, J.D.; Cocks, P.S.; Colmer, T.D. Waterlogging Tolerance Among a Diverse Range of Trifolium Accessions is Related to Root Porosity, Lateral Root Formation and ‘Aerotropic Rooting’. Ann. Bot. 2001, 88, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Arbona, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ. Exp. Bot. 2009, 66, 135–142. [Google Scholar] [CrossRef]
- Chouliaras, V.; Therios, I.; Molassiotis, A.; Patakas, A.; Diamantidis, G. Effect of Iron Deficiency on Gas Exchange and Catalase and Peroxidase Activity in Citrus. J. Plant Nutr. 2005, 27, 2085–2099. [Google Scholar] [CrossRef]
- Davis, T.D.; Jolley, V.D.; Valser, R.H.; Brown, J.C.; Blaylock, A.D. Net photosynthesis of Fe-efficient and Fe-inefficient soybean cultivars grown under varying iron levels. J. Plant Nutr. 1986, 9, 671–681. [Google Scholar] [CrossRef]
- Savé, R.; Serrano, L. Some physiological and growth responses of kiwi fruit (Actinidia chinensis) to flooding. Physiol. Plant. 1986, 66, 75–78. [Google Scholar] [CrossRef]
- Paul, M.J.; Driscoll, S.P.; Lawlor, D.W. Sink-Regulation of Photosynthesis in Relation to Temperature in Sunflower and Rape. J. Exp. Bot. 1992, 43, 147–153. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef] [Green Version]
- López-Serrano, L.; Penella, C.; San-Bautista, A.; López-Galarza, S.; Calatayud, A. Physiological changes of pepper accessions in response to salinity and water stress. Span. J. Agric. Res. 2017, 15. [Google Scholar] [CrossRef] [Green Version]
- Arbona, V.; Zandalinas, S.I.; Manzi, M.; González-Guzmán, M.; Rodriguez, P.L.; Gómez-Cadenas, A. Depletion of abscisic acid levels in roots of flooded Carrizo citrange (Poncirus trifoliata L. Raf. × Citrus sinensis L. Osb.) plants is a stress-specific response associated to the differential expression of PYR/PYL/RCAR receptors. Plant Mol. Biol. 2017, 93, 623–640. [Google Scholar] [CrossRef] [Green Version]
- García-Sánchez, F.; Syvertsen, J.P. Salinity Tolerance of Cleopatra Mandarin and Carrizo Citrange Citrus Rootstock Seedlings Is Affected by CO2 Enrichment during Growth. J. Am. Soc. Hortic. Sci. Jashs 2006, 131, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, J.G.; Syvertsen, J.P.; Botía, P.; García-Sánchez, F. Leaf Water Relations and Net Gas Exchange Responses of Salinized Carrizo Citrange Seedlings during Drought Stress and Recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Cadenas, A.; Iglesias, D.J.; Arbona, V.; Colmenero-Flores, J.M.; Primo-Millo, E.; Talón, M. Physiological and molecular responses of citrus to salinity. In Recent Research Developments in Plant Molecular Biology. Vol. 1, Part II; Research Signpost: Trivandrum, India, 2003; pp. 281–298. ISBN 8127100099. [Google Scholar]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Else, M.A.; Janowiak, F.; Atkinson, C.J.; Jackson, M.B. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soma, F.; Takahashi, F.; Suzuki, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Plant Raf-like kinases regulate the mRNA population upstream of ABA-unresponsive SnRK2 kinases under drought stress. Nat. Commun. 2020, 11, 1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Zhang, J.; Hsu, P.-K.; Ceciliato, P.H.O.; Zhang, L.; Dubeaux, G.; Munemasa, S.; Ge, C.; Zhao, Y.; Hauser, F.; et al. MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat. Commun. 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Gómez-Cadenas, A.; Arbona, V. Abscisic Acid as an Emerging Modulator of the Responses of Plants to Low Oxygen Conditions. Front. Plant Sci. 2021, 12, 755. [Google Scholar] [CrossRef]
- Yeung, E.; Bailey-Serres, J.; Sasidharan, R. After The Deluge: Plant Revival Post-Flooding. Trends Plant Sci. 2019, 24, 443–454. [Google Scholar] [CrossRef]
- Vandeleur, R.; Niemietz, C.; Tilbrook, J.; Tyerman, S.D. Roles of Aquaporins in Root Responses to Irrigation. Plant Soil 2005, 274, 141. [Google Scholar] [CrossRef]




| Annotation | Code a | Forward/Reverse Primer (5′–3′) | Reference |
|---|---|---|---|
| PIP1 | Ciclev10012384 | AGGATTACACGGAGCCACCT | [20] |
| TGCTTTTGGATTTGGACACG | |||
| PIP2 | Ciclev10029003 | TGTGTTCATGGTTCACTTGG | [20] |
| TGAATGGTCCAACCCAGAAG | |||
| Actin | Ciclev10025866 | CAGTGTTTGGATTGGAGGATCA | [42] |
| TCGCCCTTTGAGATCCACAT | |||
| UBC4 | Ciclev10009771 | TGGACGCTTCAGTCTGTTTG | [43] |
| TCGTCAATCACCCCTTCTTT |
| E | Ci | gs | An/Ci | An/E | Fv/Fm | Chl a + b | Leaf ABA | Root ABA | Ψω | Ψπ | Pip1 | Pip2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An | 0.868 *** | −0.918 *** | 0.848 *** | 0.960 *** | 0.938 *** | 0.687 * | 0.843 *** | −0.130 | 0.416 | 0.723 ** | 0.621 * | 0.2296 | 0.455 |
| E | −0.728 ** | 0.976 *** | 0.743 ** | 0.789 ** | 0.750 ** | 0.722 ** | −0.154 | 0.505 | 0.798 ** | 0.729 ** | 0.5779 * | 0.712 ** | |
| Ci | −0.6672 * | −0.924 *** | −0.992 *** | −0.640 * | −0.871 *** | 0.293 | −0.567 | −0.779 ** | −0.554 | −0.092 | −0.334 | ||
| gs | 0.726 ** | 0.719 ** | 0.737 ** | 0.699 * | −0.128 | 0.479 | 0.707 * | 0.701 * | 0.577 * | 0.7251 ** | |||
| An/Ci | 0.923 *** | 0.578 * | 0.7629 ** | −0.085 | 0.352 | 0.634 * | 0.574 * | 0.140 | 0.411 | ||||
| An/E | 0.688 * | 0.877 *** | −0.295 | 0.559 | 0.819 ** | 0.588 * | 0.154 | 0.384 | |||||
| Fv/Fm | 0.818 ** | −0.393 | 0.594 * | 0.791 ** | 0.683 * | 0.333 | 0.428 | ||||||
| Chl a + b | −0.417 | 0.672 * | 0.767 ** | 0.489 | 0.117 | 0.256 | |||||||
| Leaf ABA | −0.442 | −0.362 | 0.042 | 0.143 | −0.078 | ||||||||
| Root ABA | 0.569 | 0.524 | 0.327 | 0.415 | |||||||||
| Ψω | 0.656 * | 0.296 | 0.381 | ||||||||||
| Ψπ | 0.469 | 0.578 * | |||||||||||
| Pip1 | 0.890 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cuenca, M.-R.; Primo-Capella, A.; Forner-Giner, M.Á. Screening of ‘King’ Mandarin Hybrids as Tolerant Citrus Rootstocks to Flooding Stress. Horticulturae 2021, 7, 388. https://doi.org/10.3390/horticulturae7100388
Martínez-Cuenca M-R, Primo-Capella A, Forner-Giner MÁ. Screening of ‘King’ Mandarin Hybrids as Tolerant Citrus Rootstocks to Flooding Stress. Horticulturae. 2021; 7(10):388. https://doi.org/10.3390/horticulturae7100388
Chicago/Turabian StyleMartínez-Cuenca, Mary-Rus, Amparo Primo-Capella, and María Ángeles Forner-Giner. 2021. "Screening of ‘King’ Mandarin Hybrids as Tolerant Citrus Rootstocks to Flooding Stress" Horticulturae 7, no. 10: 388. https://doi.org/10.3390/horticulturae7100388
APA StyleMartínez-Cuenca, M.-R., Primo-Capella, A., & Forner-Giner, M. Á. (2021). Screening of ‘King’ Mandarin Hybrids as Tolerant Citrus Rootstocks to Flooding Stress. Horticulturae, 7(10), 388. https://doi.org/10.3390/horticulturae7100388






