Abstract
Cherry orchards are transitioning to high-density plantings and dwarfing rootstocks to maximize production, but the response of these rootstocks to drought stress is poorly characterized. We used a 16-container, automated lysimeter system to apply repeated water stress to ungrafted Krymsk® 5 and 6 rootstocks during two growing cycles. Drought stress was imposed by withholding irrigation until the daily transpiration rate of each tree was 25% and 30% of the unstressed rate during the first trial and second trial, respectively. After this point was reached, the root-zone water status was restored to field capacity. Whole-tree transpiration measurements were supplemented with leaf-level gas-exchange measurements. Krymsk® 6 had a higher rate of photosynthesis, more vigorous vegetative growth and less conservative stomatal regulation during incipient drought than Krymsk® 5. At harvest, carbon partitioning to roots was greater in Krymsk® 6 than Krymsk® 5. The conservative rate of water use in Krymsk® 5 could be a function of greater stomatal control or reduced carbon partitioning to roots, which thereby limited transpiration rates. Further studies are needed to confirm that these results are applicable to trees grown using a common grafted scion under field conditions.
1. Introduction
Sweet (Prunus avium L.) and tart (Prunus cerasus L.) cherries are significant economic crops in many regions of the world with the top five producers being Turkey, the U.S., China, Iran, and Chile [1,2]. Commercial orchard production of sweet cherry has shifted to the use of dwarfing rootstocks and high-density plantings to maximize yields per hectare [3,4]. Dwarfing rootstocks increase precocity and reduce costs associated with pruning and harvesting while shortening return on investment times compared to full-sized trees [5,6]. At the same time, climate change and increasing competition from population growth is making water scarcer for irrigation [7]. Plants can overcome drought through avoidance or adaptation. Morphological adaptations can include reduced leaf area, greater stomatal regulation, more vertical leaf orientation, and larger root–shoot biomass ratios. Physiological adaptive measures can also play a role in drought tolerance, including osmotic adjustment, antioxidant production, and hormone regulation [8]. In the field, vigorous rootstocks may be less susceptible to drought because large root-zone volumes and deeper root penetration allow greater access to soil moisture [9]. However, some studies indicate that dwarfing rootstocks can confer drought tolerance by conserving water through greater stomatal regulation, increased ABA production, and increasing leaf and stem water potentials. The dwarfing nature of the rootstocks also serves to reduce vegetative growth, which limits transpiration [10,11,12]. Greater drought tolerance is required as climate change makes precipitation patterns less predictable and extreme weather events more common [13]. Tolerance of water stress is also desirable to facilitate implementation of deficit irrigation to improve orchard management and fruit quality [14].
The degree to which dwarfing rootstocks can withstand drought can vary significantly among dwarfing lines. The Krymsk® series rootstocks were selected by Dr. Guennadi Eramin of the Krymsk Experimental Breeding Station in the Krasnodar Krai region in Russia near the Black Sea [15]. Krymsk® 5 (K5) (Prunus fruticosa × Prunus serrulata var. lannesiana) is a semi-dwarfing rootstock that performs well under a range of soil types and has been reported to tolerate heat and cold stress. Krymsk® 6 (K6) (Prunus cerasus × (Prunus cerasus × Prunus maackii)) was originally reported to be more dwarfing than K5 but it has subsequently been found to exhibit variable degrees of dwarfing in relation to K5 [16]. Krymsk® 6 is tolerant of water stress and adapted to cold and heat stress. Both cultivars are sensitive to prune dwarf virus and Prunus necrotic ringspot virus [17]. Despite widespread use, there is little quantitative information on Krymsk® series rootstocks’ response to drought.
Common physiological parameters for drought assessment include stem and leaf water potentials, canopy temperature, stomatal conductance, sap flow, and trunk diameter variation [18]. However, when focusing on any one method, there is the possibility of losing an understanding of the whole plant response to water stress [19]. Weighing lysimeters incorporate whole tree responses and results can be interpreted directly without scaling or extensive data processing [20]. Lysimeters also avoid the problems of localized sensor response and are unaffected by growth rate.
Our objective was to investigate the response of K5 and K6 rootstocks to acute severe drought and their ability to recover using a weighing lysimeter. Results provide insights into the differing responses of both cultivars during drought conditions and will help breeders and growers make informed decisions in rootstock selections.
2. Materials and Methods
Ungrafted K5 and K6 rootstocks grown in Ellepot™ containers were obtained from a commercial nursery (Sierra Gold Nurseries, Yuba City, CA, USA) in the fall. Trees for the first trial were transplanted into 20 L containers filled with an 80% silty clay loam, 20% peat media amended with 4.15 g/L slow-release fertilizer (Polyon 18-6-12, 5-6 Mo.) and allowed to establish for 3 months in a greenhouse environment. Trees for the second trial were transplanted into 3 L containers with the same media and allowed to overwinter outdoors. The following spring, these trees were transplanted into 20 L containers and grown for 1.5 months. For both trial establishment periods, trees were watered as needed (generally every 2–3 days) to maintain well-watered conditions. In both trials, once trees were well-established in the 20 L containers, eight trees per cultivar were selected for uniformity and transferred onto a 16-cell weighing lysimeter system (Figure 1). This greenhouse system has been described by [21,22] but will be briefly reviewed here. Each load cell platform consisted of a 35 kg beam load cell anchored between two aluminum plates (Figure 2). Load cells were spaced approximately 1.5 m away from each other in all directions. Containers were white plastic to minimize thermal loading. During the second trial, containers were wrapped with insulation to further minimize thermal changes in the root zone. After establishing field capacity, drain holes in the bottom were plugged to prevent leaching. The soil surface was covered with aluminum foil to minimize evaporation. Load cells were connected to a data logger that continuously recorded container mass. Load cells were able to provide resolution down to 0.1 g and after calibration had an average error rate of less than 1% due to temperature effects. Daily transpiration was calculated from change in initial and final mass every 30 min and summed over 24 h. The root zone was brought to field capacity by irrigating until leachate was observed from the drainage holes and then noting the container mass after 24 h from when leaching stopped. Individual container masses were programmed into the data logger as the upper soil moisture threshold. For both trials, trees were lightly pruned to equalize initial transpiration rates. Peak transpiration rates under field capacity were noted for each individual tree before the start of each trial. Transpiration rates were then allowed to decline to 25% of peak values before irrigation was triggered during the first trial. This was increased to 30% of peak transpiration rates for the second trial because of leaf burn and defoliation observed during the first trial. Transpiration responses were compared to initial transpiration rates, before drought was imposed, for each tree. A relay driver was used to control 16 solenoid valves that were activated when individual load cells fell below a pre-programmed lower threshold.

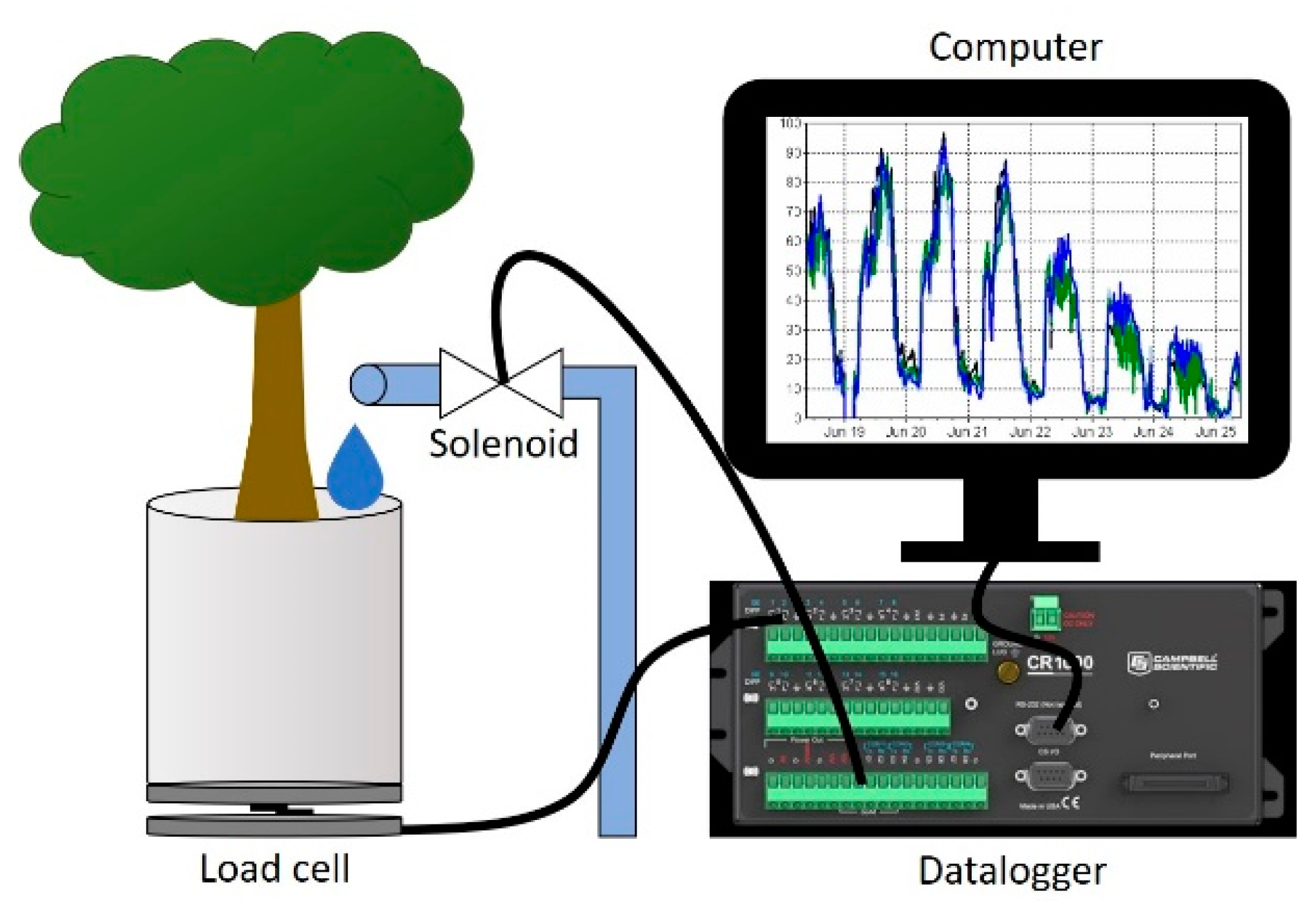
Figure 1.
The 16-cell lysimeter system used to assess transpiration rates for Krymsk® 5 and 6 rootstocks for Trial 1 (A) and Trial 2 (B). Containers were covered with aluminum foil to minimize evaporation from the soil surface. Three drought cycles were imposed during each trial period.
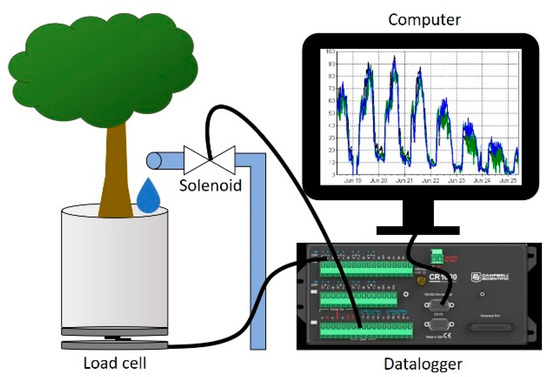
Figure 2.
Diagram of individual lysimeter cell consisting of a 35 kg weighing load cell enclosed by two aluminum plates and connected to a data logger. Data loggers also controlled irrigation through solenoid valves and drip tubes connected to each bucket. Rootstocks had water withheld until transpiration rates declined by 75% during Trial 1, and 70% in Trial 2. Once transpiration rates declined to these thresholds irrigation was independently triggered for each individual tree, returning the soil to field capacity.
During the second trial, gas exchange measurements were made with a portable photosynthesis system (Li-Cor 6800, Li-Cor, Lincoln, NE, USA) the day of peak drought stress, and two days after irrigation when recovery transpiration rates had peaked. Chamber conditions were set to the following parameters: fan speed of 5000 rpm, flow rate of 600 μmol s−1, CO2 concentration of 400 μmol mol−1, leaf temperature of 25 °C, leaf vapor pressure of 1.5 kPa, and irradiance of 700 μmol m−2 s−1 with 10% blue and 90% red. Three trees per treatment were measured with three leaves sampled per tree. After clamping on a leaf, the chamber was allowed 10 min to come to equilibrium before taking a running average of gas exchange parameters for 20 min.
For both trials, supplemental lighting was provided by nine 1000-watt high-pressure sodium lights. During the first trial, average day/night greenhouse temperatures were 25/18 °C and daily light integral averaged 13 mol m−2 d−1. During the second trial, average day/night greenhouse temperatures were 30/19 °C and daily light integral averaged 21 mol m−2 d−1. Trees were destructively harvested at the end of each trial. Trunk diameter at 3 cm above the soil surface was measured and trunk cross-sectional area was calculated. Leaves were removed and weighed and area (LI-COR, model LI-3100C area meter, Lincoln, NE) of leaves representing 10% of leaf fresh weight was determined, and whole-canopy leaf area extrapolated. Leaf and woody tissue dry mass were measured after drying for three days at 80 °C. Root balls were washed to remove all bound soil with care taken to retain as many fine roots as possible. All tissues were oven dried for three days at 80 °C before final measurement of dry mass. The percentage of root mass was calculated as a function of dry root mass over total dry mass.
Both trials were completely randomized on the lysimeter system with rootstock cultivar treated as a fixed effect. Each rootstock cultivar had eight replicate trees. Cumulative transpiration, number of dry-down days, and gas exchange parameters were analyzed utilizing two-way repeated measures multivariate analysis of variance (MANOVA). All other variables were analyzed using a two-way analysis of variance (ANOVA). All data were analyzed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
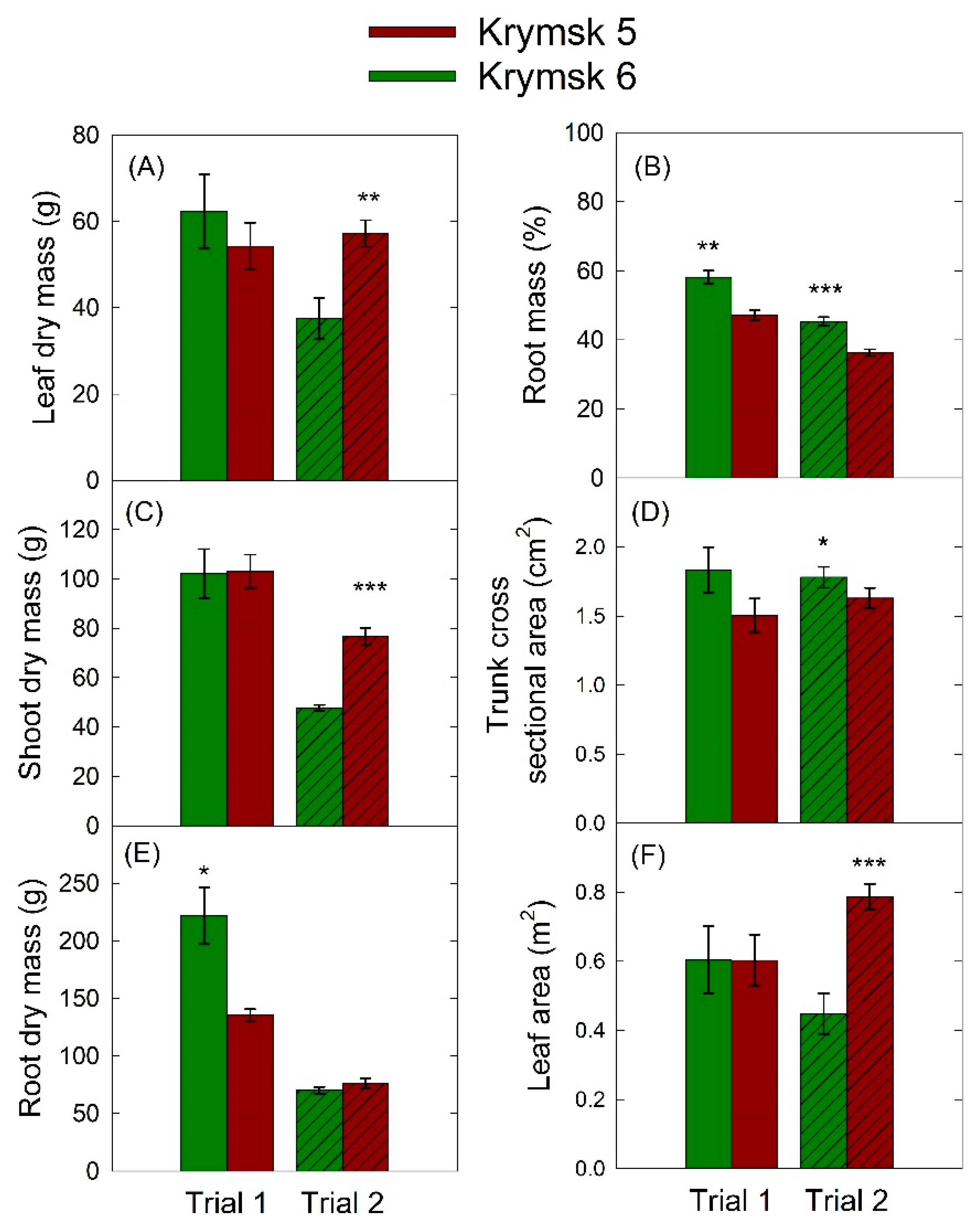
In the first trial, K6 had significantly more dry root mass (p = 0.01) and a higher percentage of root mass (p < 0.01) than K5 rootstocks (Figure 3), but there were no statistical differences among leaf or shoot dry mass, trunk cross-sectional area, or leaf area. In the second trial, trunk cross-sectional area (p = 0.04) and percentage of root mass (p < 0.001) were higher in the K6, though root dry mass was not significantly different between cultivars (p = 0.22). Canopy harvest parameters were higher in the K5 series including leaf dry mass (p < 0.01), leaf area (p < 0.001), and total shoot dry mass (p < 0.001).
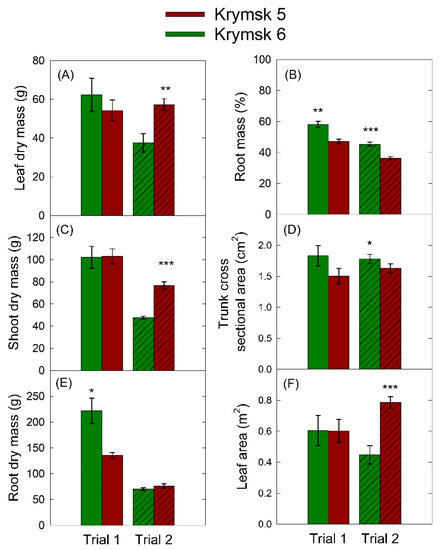
Figure 3.
Selected average destructive harvest parameters: (A) leaf dry mass, (B) percentage root mass, (C), shoot dry mass, (D) trunk cross-sectional area, (E) root dry mas, and (F) leaf area for rootstocks Krymsk® 5 (red) and 6 (green) over the course of two trials in which drought was imposed using a 16-cell lysimeter system. Each load cell was independently controlled, and when cumulative transpiration fell below 75% of peak transpiration for the first trial, and 70% for the second trial, irrigation was triggered. Bars represent standard error from 8 replicate trees. *** p < 0.001; ** p < 0.01; * p < 0.05.
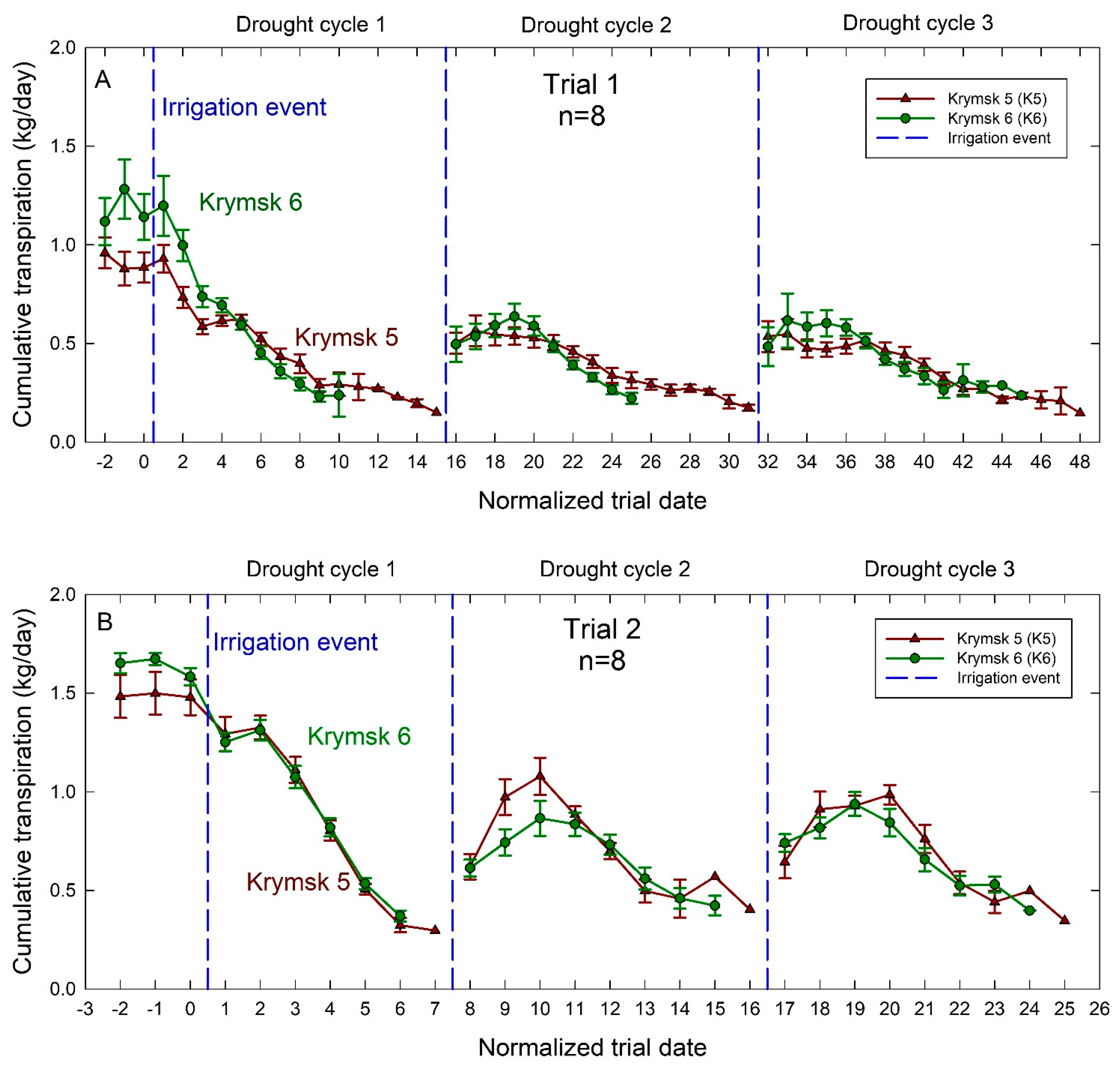
The number of days to reach the minimum transpiration threshold, thus depleting soil available water, was significantly greater (p = 0.02) during the first trial with K5 averaging 12 days and K6 averaging 8.8 days across all trees over three dry-down events (Figure 4). During the second trial, there was no significant difference between rootstocks in the number of days to dry down to the minimum threshold between treatments with both rootstocks taking approximately 6 days.
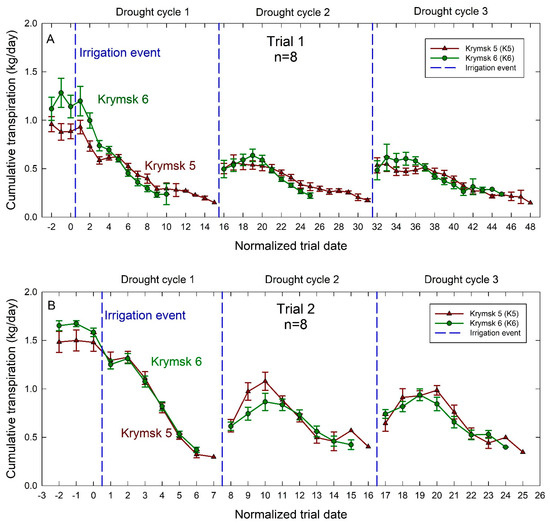
Figure 4.
Average daily transpiration rates for Krymsk® 5 (red triangles) and Krymsk® 6 (green circles) rootstocks during Trial 1 (A) and Trial 2 (B). Rootstocks had water withheld until transpiration rates declined by 75% during Trial 1 (top), and 70% in Trial 2 (bottom). Once transpiration rates declined to these thresholds, irrigation (blue dotted lines) was independently triggered for each individual tree, returning the soil to field capacity. Normalized trial date is used based on when each tree began its drought cycle. Bars represent standard error from 8 replicate trees.
In both trials, there was no statistical difference between K5 and K6 transpiration rates. For the first trial, pre-trial transpiration rates had an average of 1.2 kg/day in the K6, and 0.9 kg/day in the K5. For the second trial, pre-dry-down transpiration rates in K6 reached 1.7 kg/day and K5 transpiration rates peaked at 1.5 kg/day. For both the first and second trials, transpiration rates did not recover to their pre-drought levels after the first drought event (Figure 4). Both rootstocks had similar recovery (peak) transpiration rates following the second and third drought events in both trials. However, during the second trial after the first drought event, K5 tended to have higher average transpiration rates than K6 on the day before peak transpiration rates were recorded.
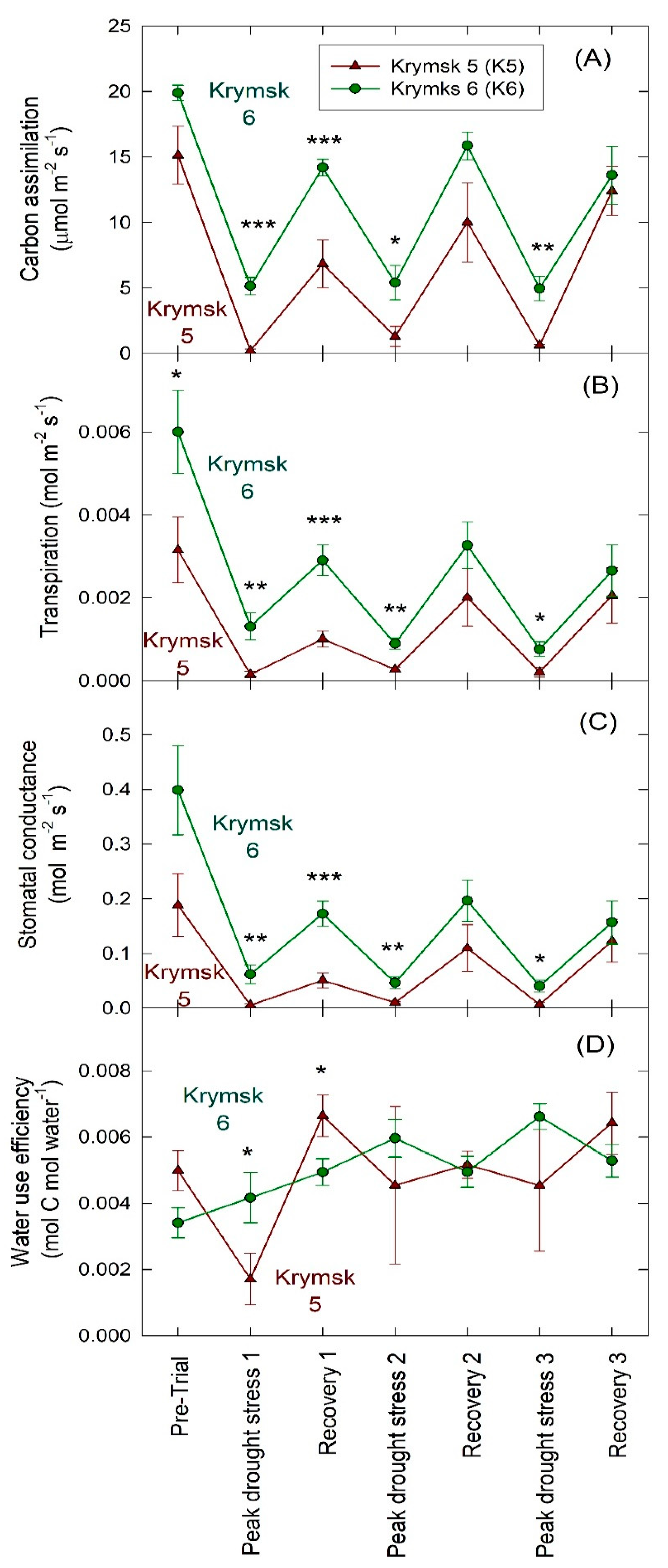
K6 rootstocks had higher rates of carbon assimilation (p = 0.01), transpiration (p < 0.01), and stomatal conductance (p < 0.01) in the second trial (Figure 5). There was no difference in water use efficiency (p = 0.67) between rootstocks; however, water efficiency increased significantly over the course of the trial in K6 (p = 0.01) while no significant increase in water use efficiency over time was noted in K5 (p = 0.10).
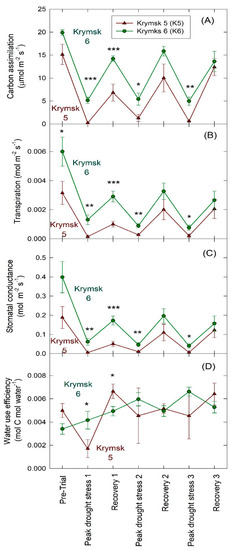
Figure 5.
Leaf level gas exchange parameters: (A) carbon assimilation, (B) transpiration, (C) stomatal conductance (gs), and (D) water use efficiency (WUE) for rootstocks Krymsk® 5 (red triangles) and 6 (green circles) during Trial 2. Three trees per treatment were measured with three leaves sampled per tree. Gas exchange chamber was allowed to come to equilibrium for 10 min before collecting gas exchange parameters for 20 min. Trees were sampled before drought imposition, the day of peak drought stress, and two days after irrigation when recovery transpiration rates peaked through three dry-down periods. *** p < 0.001; ** p < 0.01; * p < 0.05.
4. Discussion
There were significant interactions between trials and leaf dry mass, shoot dry mass, root dry mass, and leaf area (Figure 3). Differences in seasonality and overwintering of the trees used in the second trial may have impacted trial results. Trees for the first trial were received in the late fall, transplanted and allowed to establish for three months before being placed on the lysimeter system. Because it was already late in the season, it is possible that these trees had begun downregulation of their metabolism in preparation for winter dormancy. Artificial extension of the growing season in a greenhouse setting may have resulted in more muted physiological responses, as can be seen in lowered transpiration rates for the first trial (Figure 4A). Trees for the first trial had a higher shoot and root mass than those in the second trial; however, this was a result of being given three months to establish before the start of the trial, partially due to their slower growth rate. For the second trial, vigorous growth occurred in both rootstocks after emerging from the over wintering period. This vigorous growth rate continued when plants were placed on the lysimeter system 45 days later. The shorter establishment time was beneficial as smaller trees were better accommodated by the lysimeter system. Despite these limitations, several patterns were apparent in both trials.
Over the course of the first trial, K6 had significantly fewer days to minimum transpiration thresholds (p = 0.02). During the second trial, while the difference in average days to minimum transpiration was not significant (p = 0.75), K6 trees showed a pattern of using available water more quickly than K5 (Figure 4B). This was corroborated by higher leaf-level carbon assimilation (Figure 5A), transpiration (Figure 5B), and stomatal conductance (Figure 5C) in K6 measured by gas exchange throughout the second trial. K6 also showed a significant increase in WUE (Figure 5D) over the course of the second trial. Canopy effects can play a significant role in plant water use strategy and adaptation to drought stress through increased stomatal regulation and decreased leaf area [23,24]. Given the role of canopy effects in drought responses, consideration of physiological changes in grafted trees is necessary before extrapolating from studies on ungrafted rootstocks. However, previous studies on Prunus species have found that more vigorous rootstocks increase stem water potential, CO2 assimilation rate, stomatal conductance, intercellular CO2 concentration, and photochemical efficiency in their grafted scions [25]. Rootstock growth potential has also been positively correlated to cytokinin levels in xylem sap, which in turn impacts shoot vigor [26]. These changes to whole-plant physiology suggest that responses of ungrafted rootstocks can provide a good indication of responses in grafted trees. Furthermore, research in the Netherlands found Kordia scions grafted to K6 rootstocks produced more fruit per tree than those grafted to K5, which suggests that greater carbon assimilation may be conferred by K6 rootstocks [27]. Carbon assimilation rates of K6 were also higher than those noted in the sweet cherry variety Black Star grafted to semi-dwarfing rootstock Gisela™ 6, again suggesting more vigorous responses from K6 compared to other dwarfing rootstocks [28].
Consideration of parent species is useful in understanding results from this study. K6 is a hybrid between the domesticated P. cerasus and another hybrid consisting of P. cerasus × P. maackii. Prunus cerasus is thought to have originated through a natural cross between P. avium and P. fruticosa, which has been used in domesticated settings since 300 BCE. This is thought to make it more responsive to intensive management [29]. K5, by contrast, is a hybrid between the relatively wild species of P. fruticosa and the ornamental P. serrulata. Prunus fruticosa is a relatively short, wild shrub (0.5–1 m) that confers dwarfing qualities and drought and frost tolerance when crossed with other Prunus species [30]. Prunus serrulata, while cultivated in landscaped settings, has not been the focus of agronomic breeding and may be less developed for vigorous growth [31].
During the first study, there was no significant difference in leaf area (p = 0.99) or leaf dry mass (p = 0.47) between cultivars, indicating equivalent canopy sizes, however K5 took significantly longer to reach irrigation thresholds. During the second trial, differences in time to reach irrigation thresholds was not significant between K5 and K6 but K5 rootstocks had a significantly larger canopy with greater leaf area (p < 0.01) and leaf dry mass (p < 0.01) (Figure 3). In both trials, K5 had a lower percentage of root mass as a function of total dry weight and lower leaf level transpiration rates, except for in the second recovery phase during the second trial (Figure 5B). This pattern of more conservative water use in K5 could be a function of either greater stomatal control or a smaller root system, which in turn limited transpiration rates. More conservative water usage by K5 rootstocks may be due to their relatively less domesticated lineage, as prolonged severe drought is more common in wild land settings. K6 rootstocks, with a predominance of P. cesarus genetics, may be more adapted to high-input agricultural settings in which resources are generally abundant and trees are bred for vigor.
Drought events in the field often occur gradually, whereas the imposition of individual drought events in this study occurred over 6 to 9 days. Osmotic adjustment is dependent on the rate of stress development with more progressive water stress allowing for greater upregulation of osmotic potential in plant tissues [32,33]. Significantly reduced leaf osmotic potentials (Ψπ) have been observed in peach scions grafted to Prunus rootstocks after 16 days and one month of progressive drought when compared to well-watered controls [34,35]. This contrasts with findings in ungrafted P. persica where, following rapid imposition of drought over the course of 8 days, stressed trees showed no significant differences in Ψπ when compared to well-watered controls [36]. The more rapid onset of individual drought events in this study may have limited the initial degree of osmotic adjustment. However, the repeated cycling of drought and rewatering should have allowed for osmotic adjustment over the course of the trials, which lasted 48 days for the first trial and 25 days in the second trial.
5. Conclusions
Neither K5 nor K6 returned to pre-drought transpiration rates after water was restored, but Krymsk® 6 maintained higher rates of photosynthesis, transpiration, and growth during drought. Krymsk® 6 had a greater root mass fraction in both trials and appears to be better adapted to repeated cycles of drought stress. Further studies are needed to determine if these patterns in ungrafted rootstocks are consistent when rootstocks are grown with a common grafted scion and under field conditions.
Author Contributions
Conceptualization, B.B. (Bruce Bugbee) and B.B. (Brent Black); methodology, B.B. (Bruce Bugbee) and B.B. (Brent Black); formal analysis, W.W.; investigation, W.W.; resources, B.B. (Bruce Bugbee) and B.B. (Brent Black); data curation, W.W.; writing—original draft preparation, W.W.; writing—review and editing, W.W., B.B. (Bruce Bugbee) and B.B. (Brent Black); visualization, W.W.; supervision, B.B. (Bruce Bugbee) and B.B. (Brent Black); project administration, B.B. (Bruce Bugbee) and B.B. (Brent Black); funding acquisition, B.B. (Bruce Bugbee) and B.B. (Brent Black). All authors have read and agreed to the published version of the manuscript.
Funding
This project was made possible by funding from a USDA-NIFA Specialty Crop Block Grant administered by the Utah Department of Agriculture, the Utah Water Initiative grant program, and by the Utah Agriculture Experiment Station, Utah State University, journal paper number UAES #9460.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank Sierra Gold Nurseries for their donation of trees and Alec Hay for his extensive technical support and intellectual contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milošević, T.; Milošević, N.; Mladenović, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 265, 109236. [Google Scholar] [CrossRef]
- Lang, G.A. The cherry industries in the USA: Current trends and future perspectives. In Proceedings of the VIII International Cherry Symposium, Yamagata, Japan, 28 February 2019; pp. 119–132. [Google Scholar]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Micheli, A.; Boini, A.; Perulli, G.; Bresilla, K.; Corelli Grappadelli, L. Physiological responses to rootstocks vigor in cherry: Why dwarfing is efficient? In Proceedings of the XXX International Horticultural Congress IHC2018: International Symposium on Cultivars, Rootstocks and Management Systems of 1281, Istanbul, Turkey, 12 August 2018; pp. 487–492. [Google Scholar]
- Robinson, T. The Evolution towards More Competitive Apple Orchard Systems in the USA. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Enhancing Economic and Environmental 772, Seoul, Korea, 13 August 2006; pp. 491–500. [Google Scholar] [CrossRef]
- Cline, J.A. Planting density and size-controlling rootstocks influence the performance of Montmorency tart cherry (Prunus cerasus L.). Can. J. Plant Sci. 2020, 100, 16–28. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Rodriguez-Gamir, J.; Martinez-Alcantara, B.; Quinones, A.; Iglesias, D.J.; Primo-Millo, E.; Forner, J. Performance of Navel orange trees grafted onto two new dwarfing rootstocks (Forner-Alcaide 517 and Forner-Alcaide 418). Sci. Hortic. 2014, 179, 376–387. [Google Scholar] [CrossRef]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3239–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. ISBN 9783642326530. [Google Scholar]
- Tworkoski, T.; Fazio, G. Effects of Size-Controlling Apple Rootstocks on Growth, Abscisic Acid, and Hydraulic Conductivity of Scion of Different Vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Policarpo, M.; Webster, A.D.; Kingswell, G. Drought tolerance of clonal Malus determined from measurements of stomatal conductance and leaf water potential. Tree Phys. 2000, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hajagos, A.; Vegvari, G. Investigation of tissue structure and xylem anatomy of eight rootstocks of sweet cherry (Prunus avium L.). Trees 2013, 27, 53–60. [Google Scholar] [CrossRef]
- Ballesta, M.M.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Naor, A. Irrigation Scheduling and Evaluation of Tree Water Status in Deciduous Orchards. Hortic. Rev. 2006, 32, 111–165. [Google Scholar] [CrossRef]
- Eremin, G. Prunus Plant Named “VSL-2”. U.S. Patent Application 09/880953, 20 November 2003. [Google Scholar]
- Roper, T.; Black, B.; Stasiak, R.; Marini, M.; Cline, J.; Robinson, T.; Lang, G.; Anderson, L.; Anderson, R.; Freer, J.; et al. Performance of “Montmorency” Sour Cherry (Prunus cerasus L.) on Size-Controlling Rootstocks at Six NC140 Trial Locations in North America. J. Am. Pomol. Soc. 2019, 73, 168–177. [Google Scholar]
- Long, L.E.; Brewer, L.J.; Kaiser, C. Cherry Rootstocks for the Modern Orchard. OSU Extension Service. Oregon State University: Pacific Northwest Extension Publication. 2014. Available online: http://extension.oregonstate.edu/wasco/sites/default/files/cherryrootstocksmodern-long.pdf (accessed on 15 November 2020).
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Ben-Gal, A.; Kool, D.; Agam, N.; van Halsema, G.E.; Yermiyahu, U.; Yafe, A.; Presnov, E.; Erel, R.; Majdop, A.; Zipori, I.; et al. Whole-tree water balance and indicators for short-term drought stress in non-bearing “Barnea” olives. Agric. Water Manag. 2010, 98, 124–133. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Weighing lysimeter systems for quantifying water use and studies of controlled water stress for crops grown in low bulk density substrates. Agric. Water Manag. 2011, 98, 967–976. [Google Scholar] [CrossRef]
- Chard, J.; van Iersel, M.; Bugbee, B. Mini-Lysimeters to Monitor Transpiration and Control Drought Stress: System Design and Unique Applications. 2010. Available online: https://digitalcommons.usu.edu/cpl_techniquesinstruments/18/ (accessed on 15 November 2020).
- Wheeler, W.; Wytsalucy, R.; Black, B.; Cardon, G.; Bugbee, B. Drought Tolerance of Navajo and Lovell Peach Trees: Precision Water Stress Using Automated Weighing Lysimeters. HortScience 2019, 54, 799–803. [Google Scholar] [CrossRef]
- Dos Santos, I.C.; de Almeida, A.-A.F.; Pirovani, C.P.; Costa, M.G.C.; Silva, M.F.D.G.F.D.; Bellete, B.S.; Freschi, L.; Filho, W.S.; Filho, M.A.C.; Gesteira, A.D.S. Differential accumulation of flavonoids and phytohormones resulting from the canopy/rootstock interaction of citrus plants subjected to dehydration/rehydration. Plant Physiol. Biochem. 2017, 119, 147–158. [Google Scholar] [CrossRef]
- Rieger, M.; Duemmel, M.J. Comparison of drought resistance among Prunus species from divergent habitats. Tree Physiol. 1992, 11, 369–380. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Sorce, C.; Mariotti, L.; Lorenzi, R.; Massai, R. Hormonal factors involved in the control of vigor of grafted peach [Prunus persica (L.) Batsch] trees and hybrid rootstocks. Adv. Hort. Sci. 2007, 21, 68–74. [Google Scholar]
- Maas, F.M.; Balkhoven-Baart, J.; van der Steeg, P.A.H. Evaluation of Krymsk® 5 (VSL-2) and Krymsk® 6 (LC-52) as rootstocks for sweet cherry “Kordia”. Acta. Hortic. 2014, 1058, 531–536. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Boini, A.; Perulli, G.D.; Bresilla, K.; Venturi, M.; Grappadelli, L.C. Sweet cherry water relations and fruit production efficiency are affected by rootstock vigor. J. Plant Physiol. 2019, 237, 43–50. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Hernández, A.; López-Corrales, M.; Ruiz-Moyano, S.; Córdoba, M.G.; Martín, A. Composition of the cherry (Prunus avium L. and Prunus cerasus L.; Rosaceae). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: London, UK, 2016; pp. 127–147. [Google Scholar]
- Barać, G.; Ognjanov, V.; Vidaković, D.O.; Dorić, D.; Ljubojević, M.; Dulić, J.; Miodragović, M.; Gašić, K. Genetic diversity and population structure of European ground cherry (Prunus fruticosa Pall.) using SSR markers. Sci. Hortic. 2017, 224, 374–383. [Google Scholar] [CrossRef]
- Kato, S.; Matsumoto, A.; Yoshimura, K.; Katsuki, T.; Iwamoto, K.; Kawahara, T.; Mukai, Y.; Tsuda, Y.; Ishio, S.; Nakamura, K.; et al. Origins of Japanese flowering cherry (Prunus subgenus Cerasus) cultivars revealed using nuclear SSR markers. Tree Genet. Genomes 2014, 10, 477–487. [Google Scholar] [CrossRef]
- Jones, M.M.; Rawson, H.M. Influence of Rate of Development of Leaf Water Deficits upon Photosynthesis, Leaf Conductance, Water Use Efficiency, and Osmotic Potential in Sorghum. Physiol. Plant. 1979, 45, 103–111. [Google Scholar] [CrossRef]
- Wang, Z.; Quebedeaux, B.; Stutte, G.W. Osmotic Adjustment: Effect of Water Stress on Carbohydrates in Leaves, Stems and Roots of Apple. Funct. Plant Biol. 1995, 22, 747–754. [Google Scholar] [CrossRef]
- Jiménez, S.; Dridi, D.J.; Gutiérrez, D.; Moret, J.J.; Irigoyen, M.A.; Moreno, Y.; Gogorcena, Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Phys. 2013, 33, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Mellisho, C.D.; Cruz, Z.N.; Conejero, W.; Ortuño, M.F.; Rodríguez, P. Mechanisms for drought resistance in early maturing cvar Flordastar peach trees. J. Agric. Sci. 2011, 149, 609–616. [Google Scholar] [CrossRef]
- Escobar-Gutiérrez, A.J.; Zipperlin, B.; Carbonne, F.; Moing, A.; Gaudillère, J.P. Photosynthesis, carbon partitioning and metabolite content during drought stress in peach seedlings. Funct. Plant Biol. 1998, 25, 197–205. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).