Sap Analysis: A Powerful Tool for Monitoring Plant Nutrition
Abstract
:1. Introduction
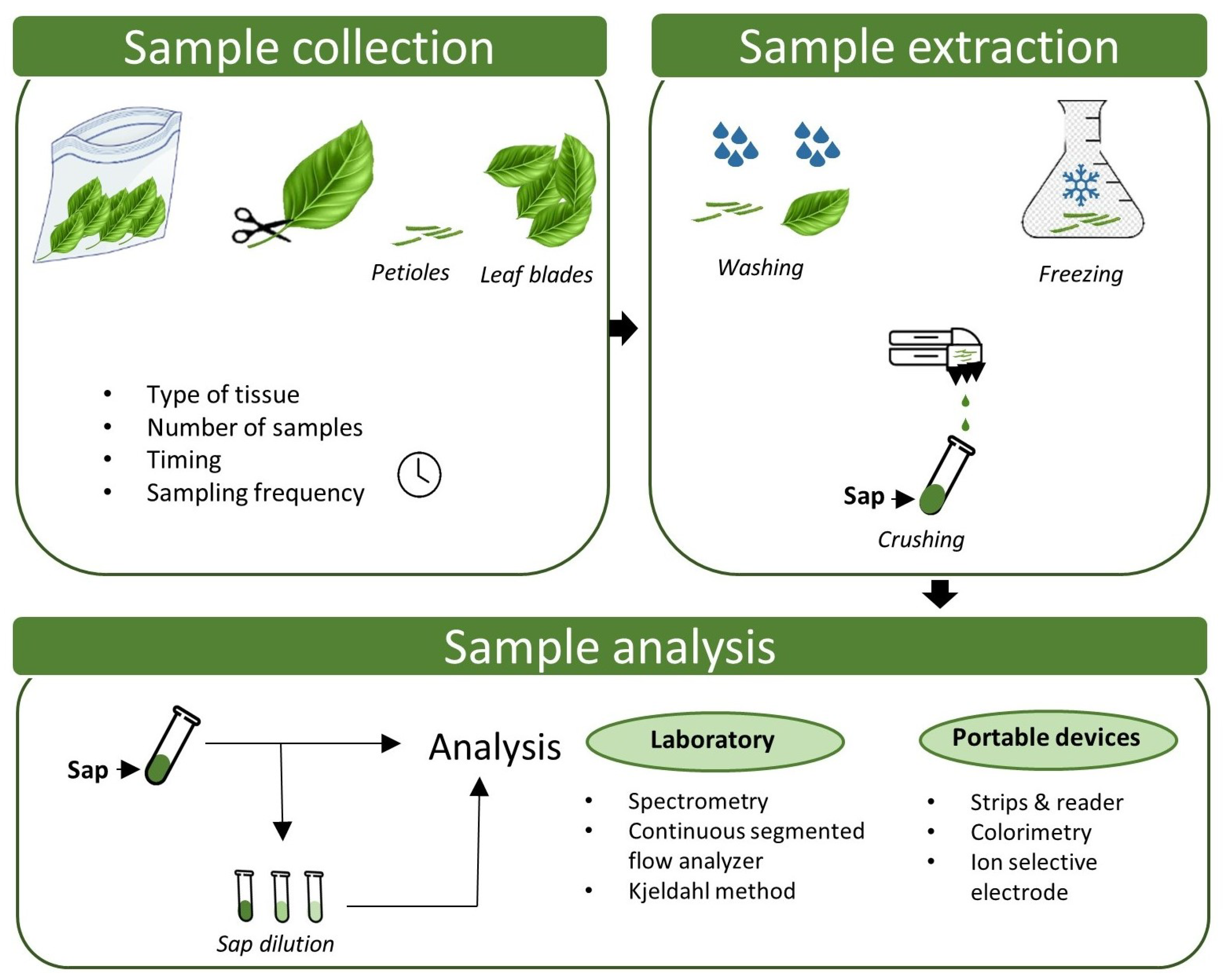
2. Procedures for Sap Analysis
2.1. Sample Collection
2.1.1. Type of Tissue
2.1.2. Timing and Frequency
2.2. Sample Extraction
2.3. Sample Analysis
3. Sap as a Potential Nutrition Index for Citrus
4. Sap as a Nutrition Index for Other Crops
4.1. Vegetables
4.2. Strawberry
4.3. Grapevine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Incrocci, L.; Massa, D.; Pardossi, A. New trends in the fertigation management of irrigated vegetable crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peña-Fleitas, M.T.; Padilla, F.M.; Gallardo, M.; Thompson, R.B. Petiole sap nitrate concentration to assess crop nitrogen status of greenhouse sweet pepper. Sci. Hortic. 2021, 285, 110157. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Jani, A.D.; Thomas James, H.; Gil, C.; Ritenour, M.A.; Wright, A.L. Sweet orange orchard architecture design, fertilizer, and irrigation management strategies under Huanglongbing-endemic conditions in the Indian River citrus district. HortScience 2020, 55, 2028–2036. [Google Scholar] [CrossRef]
- Thompson, R.B.; Incrocci, L.; Voogt, W.; Pardossi, A.; Magán, J.J. Sustainable irrigation and nitrogen management of fertigated vegetable crops. Acta Hortic. 2017, 1150, 363–378. [Google Scholar] [CrossRef]
- Rouse, R.; Irey, M.; Gast, T.; Boyd, M.; Willis, T. Fruit Production in a Southwest Florida citrus grove using the boyd nutrient / SAR Foliar Spray. Proc. Fla. State Hortic. Soc. 2012, 125, 61–64. [Google Scholar]
- Zambon, F.T.; Kadyampakeni, D.M.; Grosser, J.W. Ground application of overdoses of manganese have a therapeutic effect on sweet orange trees infected with Candidatus Liberibacter asiaticus. HortScience 2019, 54, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Morgan, K.T.; Kadyampakeni, D.M. Nutrition of Florida Citrus Trees, 3rd ed.; University of Florida IFAS Extension: Gainesville, FL, USA, 2020. [Google Scholar]
- Quaggio, J.A.; Souza, T.R.; Zambrosi, F.C.B.; Mattos, D.; Boaretto, R.M.; Silva, G. Citrus fruit yield response to nitrogen and potassium fertilization depends on nutrient-water management system. Sci. Hortic. 2019, 249, 329–333. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Nkedi-Kizza, P.; Kasozi, G.N. Nutrient management options for Florida citrus: A review of NPK application and analytical methods. J. Plant Nutr. 2015, 38, 568–583. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W. Citrus fertigation and irrigation practices for managing HLB in Florida. Proc. Fla. State Hortic. Soc. 2016, 129, 64–68. [Google Scholar]
- Spann, T.M.; Schumann, A.W. The role of plant nutrients in disease development with emphasis on citrus and Huanglongbing. Proc. Fla. State Hortic. Sci. 2009, 122, 169–171. [Google Scholar]
- Esteves, E. Nitrogen, Calcium and Magnesium Management for Improving the Performance of HLB-Affected Citrus Trees; University of Florida: Gainesville, FL, USA, 2020. [Google Scholar]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M.; Mahmoud, K.A. Water and soil nutrient dynamics of Huanglongbing-Affected citrus trees as impacted by ground-Applied nutrients. Agronomy 2020, 10, 1485. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M.; Kamal, M.A. Effect of soil and / or foliar applied nutrients on leaf nutrient accumulation and water uptake on Huanglongbing affected ‘ Valencia ’ citrus trees. Proc. Fla. State Hortic. Soc. 2018, 131, 58–64. [Google Scholar]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M. Effect of essential nutrients on roots growth and lifespan of Huanglongbing affected citrus trees. Plants 2020, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P.; Barlas, N.T.; Atta, A.A.; Morgan, K.T. Comparative response of Huanglongbing-affected sweet orange trees to nitrogen and zinc fertilization under microsprinkler irrigation. Agriculture 2020, 10, 489. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Zanotelli, D.; Allegra, M.; Giuffrida, A.; Torrisi, B.F.; Leonardi, A.; Quiñones, A.; Intrigliolo, F.; Tagliavini, M. Assessing nutrient uptake by field-grown orange trees. Eur. J. Agron. 2012, 41, 73–80. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P.; Mahmoud, K. Ammonium and nitrate distribution in soil using drip and microsprinkler irrigation for citrus production. Soil Sci. Soc. Am. J. 2014, 78, 645. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquinto, G.; Tei, F.; Thompson, R.B. Monitoring nitrogen status of vegetable crops and soils for optimal nitrogen management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Zekri, M.; Schumann, A.; Vashisth, T.; Kadyampakeni, D.; Morgan, K.; Boman, B.; Obreza, T. Fertilizer application methods. In Florida Citrus Production Guide; Diepenbrock, L.M., Dewdney, M.M., Vashisth, T., Eds.; University of Florida IFAS: Gainesville, FL, USA, 2018; pp. 59–61. [Google Scholar]
- Obreza, T.A.; Zekri, M.; Futch, S.H. General soil fertility and citrus tree nutrition. In Nutrition of Florida Citrus Trees; Morgan, K.T., Kadyampakeni, D.M., Eds.; University of Florida IFAS Extension: Gainesville, FL, USA, 2020; p. 108. [Google Scholar]
- Morgan, K.T.; Kadyampakeni, D.M.; Zekri, M.; Schumann, A.W.; Vashisth, T.; Obreza, T.A. 2020–2021 Florida citrus production guide: Nutrition management for citrus trees. In 2020–2021 Florida Citrus Production Guide; University of Florida IFAS Extension: Gainesville, FL, USA, 2020; p. 264. [Google Scholar]
- Zekri, M. The Critical Importance of Citrus Tree Nutrition, 1st ed.; AgNet Media: Newberry, FL, USA, 2016; ISBN 978-0-9978348-0-2. [Google Scholar]
- Obreza, T.A.; Zekri, M.; Hanlon, E.A. Soil and leaf tissue testing for commercial citrus production. In Nutrition of Florida Citrus Trees; Morgan, K.T., Kadyampakeni, D.M., Eds.; UF/IFAS: Gainesville, FL, USA, 2020; p. 108. [Google Scholar]
- Cadahía, C. Fertirrigación, 3rd ed.; Ediciones Mundi-Prensa: Madrid, Spain, 2005. [Google Scholar]
- Cadahía, C.; Frutos, I.; Eymar, E. La Savia Como Indice de Fertilización, 1st ed.; Ediciones Mundi-Prensa: Madrid, Spain, 2008. [Google Scholar]
- Eymar, E.; Cadahia, C.; Sanchez, A.; Lopez-Vela, D. Nutritional diagnosis based on sap analysis for containerized Cupressus glabra and Cupressocyparis leylandii with fertigation systems. Commun. Soil Sci. Plant Anal. 2000, 31, 1225–1248. [Google Scholar] [CrossRef]
- Olsen, J.K.; Lyons, D.J. Petiole sap nitrate is better than total nitrogen in dried leaf for indicating nitrogen status and yield responsiveness of capsicum in subtropical Australia. Aust. J. Exp. Agric. 1994, 34, 835–843. [Google Scholar] [CrossRef]
- Farneselli, M.; Tei, F.; Simonne, E. Reliability of petiole sap test for N nutritional status assessing in processing tomato. J. Plant Nutr. 2014, 37, 270–278. [Google Scholar] [CrossRef]
- Llanderal, A.; García-Caparrós, P.; Segura, M.L.; Contreras, J.I.; Lao, M.T. Nutritional changes in petiole sap over space and time in a tomato crop greenhouse. J. Plant Nutr. 2019, 42, 1205–1217. [Google Scholar] [CrossRef]
- Hochmuth, G.J. Efficiency ranges for nitrate-nitrogen and potassium for vegetable petiole sap quick tests. Horttechnology 1994, 4, 218–222. [Google Scholar] [CrossRef]
- Killiny, N.; Hijaz, F. Chemical composition of xylem sap of Citrus sinensis L. Osbeck (sweet orange). Trends Plant Sci. 1998, 3, 188–195. [Google Scholar]
- Ceballos-Laita, L.; Gutierrez-Carbonell, E.; Takahashi, D.; Abadía, A.; Uemura, M.; Abadía, J.; López-Millán, A.F. Effects of Fe and Mn deficiencies on the protein profiles of tomato (Solanum lycopersicum) xylem sap as revealed by shotgun analyses. J. Proteom. 2018, 170, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.N.S.; Oliva, M.A.; Martínez, C.A.; Silva, M.A.P. e Variação diurna e sazonal do pH e composição mineral da seiva do xilema em tomateiro. Hortic. Bras. 2003, 21, 10–14. [Google Scholar] [CrossRef]
- Palmer, L.J.; Stangoulis, J.C.R. Changes in the elemental and metabolite profile of wheat phloem sap during grain filling indicate a dynamic between plant maturity and time of day. Metabolites 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killiny, N. Metabolomic comparative analysis of the phloem sap of curry leaf tree (Bergera koenegii), orange jasmine (Murraya paniculata), and Valencia sweet orange (Citrus sinensis) supports their differential responses to Huanglongbing. Plant Signal. Behav. 2016, 11, e1249080. [Google Scholar] [CrossRef] [Green Version]
- Hijaz, F.; Manthey, J.A.; Van der Merwe, D.; Killiny, N. Nucleotides, micro- and macro-nutrients, limonoids, flavonoids, and hydroxycinnamates composition in the phloem sap of sweet orange. Plant Signal. Behav. 2016, 11, e1183084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, C.J.; Eliason, R. Nutrient Management in Minnesota Fruit & Vegetable Crops for Commercial; University of Minnesota: Minneapolis, MN, USA, 2005. [Google Scholar]
- Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B.; Farneselli, M.; Padilla, F.M. Assessing crop N status of fertigated vegetable crops using plant and soil monitoring techniques. Ann. Appl. Biol. 2015, 167, 387–405. [Google Scholar] [CrossRef]
- Goffart, J.P.; Olivier, M.; Frankinet, M. Potato crop nitrogen status assessment to improve N fertilization management and efficiency: Past-present-future. Potato Res. 2008, 51, 355–383. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Elmer, W.H.; Huber, D.M. Mineral Nutrition and Plant Disease; The American Phytopathological Society: Saint Paul, MN, USA, 2007. [Google Scholar]
- García-Mina, J.M. Plant nutrition and defense mechanism: Frontier knowledge. In Advances in Citrus Nutrition; Srivastava, A.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; p. 477. [Google Scholar]
- Arnon, D.I. Effect of ammonium and nitrate nitrogen on the mineral composition and sap characteristics of barley. Soil Sci. 1939, 48, 295–307. [Google Scholar] [CrossRef]
- Pettinger, N.A. The expressed sap of corn plants as an indicator or nutrient needs. J. Agric. Res. 1931, 43, 95–119. [Google Scholar]
- Cadahía, C.; Hernando, V. Diagnosis of the evolution of mineral nutrition in plants using sap analysis. In Proceedings of the Proceedings of the 7th International Colloquium on Plant Analysis and Fertilizer Problems, Hannover, Germany, 2–6 September 1974; pp. 157–165. [Google Scholar]
- Studstill, D.W.; Simonne, E.H.; Hutchinson, C.M.; Hochmuth, R.C.; Dukes, M.D.; Davis, W.E. Petiole sap testing sampling procedures for monitoring pumpkin nutritional status. Commun. Soil Sci. Plant Anal. 2003, 34, 2355–2362. [Google Scholar] [CrossRef]
- Hochmuth, G. Plant Petiole Sap-Testing for Vegetable Crops; University Florida: Gainesville, FL, USA, 1999; Volume CIR1144, pp. 1–6. [Google Scholar]
- Hochmuth, G.; Maynard, D.; Vavrina, C.; Hanlon, E.; Simonne, E. HS964/EP081: Plant Tissue Analysis and Interpretation for Vegetable Crops in Florida; UF/IFAS Extension University Florida: Gainesville, FL, USA, 2012; Volume HS 964, p. 55. [Google Scholar]
- Thompson, R.; Tremblay, N.; Fink, M.; Gallardo, M.; Padilla, F. Tools and strategies for sustainable nitrogen fertilisation of vegetable crops. In Advances in Research on Fertilization Management of Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 183–219. ISBN 978-3-319-53624-8. [Google Scholar]
- Hartz, T.K. The assessment of soil and crop nutrient status in the development of efficient fertilizer recommendations. Acta Hortic. 2003, 627, 231–240. [Google Scholar] [CrossRef]
- Wu, J.; Wang, D.; Rosen, C.J.; Bauer, M.E. Comparison of petiole nitrate concentrations, SPAD chlorophyll readings, and QuickBird satellite imagery in detecting nitrogen status of potato canopies. Field Crop. Res. 2007, 101, 96–103. [Google Scholar] [CrossRef]
- Opstad, N. Mineral Concentrations in leaf dry matter and leaf and petiole sap in strawberry depend on leaf age and plant developmental stage. Acta Hortic. 2010, 868, 143–148. [Google Scholar] [CrossRef]
- Lacroix, C.R.; Cousin, I. Petiolar sap nitrate as a guide in the fertilization of strawberry. Acta Hortic. 1997, 439, 753–762. [Google Scholar] [CrossRef]
- Farneselli, M.; Simonne, E.H.; Studstill, D.W.; Tei, F. Washing and/or cutting petioles reduces nitrate-nitrogen and potassium sap concentrations in vegetables. J. Plant Nutr. 2006, 29, 1975–1982. [Google Scholar] [CrossRef]
- Bélec, C.; Villeneuve, S.; Coulombe, J.; Tremblay, N. Influence of nitrogen fertilization on yield, hollow stem incidence and sap nitrate concentration in broccoli. Can. J. Plant Sci. 2001, 81, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Joris, H.A.W.; Souza, T.R.; Montezano, Z.F.; Vargas, V.P.; Cantarella, H. Evaluating nitrogen behavior in sugarcane after fertilization using leaf and sap extract analyzes. Am. J. Plant Sci. 2014, 05, 2655–2664. [Google Scholar] [CrossRef] [Green Version]
- De Souza, T.R.; Bôas, R.L.V.; Quaggio, J.A.; Salomão, L.C. Nutrientes na Seiva de plantas cítricas fertirrigadas. Rev. Bras. Frutic. 2012, 34, 482–492. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.R.; de Alvarenga, F.V.; Boaretto, R.M.; Lopes, J.R.S.; Quaggio, J.A.; Coletta Filho, H.D.; Mattos, D. Following the effects of micronutrient supply in HLB-infected trees: Plant responses and ‘Candidatus Liberibacter asiaticus’ acquisition by the Asian citrus psyllid. Trop. Plant Pathol. 2020, 45, 597–610. [Google Scholar] [CrossRef]
- Daugaard, H.; Todsen, T.T. Nitrogen fertilization of strawberries: N(min), leaf dry matter, and leaf sap analyses as control methods. J. Plant Nutr. 1999, 22, 1679–1685. [Google Scholar] [CrossRef]
- Vitosh, M.L.; Silva, G.H. Factors affecting potato petiole sap nitrate tests. Commun. Soil Sci. Plant Anal. 1996, 27, 1137–1152. [Google Scholar] [CrossRef]
- Nagarajah, S. A petiole sap test for nitrate and potassium in ’Sultana’ grapevines. Aust. J. Grape Wine Res. 1999, 5, 56–60. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers, 8th ed.; Pearson: London, UK, 2013. [Google Scholar]
- Killiny, N. Collection of the phloem sap, pros, and cons. Plant Signal. Behav. 2019, 14, 1618181. [Google Scholar] [CrossRef]
- Errebhi, M.; Rosen, C.J.; Birong, D.E. Calibration of a petiole sap nitrate test for irrigated “Russet Burbank” potato. Commun. Soil Sci. Plant Anal. 1998, 29, 23–35. [Google Scholar] [CrossRef]
- Parks, S.E.; Irving, D.E.; Milham, P.J. A critical evaluation of on-farm rapid tests for measuring nitrate in leafy vegetables. Sci. Hortic. 2012, 134, 1–6. [Google Scholar] [CrossRef]
- Peña-Fleitas, M.T.; Gallardo, M.; Padilla, F.M.; Rodríguez, A.; Thompson, R.B. Use of a portable rapid analysis system to measure nitrate concentration of nutrient and soil solution, and plant sap in greenhouse vegetable production. Agronomy 2021, 11, 819. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 1999, 78, 127–157. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Casado, M. Estudio de la Fertilización y Nutrición del Fresón Mediante el Análisis de Savia; Universidad Complutense de Madrid: Madrid, Spain, 1974. [Google Scholar]
- Peñalosa, J.M.; Cadahia, C.; Sarro, M.J.; Masaguer, A. Improvement of strawberry nutrition in sandy soils by addition of manure, calcium and magnesium. J. Plant Nutr. 1994, 17, 147–153. [Google Scholar] [CrossRef]
- Haghshenas, M.; Arshad, M.; Nazarideljou, M.J. Different K:Ca ratios affected fruit color and quality of strawberry ‘Selva’ in soilless system. J. Plant Nutr. 2018, 41, 243–252. [Google Scholar] [CrossRef]
- Pino, P.; Callejas, R.; Razeto, B.; Reginato, G. Análisis químico del extracto peciolar para evaluar el estado nutricional en la vid. Pesqui. Agropec. Bras. 2012, 47, 111–117. [Google Scholar] [CrossRef] [Green Version]
| Nutrients Analyzed | Solvent | Ratio | Type of Analysis | Authors |
|---|---|---|---|---|
| NO3−-N, NH4+, P, B, Ca, K, Mg, and Na | HCl 2% | 1:25 | Spectrometry | [26] |
| Fe, Cu, Mn, and Zn | HCl 2% | 1:10 | Spectrometry | |
| Cl− | HCl 2% | 1:25 | Ion selective electrode | |
| Total N | - | - | Kjeldahl method | |
| NO3−-N | Deionized water | 1:200 | Colorimetry | [28] |
| K | Deionized water | 1:20 | Spectrometry | |
| NO3−-N | Distilled water | 1:20 | Strips and reader | [55] |
| NO3−-N and K | Distilled/deionized water | 1:50 | Strips and reader, colorimetry, and electrodes | [48] |
| Cultivar | pH | NH4+ | NO3−-N | Total N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sap Nutrient Concentration (mg L−1) | ||||||||||||||
| ‘Valencia’ | 5.4 | 23.6 | 62.8 | 86.4 | 3600 | 4000 | 596.8 | 474.4 | 156.8 | 4.0 | 2.1 | 1.7 | 0.9 | 2.6 |
| ‘Hamlin’ | 5.5 | 22.8 | 61.6 | 84.4 | 3500 | 3800 | 581.8 | 468.5 | 139.4 | 3.6 | 2.1 | 1.3 | 0.9 | 2.4 |
| Crop Stage | Sap Nutrient Concentration (mg L−1) | Authors | ||||||
|---|---|---|---|---|---|---|---|---|
| NO3−-N | H2PO4−-P | K+ | Ca2+ | Mg2+ | Na+ | Cl− | ||
| Throughout the crop cycle | 1253 | 39.5 | 4533 | 555 | 1688 | 5512 | 3120 | [30] |
| Harvest | 700 | - | 3500 | - | - | - | - | [31] |
| Crop Stage | Sap Nutrient Concentration (mg L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| NO3−-N | P | K+ | Ca2+ | Mg2+ | Na+ | Cl− | Authors | |
| Blooming summer | 350–500 | 295–425 | 4500–5000 | 850–1000 | 300–450 | 40–50 | - | [69] |
| Fruit set summer | 600–800 | 140–210 | 4300–4800 | 450–600 | 200–300 | 30–40 | 500 | |
| March | 500–700 | 250–360 | 4200–5600 | 700–1200 | 300–610 | - | 500–780 | [26] |
| May | 300–550 | 220–330 | 4200–5800 | 500–610 | 190–310 | - | 330–500 | |
| March | 200–500 | - | 1800–2500 | - | - | - | - | [31] |
| April | 200–500 | - | 1500–2000 | - | - | - | - | |
| Crop Stage | Sap Nutrient Concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| NO3−-N | P | K+ | Ca2+ | Mg2+ | |
| Vegetative flush | 1700 | 155 | 2800 | 600 | 480 |
| Blooming | 300 | 530 | 2000 | 1200 | 1000 |
| Veraison | 550 | 870 | 3350 | 1400 | 1400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, E.; Locatelli, G.; Bou, N.A.; Ferrarezi, R.S. Sap Analysis: A Powerful Tool for Monitoring Plant Nutrition. Horticulturae 2021, 7, 426. https://doi.org/10.3390/horticulturae7110426
Esteves E, Locatelli G, Bou NA, Ferrarezi RS. Sap Analysis: A Powerful Tool for Monitoring Plant Nutrition. Horticulturae. 2021; 7(11):426. https://doi.org/10.3390/horticulturae7110426
Chicago/Turabian StyleEsteves, Eduardo, Guilherme Locatelli, Neus Alcon Bou, and Rhuanito Soranz Ferrarezi. 2021. "Sap Analysis: A Powerful Tool for Monitoring Plant Nutrition" Horticulturae 7, no. 11: 426. https://doi.org/10.3390/horticulturae7110426
APA StyleEsteves, E., Locatelli, G., Bou, N. A., & Ferrarezi, R. S. (2021). Sap Analysis: A Powerful Tool for Monitoring Plant Nutrition. Horticulturae, 7(11), 426. https://doi.org/10.3390/horticulturae7110426







