Abstract
Ecklonia maxima is a brown algae seaweed largely harvested over the last years and used to produce alginate, animal feed, fertilizers, and plant biostimulants. Their extracts are commercially available in various forms and have been applied to many crops for their growth-promoting effects which may vary according to the treated species and doses applied. The aim of the study was to characterize the effect of adding an Ecklonia maxima commercial extract (Basfoliar Kelp; 0, 1, 2, and 4 mL L−1) to the nutrient solution of a hydroponic floating system on growth, yield, and quality of leaf lettuce at harvest and during cold storage (21 days at 4 °C). The supplementation of the E. maxima extract through the mineral nutrient solutions, especially between 2 and 4 mL L−1, enhanced plant growth and improved the yield and many morphological and physiological traits (biomass accumulation, leaf expansion, stomatal conductance, water use efficiency, nitrogen use efficiency, etc.). Preharvest treatments with E. maxima extract were effective in delaying leaf senescence and extending the shelf-life of fresh-cut leaf lettuce. The delay in leaf decay of treated samples allowed to retain an overall quality over the threshold of marketability for up to 21 d of cold storage, especially using 2 mL L−1 of extract.
1. Introduction
In the last decades, the rising awareness of consumers on the importance of consuming healthy foods [1] has led to the increase in the demand for vegetable products with the consequent increase of many folds of their production and global trade value [2]. A part of this increase is linked to the increased demand for convenience foods such as fresh-cut vegetables [3]. Ready-to-eat (RTE) products have shown the most rapid expansion among vegetable products, even in developing countries [4]. Consequently, the demand for raw vegetables for minimally processed vegetable production is increasing, and food industries have been urged to utilize new cultivation systems and techniques to produce a sufficient amount of high-quality and safe food without detrimental effects on quality maintenance after minimal processing and during cold storage. Food industries are also interested in increasing crop yield or crop growth rate to reduce raw product cost and be more competitive in pricing.
Moreover, the increasing world population is expected to reach 9 billion by 2050, of which 70% will live in urban centers, and climate change will strain the food supply chain [5]. Thus, there is a growing concern about the importance of supplying vegetables to cities in an environmentally friendly and energy-efficient way [6]. This demand for sustainable urban food could be met by designing and developing vertical farms [7]. This farming system involves large-scale food production in indoor environments, with precisely controlled light, nutrients, and temperatures where plants are stacked in layers that may reach several stories tall. This crop cultivation system is based on hydroponics using cutting-edge greenhouse technologies [8]. Soilless cultivation systems (or hydroponics) are currently used for many vegetable crops to overcome many biotic or abiotic issues [9,10,11], improve yield and quality [10,12,13,14], and extend cultivation area. In the hydroponic cultivation systems, plants are continuously nourished with mineral nutrient solutions prepared by adding mineral fertilizers and chemical elements to the water. The aim to improve the sustainability of intensive agriculture systems is pulling toward the reduction of synthetic inputs and their substitution with natural resources even in innovative cultivation systems such as hydroponics and vertical farming.
The use of growth-promoting products has been suggested for increasing crop production and sustainability and reducing the need for chemical inputs [15]. The plant growth promoters, also known as biostimulants [16], have different functions (biofertilizers, biocontrol, phytostimulation, etc.) [17,18] and are of different types (bacteria, mycorrhizae, humic acid, algae extracts, etc.).
Algae or seaweed extracts can be produced from brown algae (Phaeophyceae) [19] and are commercially available in liquid form. They can positively affect nutrient uptake, resistance to biotic and abiotic stresses, seed germination, and crop yield [20]. Seaweed extracts contain appreciable quantities of macroelements and some trace elements (Ca, Mg, K, Na, P, S, Fe) [21,22], considerable amounts of polyamines [23], abscisic acid, and brassinosteroids [24,25]. They contain also several osmoprotectants (betaine, proline, mannitol) [26,27] and other components of great biological importance, for their reported promotion of nutrient uptake, translocation, and ability to stimulate root growth [28], such as alginates and polysaccharides [26,27] or many classes of substances with hormone-like activity [29] such as auxins, cytokinins, ethylene, and gibberellins [30,31].
The mode of action of seaweed extracts on plant growth promotion is not well known yet. Understanding the mechanisms responsible for this effect is very complex due to the various interactions among the numerous bioactive compounds contained in the extracts [32]. Nevertheless, similar physiological responses to seaweed extract supplementation have been found in plants treated with plant growth regulators, so the effects on growth, development, and yield have been often related to the hormone-like compounds found in these extracts [16,20,33].
Ecklonia maxima (Osbeck) Papenfuss is a brown algae seaweed also known as sea bamboo that grows in the southern oceans and is mainly found along the southern Atlantic coast of Africa. It has been largely collected over the last years and used to produce alginate, animal feed, fertilizers, and plant biostimulants [34]. Their extracts are commercially available in various forms and have been applied to many crops for their growth-promoting effects [35]. The commercial biostimulants produced from E. maxima contain various plant growth regulators (auxins, cytokinins, polyamines, gibberellins, abscisic acid, and brassinosteroids) that play a role in the growth stimulation recorded on many crops [25,36,37]. A wide range of processes in plant development and growth can be controlled by exogenous phytohormones [18,38,39]. These effects may vary according to the treated species and doses applied [38,40,41,42].
Thus, it would be highly worthwhile to test if the supplementation of biostimulants obtained from seaweed extracts through the nutrient solution used in hydroponic cultivation systems could be a cost-effective and easy-to-use technique to increase the productivity and postharvest quality of baby leaf crops, while at the same time reducing the effect on the environment and limiting food waste [12,13,14,15,16].
Therefore, the aim of this study was to characterize the effect of adding an Ecklonia maxima commercial extract to the mineral nutrient solution of a hydroponic floating system on the growth, yield, and quality of leaf lettuce at harvest and during cold storage.
2. Materials and Methods
2.1. Leaf Lettuce Hydroponic Cultivation
The experiment was carried out in a greenhouse situated at the Department of Agricultural, Food and Forest Sciences (SAAF—University of Palermo, Italy) (38°6′28″ N 13°21′3″ E; altitude 49 m). Leaf lettuce plants (Lactuca sativa L. var. Crispa) were grown in a hydroponic floating system using nutrient solutions with four concentrations of a commercial Ecklonia maxima extract (Basfoliar® Kelp SL Compo Expert, Münster, Germany): 0, 1, 2, and 4 mL L−1. The nutrient solutions (MNS) were obtained adding the following nutrients to tap water (pH 7.6; electrical conductivity (EC) 500 μS cm−1): 19 mM NO3−, 1.25 mM NH4+, 2 mM H2PO4−, 11 mM K+, 4.5 mM Ca2+, 1 mM Mg2+, 1.1 mM SO42−, 40 μM Fe3+, 30 μM BO33−, 5 μM Mn2+, 4 μM Zn2+, 0.75 μM Cu2+, and 0.50 µM Mo [43]. MNS differed only in seaweed extract concentration and had an EC of 2.6 mS cm−1 and a pH of 6.0. Each nutrient solution was poured into four different tanks (100 cm long × 50 cm wide × 15 cm deep, containing 75 L).
Seeds of leaf lettuce (cv. ‘Lattuga da Taglio a Foglia Liscia’, Sementi Dotto—SDD SPA, Udine, Italy) were sown on 8 March 2021 into polystyrene panels (300 holes m−2) filled with a commercial substrate (Utilis, GreenView Srl, Crocetta del Montello, Italy). The panels were placed in a dark room at 22–24 °C until seed germination and were then transferred into the cold greenhouse. When the seedlings had fully expanded cotyledons (10th BBCH growth stage [44]) the panels were moved to float in the tanks (Figure 1).

Figure 1.
Graphical representation of the hydroponic floating system made of drilled polystyrene panels floating on mineral nutrient solutions (MNS) with four levels of Ecklonia maxima extract (AE) (1 Drilled polystyrene panels (300 plants m−2) floating on MNS; 2 Tanks filled with 75 L of MNS containing increasing concentrations of Ecklonia maxima extract).
During the greenhouse experiment, the average outside temperature varied between 6.1 °C (night) and 22.0 °C (day), and the average net solar radiation at noon was 623 W·m−2, with a day length that ranged between 9 and 11 h. The air temperature inside the greenhouse was on average 20.0 ± 1.3 °C and varied from 10.0 (night) to 36.4 °C (day), whereas the relative humidity was 78.8 ± 1.9% and varied from 55.0% to 100%; the light intensity at noon was 54,246 ± 3900 lux and varied from 78,801 to 12,970 lux as a function of the cloudiness.
Each treatment was composed of four replicated tanks for each seaweed extract concentration (150 plants for each tank) arranged in a randomized complete block design. The MNS were not aerated during the experiment because leaf lettuce has fast growth and does not necessitate high oxygen concentration in the nutrient solution [45]. Water utilization and the changes in EC and pH of the nutrient solutions were regularly checked and recorded and when the volume dropped by 20% the tanks were refilled with fresh MNS with the same seaweed extract concentration. The polystyrene panels completely covered the tanks so the amount of evaporated water was insignificant and was not considered for assessing the nutrient solutions consumed by the plants of each treatment.
2.2. Agronomical and Morpho-Physiological Parameters
The water use efficiency (WUE) was calculated as WUE (g DW L−1 H2O) = plant dry weight (g)/H2O (L). At the end of crop cultivation, the nutrient solution that remained in the tanks was examined and the residual N-NH4+ and N-NO3− contents were determined reflectometrically by a Merck RQflex10 reflectometer according to the company protocols (Merck, Darmstadt, Germany). This allowed to calculate the uptake of nitrogen during the plant growth and to determine Nitrogen Use Efficiency (NUE) [46] as NUE (g DW g−1 N) = plant total dry weight (g)/plant N uptake (g).
The stomatal conductance was evaluated 5 days before harvest on two recently expanded, unshaded leaves of 20 plants for each replicate with a diffusion porometer (AP4, Delta-T Devices Ltd., Cambridge, UK).
At harvest (32 days from sowing), all plants were harvested, and marketable yield was calculated.
The plants were collected 23 days after the beginning of the floating cultivation, and marketable yield was determined after removing decayed or senescent older leaves. Then, 20 plants for each replicate were randomly chosen and destructively sampled to measure plant height, leaf number, main leaf width, and leaf area. Soon after sampling the leaves were weighed, then scanned at 200 dpi (Epson Perfection 1640SU, Seiko Epson Corp., Suwa, Japan) to obtain digital images that were analyzed with the Easy Leaf Area software [47] to calculate the leaf area of each plant. Then, the leaves were dried in an oven at 85 °C until they reach a constant weight and the specific leaf area (SLA cm2 g−1) was calculated as leaf area/leaf dry weight. Another sample of 20 plants randomly selected for each replicate was divided into leaves, stems, and roots. Each fraction was weighed and then dried to constant weight at 85 °C for assessing fresh and dry biomass.
2.3. Minimal Processing and Cold Storage
Once harvested, plants were immediately transferred to the laboratory of Vegetable Analysis of the SAAF Department and minimally processed for fresh-cut leaf lettuce production. Leaves were detached from stems, retaining only those free from defects, yellowing, or decay. Then, they were immersed in tap water for 5 min two times and dried by manual centrifugation for 1 min with a handheld salad spinner. At the end of processing, the fresh-cut leaves obtained from each treatment and replicate was weighed and the yield of the minimally processed product was determined as (Crop yield weight − Fresh-cut leaves weight)/Crop yield weight × 100 and the correspondent fresh-cut leaves yield expressed in kg m−2 was calculated.
Samples of 100 g from each seaweed extract level were directly packed in polyethylene (PE) bags, sealed with a hot bar (Laica VT3112, Vicenza, Italy), and stored at 4 °C for 21 days.
2.4. Physico-Chemical and Quality Parameters
The physicochemical characteristics of four randomly selected samples for each seaweed extract treatment were evaluated at the end of processing and at each sampling time (after 7, 14, and 21 days of cold storage). The experimental design was a factorial combination of four seaweed extract levels and four storage times arranged in a randomized complete block design with four replicates.
At packaging and at each sampling time, each sample was weighed to estimate the weight loss (g 100 g−1 of initial fresh weight FW).
Fifteen leaves were randomly selected from each sample to measure upper side leaf color using a Chroma-meter CR-400 (Minolta Corp., Ltd., Osaka, Japan). The CIELAB color components L* (lightness), a* (positive values for reddish colors and negative values for greenish colors), and b* (positive values for yellowish colors and negative values for bluish colors) were recorded and Chroma (C*) and hue angle (h°) were calculated as C* = (a*2 + b*2)1/2 and h ° = 180° + arctan(b*/a*) [48]. Moreover, total color difference (ΔE) was also determined at each sampling date as ΔE = [(L* − L*0) + (a* − a*0) + (b* − b*0)]1/2, where L*0, a*0 and b*0 were the control values at the end of processing (T0).
The samples were also analyzed by an informal panel consisting of twelve people (six men and six women, aged 28–57) to evaluate their overall quality (OQ). Each panel member scored the samples from 1 to 5 where 1 = poor/unmarketable (off odors, extensive color changes, and major defects or decay symptoms), 3 = fair/still acceptable and marketable (presence of minor defects or modest color changes), and 5 = excellent (freshly harvested appearance and full visual and sensory acceptability; free from any off odors, defects, and decay).
Then, twenty grams of each sample were blended with H2O (1:5 w/v), and the suspensions were centrifuged at 3500 rpm for 10 min. The supernatants were used to assess soluble solids content (SSC), titratable acidity (TA), and nitrate (NO3−) and ascorbic acid content. SSC (°Brix) was measured with a digital refractometer (MTD-045nD, Three-In-One Enterprises Co. Ltd., New Taipei City, Taiwan). TA (mg of citric acid per 100 g of fresh weight) was determined by titrating 10 mL of the supernatants with 0.1 M NaOH up to pH 8.1. The content of NO3− and ascorbic acid (expressed as mg kg −1 FW and mg 100 g−1 FW, respectively) were estimated with a reflectometer (Merck RQflex10; Merck, Darmstadt, Germany) [48,49,50].
2.5. Statistics and Principal Component Analyses
The experimental layout consisted of four replicates for each seaweed extract concentration randomly assigned in four blocks. The effects of the Ecklonia maxima extract levels on morpho-physiological parameters and yield were determined by performing a one-way ANOVA (four replicated tanks for each biostimulant concentration). Tukey’s multiple range test at p ≤ 5% was used to determine the differences between means. The effects of seaweed extract levels and cold storage on minimally processed leaf lettuce were assessed with a two-way ANOVA (four replicated bags for each biostimulant concentration and each storage time). The least significant differences (LSD) test at p ≤ 5% was applied to detect significant interactions between factors or significant differences among treatments.
Two different principal components analyses (PCA) were carried out on cultivation and postharvest trials. The first PCA was performed to investigate the relationship between the tested seaweed extract concentrations and the morphological and quality characteristics of leaf lettuce at harvest. The parameters used for the analysis included plant height, stem diameter, whole plant fresh weight (FW), leaf FW, stem FW, roots FW, Shoot/Root FW, whole plant dry weight (DW), leaf DW, stem DW, root DW, S/R DW, epigeal dry matter percentage, root dry matter percentage, yield, leaf yield (% and kg) leaf no., leaf width, leaf area, plant area, SLA, WUE, NUE, stomatal conductance, L*, chroma, hue angle, SSC, TA, ascorbic acid and NO3−. A second PCA was carried out on morpho-physiological and phytochemical characteristic evolution of fresh-cut leaves during storage to estimate the parameters that more strongly discriminated between preharvest seaweed extract treatments and storage duration. The input matrix for this PCA included weight loss, SSC, TA, ascorbic acid and NO3− content, L*, chroma, hue angle, color difference (∆E), and overall quality (OQ). In each PCA, the best level of principal components (PCs) was evaluated by saving factors with eigenvalues higher than 1.0. The plots of the first and second PCs of each PCA enabled the study of the relationships between the variables of each input data set. Moreover, the initial parameters were projected into the subspace identified by the first and second PCs, and correlated variables were revealed. PCA were carried out with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Leaf Lettuce Yield and Quality
At harvest, plant height was significantly affected by the seaweed extract in the nutrient solution especially when the two highest doses were used (2 and 4 mL L−1). At these concentrations, plants were 2.4 cm higher on average than control (10.7 cm) but had no significant difference in stem diameter (Table 1).

Table 1.
Yield and morphological characteristics of green leaf lettuce plants grown in nutrient solutions added with different concentrations of Ecklonia maxima extract.
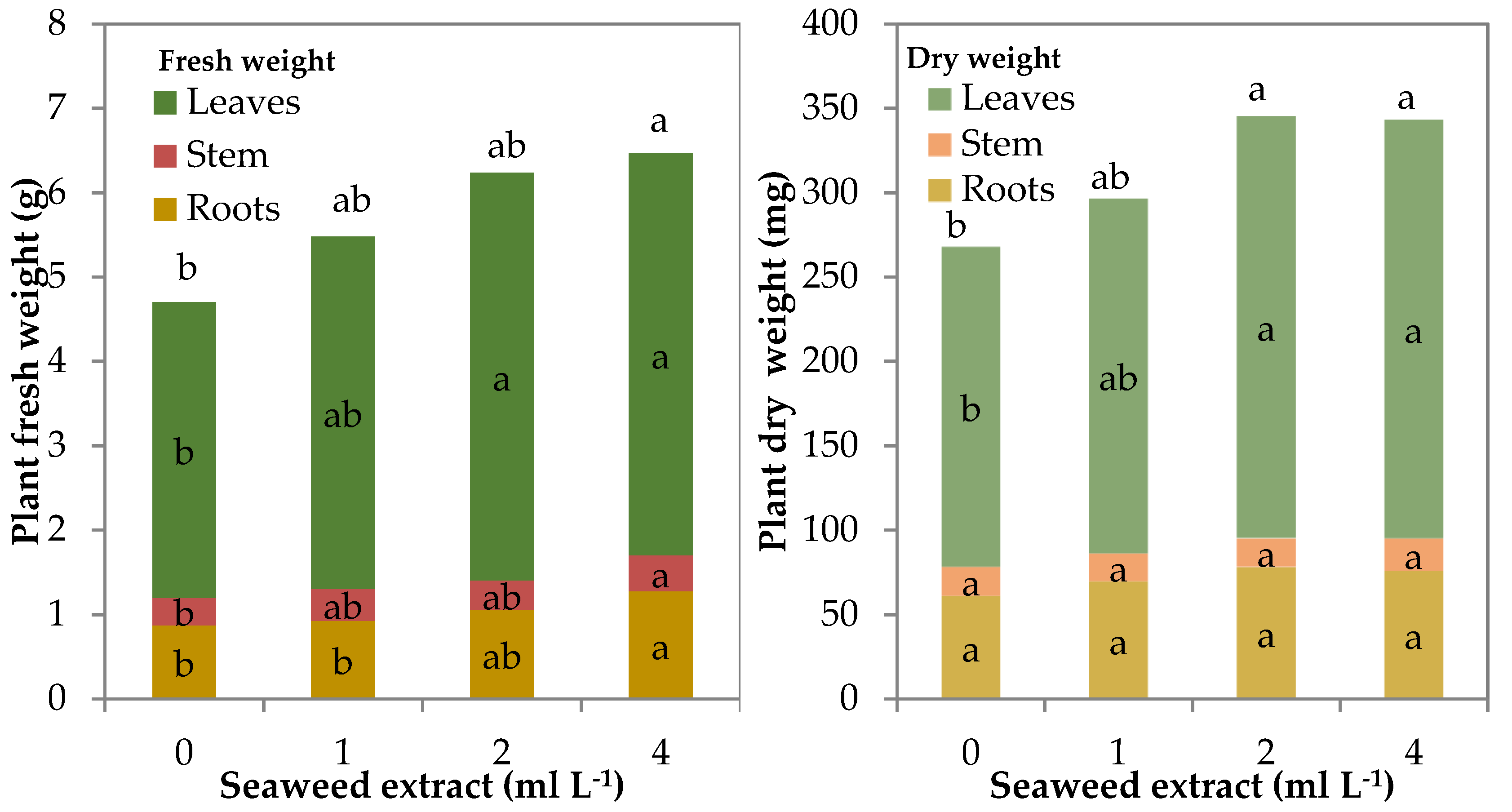
The total fresh weight (FW) of leaf lettuce plants showed a quadratic trend (R2 = 0.641 ***) as a function of the seaweed concentration in the mineral nutrient solution (MNS). The total fresh biomass of control plants was 4.7 g plant−1, whereas it was significantly higher by 32.6 and 37.5% in the plants grown with 2 and 4 mL L−1 of biostimulant in the MNS, respectively (Table 1; Figure 2). The main increase in fresh biomass was noted in the leaves with both 2 and 4 mL L−1 of seaweed extract in the MNS whereas stem and roots had a significantly higher fresh weight than control only with the highest concentration of the biostimulant. Nevertheless, the shoot/root (S/R) ratio did not significantly change by increasing the seaweed extract level in the MNS (Table 1).
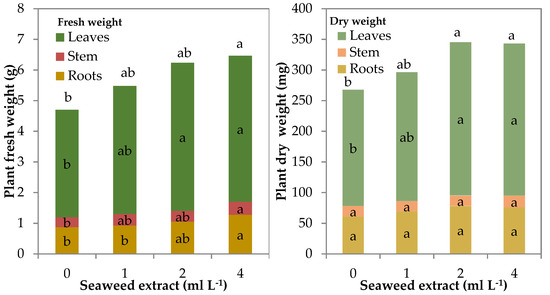
Figure 2.
Fresh and dry biomass of leaf lettuce plants grown in nutrient solutions containing different concentrations of a seaweed extract from Ecklonia maxima (bars of the same color with different letters are significantly different at p ≤ 0.05 according to Tukey’s test).
The total dry biomass of leaf lettuce plants showed a quadratic trend (R2 = 0.504 **) like that found for fresh biomass. Total dry weight increased significantly only in the plants supplemented with 2 and 4 mL L−1 of biostimulant in the MNS (Table 1; Figure 2). Stem and root dry weight did not show significant modifications due to the presence of seaweed extract in the MNS, so, the higher total dry weight should be attributed to the rise of the dry biomass of the leaves (+31.5% on average for 2 and 4 mL L−1). Even the dry matter partitioning between shoot and roots did not change as found for the fresh shoot/root ratio (Table 1). The dry matter percentage was not affected by the treatments and was 5.2% in the epigeal part and 7.0% in the roots (Table 1).
The plants of leaf lettuce not supplemented with seaweed extract yielded 1.15 kg m−2; the addition of the biostimulants to the MNS at the highest concentrations (2 and 4 mL L−1) increased the yield by 35.4% on average (about 400 g m−2 more than control plants) (Table 1). The yield (%) of fresh-cut leaves recorded a significant increase only with 2 mL L−1 of seaweed extract in the MNS (93.1%) whereas it was 91.7% on average in the other treatments (Table 1). The production of fresh-cut leaves was lowest in control plants (1.04 kg m−2) and increased up to 1.45 kg m−2 with 2 mL L−1 (Table 1).
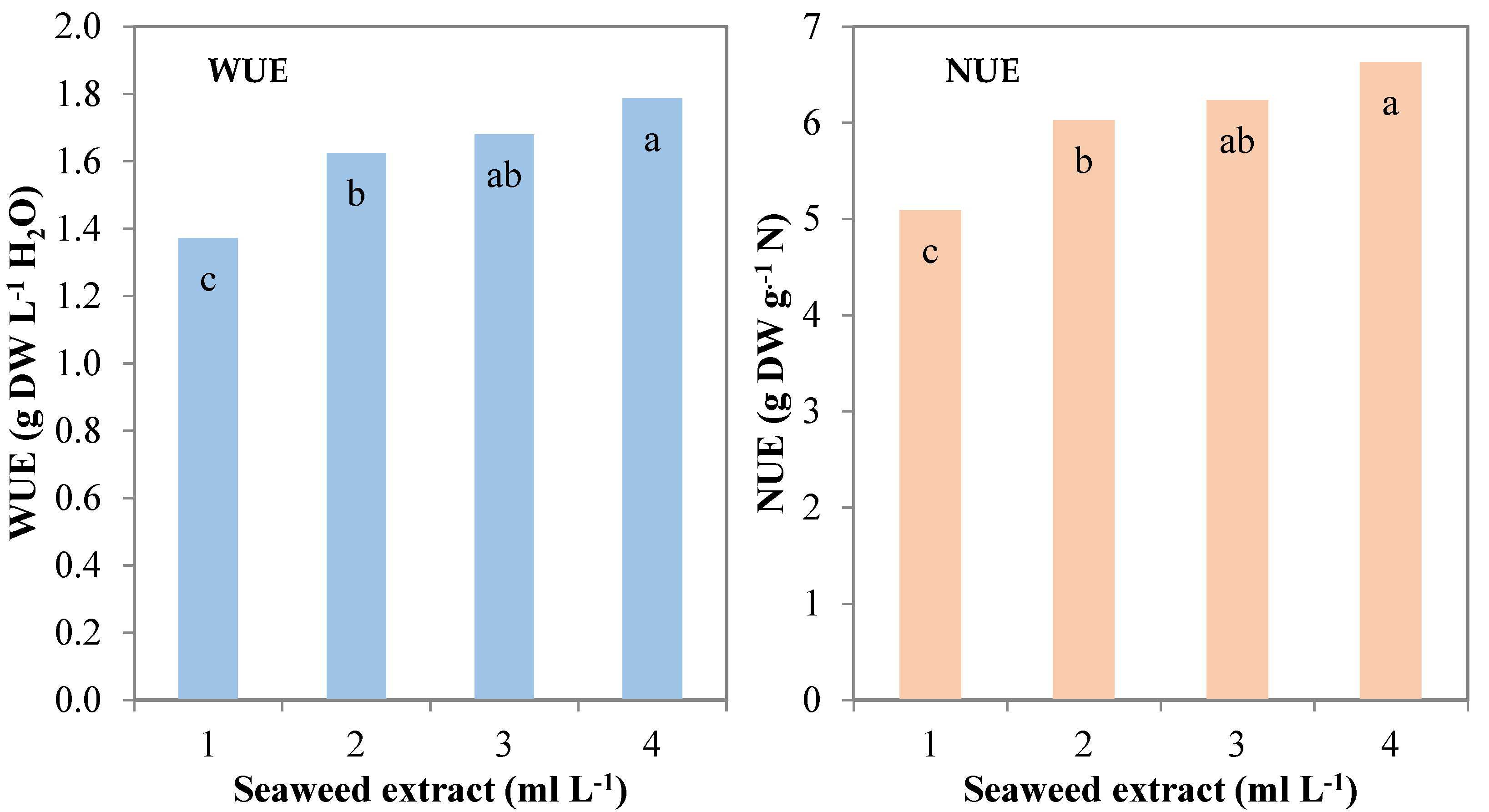
During the experiment, the consumption of nutrient solution was monitored and recorded to calculate the water use efficiency (WUE) and nitrogen use efficiency (NUE). Control plants recorded 1.4 g DW L−1 H2O and 5.1 g DW g−1 N for WUE and NUE, respectively. These parameters followed a quadratic trend (R2 = 0.735 *** and R2 = 0.729 ***, respectively) when increasing the seaweed extract concentration in the MNS and were significantly higher than control with the lowest seaweed extract level (1 mL L−1; 1.6 g DW L−1 H2O and 6.0 g DW g−1 N, respectively), and recorded the highest values at the highest concentration (4 mL L−1; 1.8 g DW L−1 H2O and 6.6 g DW g−1 N, respectively) (Table 1; Figure 3).
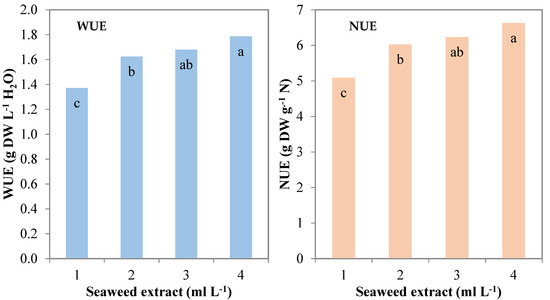
Figure 3.
Water use efficiency (WUE) and nitrogen use efficiency (NUE) of leaf lettuce plants grown in nutrient solutions containing different concentrations of a seaweed extract from Ecklonia maxima (Bars of the same color with different letters are significantly different at p ≤ 0.05 according to Tukey’s test).
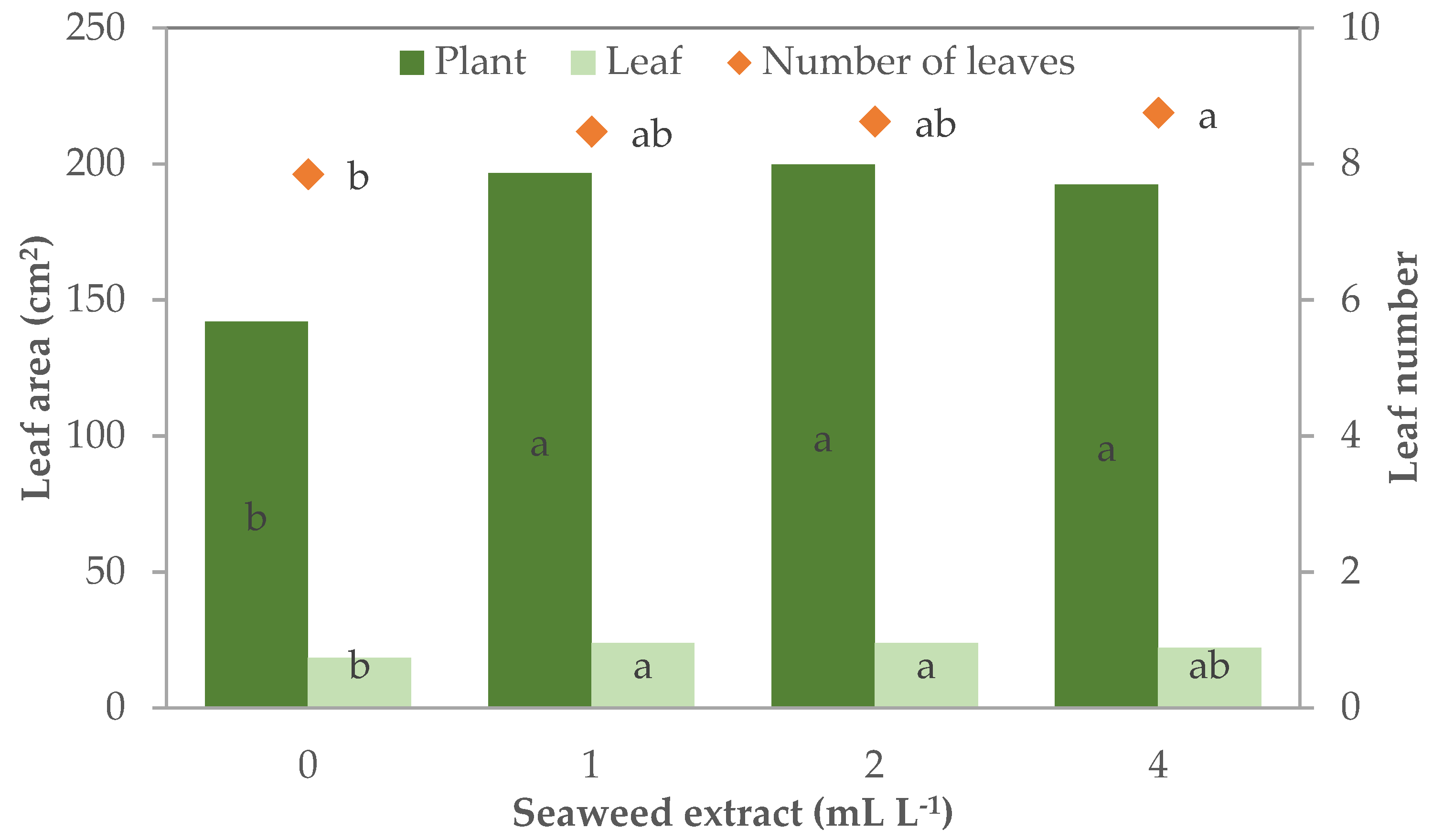
The supplementation of the E. maxima extract in the MNS also influenced the leaf characteristics of leaf lettuce plants that had 7.9 leaves per plant when grown without the biostimulant supplementation, while leaf lettuce grown with seaweed extract in the MNS were leafier, particularly with 4 mL L−1 (8.8 leaves plant−1) (Table 2; Figure 4).

Table 2.
Leaf characteristics of green leaf lettuce plants grown in nutrient solutions added with different concentrations of Ecklonia maxima extract.
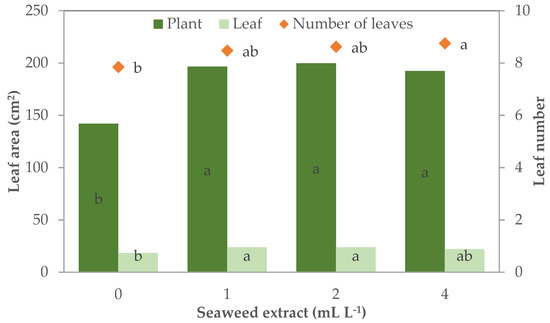
Figure 4.
Leaf number and plant and leaf area of leaf lettuce plants grown in nutrient solutions with different concentrations of a seaweed extract from Ecklonia maxima (bars of the same color or points with different letters are significantly different at p ≤ 0.05 according to Tukey’s test).
The leaf morphology was more influenced by the treatments. The effect of biostimulant concentration on leaf width and leaf and plant area followed a quadratic trend (R2 = 0.762 ***, 0.727 **, and 0.574 *, respectively). Leaf width was 4.5 cm in control plants whereas it was significantly wider with the lowest level of biostimulant in the MNS (+12.9%) and recorded the highest width in the plants grown with 2 and 4 mL L−1 in the MNS (5.6 cm; + 23.7% than control plants, on average) (Table 2). The supplementation of seaweed extract was effective in increasing the leaf and plant area to a different extent for each parameter. The leaves were significantly larger than control (18.4 cm2 leaf−1) in the plants supplemented with 1 and 2 mL L−1 of biostimulant (+30%). Control plants had a total leaf area of 142.0 cm2 plant−1, this parameter increased by 35–40% in all the treated plants (196.2 cm2 plant−1 on average).
The E. maxima extract influenced the specific leaf area only when supplemented at 1 mL L−1 (898.0 cm2 g−1 DW; 17.6% more than control) (Table 2).
The presence of the E. maxima extract in the nutrient solution affected also leaf physiology as shown by the measures of stomatal conductance that was on average 211.1 mmol m−2 s−1 in the plants supplied with the biostimulant, 40.3% higher than control plants (150.5 mmol m−2 s−1) (Table 2).
On the contrary, the biostimulant had no effect on leaf color at harvest (Table 2).
To assess the quality of leaf lettuce at harvest, soluble solid content (SSC), titratable acidity (TA), nitrate (NO3−) and ascorbic acid contents of the leaves were determined (Table 2). SSC, TA, and ascorbic acid content showed no significant effect due to the seaweed extract. The nitrate content of leaf lettuce leaves was highest in control plants, which accumulated 3120.0 mg kg−1 FW and was reduced by biostimulant supplementation, especially when added to the MNS at 1 mg L−1 (2540.0 mg kg−1 FW) (Table 2).
3.2. Storage of Fresh-Cut Leaf Lettuce
After harvesting, the minimally processed leaf lettuce was stored for 21 days at 4 °C to assess the effect of E. maxima extract supplementation and cold storage on postharvest quality changes.
The weight loss of stored samples was not influenced by the treatments during the first week of storage (0.64 g 100 g−1 FW on average). Afterward, control samples showed a higher weight loss that reached 2.14 g 100 g−1 FW after 21 days. The treated samples recorded a significant increase of weight loss only at the end of cold storage, but to a lower extent compared to the control (1.41 g 100 g−1 FW on average) (Table 3).

Table 3.
Effect of different concentrations of Ecklonia maxima extract in the nutrient solution and cold storage on weight loss, soluble solids content (SSC), titratable acidity (TA), ascorbic acid and nitrate (NO3−) content of fresh-cut leaf lettuce.
Seaweed extract did not affect the soluble solids and ascorbic acid content that showed no significant change during storage.
The titratable acidity raised during the second part of the storage period in all the samples. It increased by 27.3% in control samples, whereas the treated samples showed a significantly higher level of titratable acidity especially in the samples treated with the highest level of E. maxima extract (+70%) (Table 3).
The nitrate content (NO3−) of control samples was 2831.9 mg kg−1 FW on average and was slightly but significantly reduced by the treatments (−4.2% on average), whereas no significant change was recorded due to storage time (Table 3).
Color parameters appeared to be variously affected by seaweed extract and storage time. Color lightness (L*) increased from 55.0 to 56.6 during storage on average (Table 4). The color saturation (Chroma) recorded significantly lower values in the treatment with biostimulant in the MNS compared to the control. This parameter recorded a decrease during the initial 14 days of storage followed by a slight increase in the third week. At the beginning of storage, the hue angle was slightly higher than control in the samples treated with the two highest levels of biostimulant. The value of this parameter dropped during storage in control samples down to 118.8 whereas the reduction was lower in the treated samples (119.8 on average). A similar trend was recorded in the changes of color evaluated by calculating the color difference (∆E) which increased during the storage up to 2.9 in control samples, whereas it ranged from 1.3 to 1.9 in the treated samples at the end of storage time (Table 4).

Table 4.
Effect of different concentrations of Ecklonia maxima extract in the nutrient solution and cold storage on leaf appearance of fresh-cut leaf lettuce.
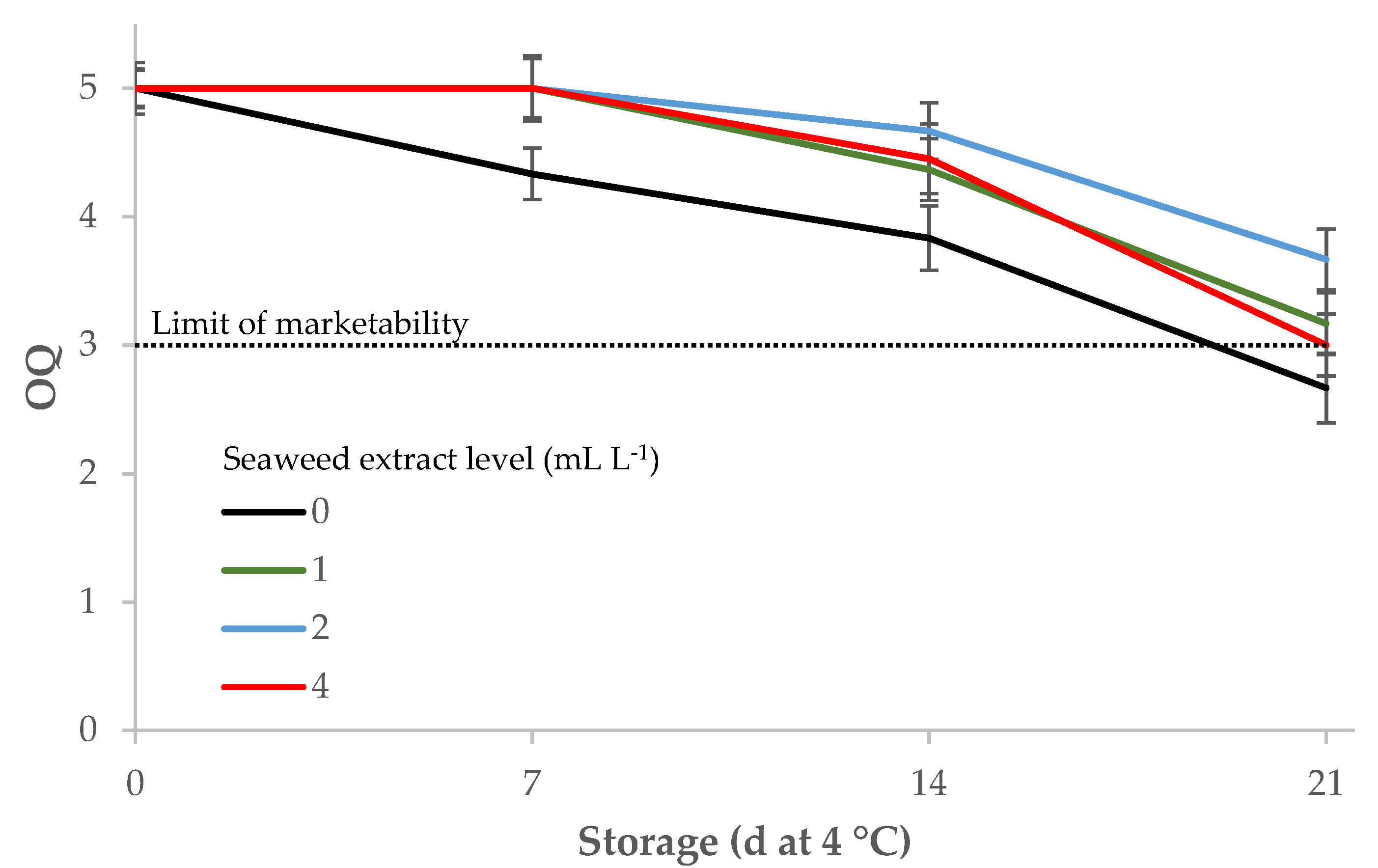
The scores for overall quality assessed by an informal panel dropped during storage, but the samples treated with E. maxima extract had a significantly better visual quality than the control during the first two weeks of storage, and maintained the marketability score until the end of the trial, while the overall quality of the control samples was under this threshold at day 21 (Table 4; Figure 5).
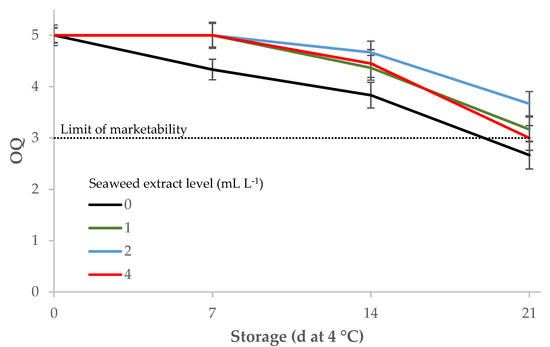
Figure 5.
Influence of Ecklonia maxima extract levels in the nutrient solution and storage at 4 °C on the overall quality of fresh-cut leaf lettuce (1: unmarketable; 3: average—limit of marketability; 5: excellent or having a fresh appearance).
3.3. Principal Components Analyses
The principal component analysis performed on the agronomic and quality characteristics of leaf lettuce at harvest presented three principal components (PCs) with eigenvalues higher than 1 (Table 5), representing 65.98%, 24.57%, and 9.45% of the total variance, respectively. This suggested that the initial 32 parameters could be represented by three PCs, explaining 100% of the total variance. PC1 was mostly correlated to plant height, stem diameter, whole plant, leaf, stem and root FW, whole plant, leaf, and root DW, yield, leaf yield (kg), WUE, NUE, leaf number, plant and leaf area, stomatal conductance, L*, hue angle, TA and ascorbic acid content; PC2 was correlated to S/R FW, stem DW, S/R DW, epigeal and root dry matter percentage, SLA, SSC, and nitrates; PC3 was correlated to leaf yield percentage (Table 5).

Table 5.
Correlation of variables to the factors of the principal components analysis (PCA) based on the agronomic and quality characteristics of leaf lettuce at harvest.
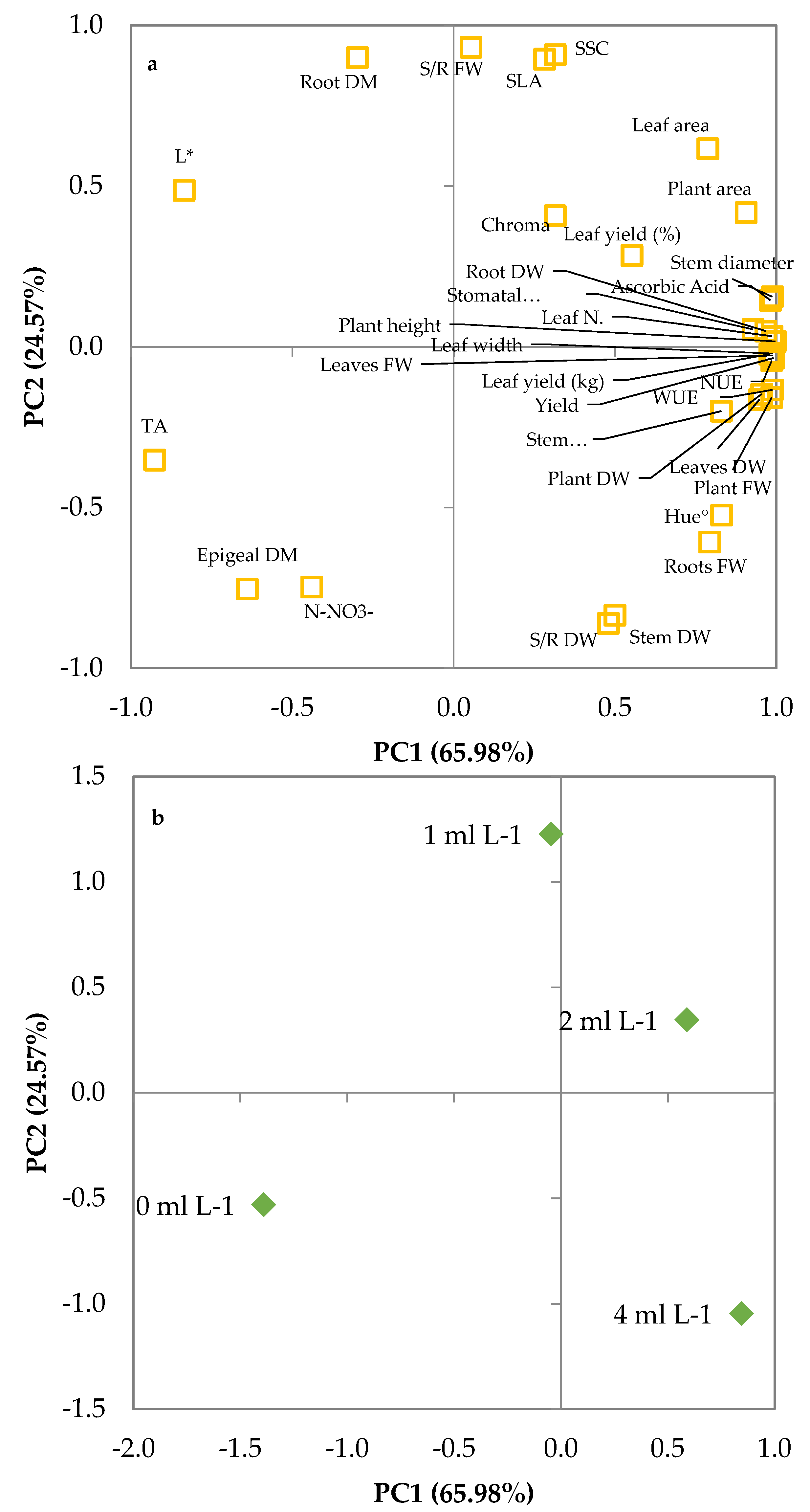
Such a relationship can be clearly evidenced by projecting the original parameters on the plane of the first two PCs, as shown in the plot of loadings (Figure 6a). The different E. maxima extract levels can be discriminated in the plot of scores (Figure 6b), where each biostimulant level is clearly separated from the others. The score of control had negative values of PC1 and PC2 (quadrant III; −, −) and was visibly distinguished by the score of the treatments with seaweed extract that were located in the positive part of the PC1 axis (2 and 4 mL L−1 of E. maxima extract) or very close to it (1 mL L−1 of E. maxima extract). Increasing the concentration of seaweed extract in the MNS from 1 to 4 mL L−1 was negatively related to PC2 and positively related to PC1 so that the score of 1 mL L−1 was located in the quadrant II (−, +), the score of 2 mL L−1 was located in the quadrant I (+, +) and the score of 4 mL L−1 was located in the quadrant IV (+, −). Merging the data from the plot of scores and loadings, it can be concluded that the rise of seaweed extract concentration in the nutrient solution was positively correlated to plant fresh and dry weight, WUE, NUE, stomatal conductance, leaf number, S/R ratios, plant height and yield, and negatively related to L*, nitrate content, and dry matter.
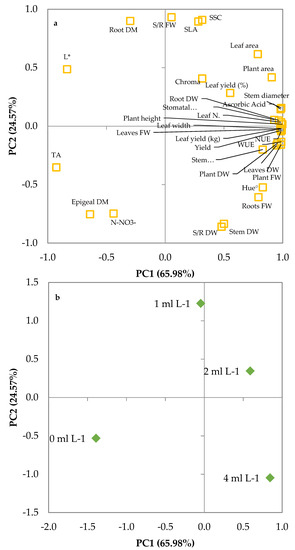
Figure 6.
Plot of (a) loadings (morpho-physiological and quality characteristics of leaf lettuce plants at harvest) and (b) scores (trials) formed by the two principal components from the PCA analysis on leaf lettuce grown on nutrient solutions with 0, 1, 2 and 4 mL L−1 of Ecklonia maxima extract.
To have an outlook of the morpho-physiological and biochemical variables characterizing leaf lettuce treated with different biostimulant levels during cold storage, a second PCA was carried out. It showed three PCs with eigenvalues higher than 1.00 (Table 6), representing 53.95%, 14.45%, and 13.41% of the total variance, respectively. Thus, the initial 10 parameters could be expressed by three PCs, explaining 81.81% of the total variance. PC1 was positively correlated to weight loss, titratable acidity, L* and ∆E and was negatively related to hue angle and overall quality; PC2 was positively correlated to SSC, and PC3 was positively correlated to ascorbic acid and nitrate content (Table 6).

Table 6.
Correlation of variables to the factors of the principal components analysis (PCA) based on morpho-physiological and biochemical parameters changes during cold storage of leaf lettuce treated with different Ecklonia maxima extract levels.
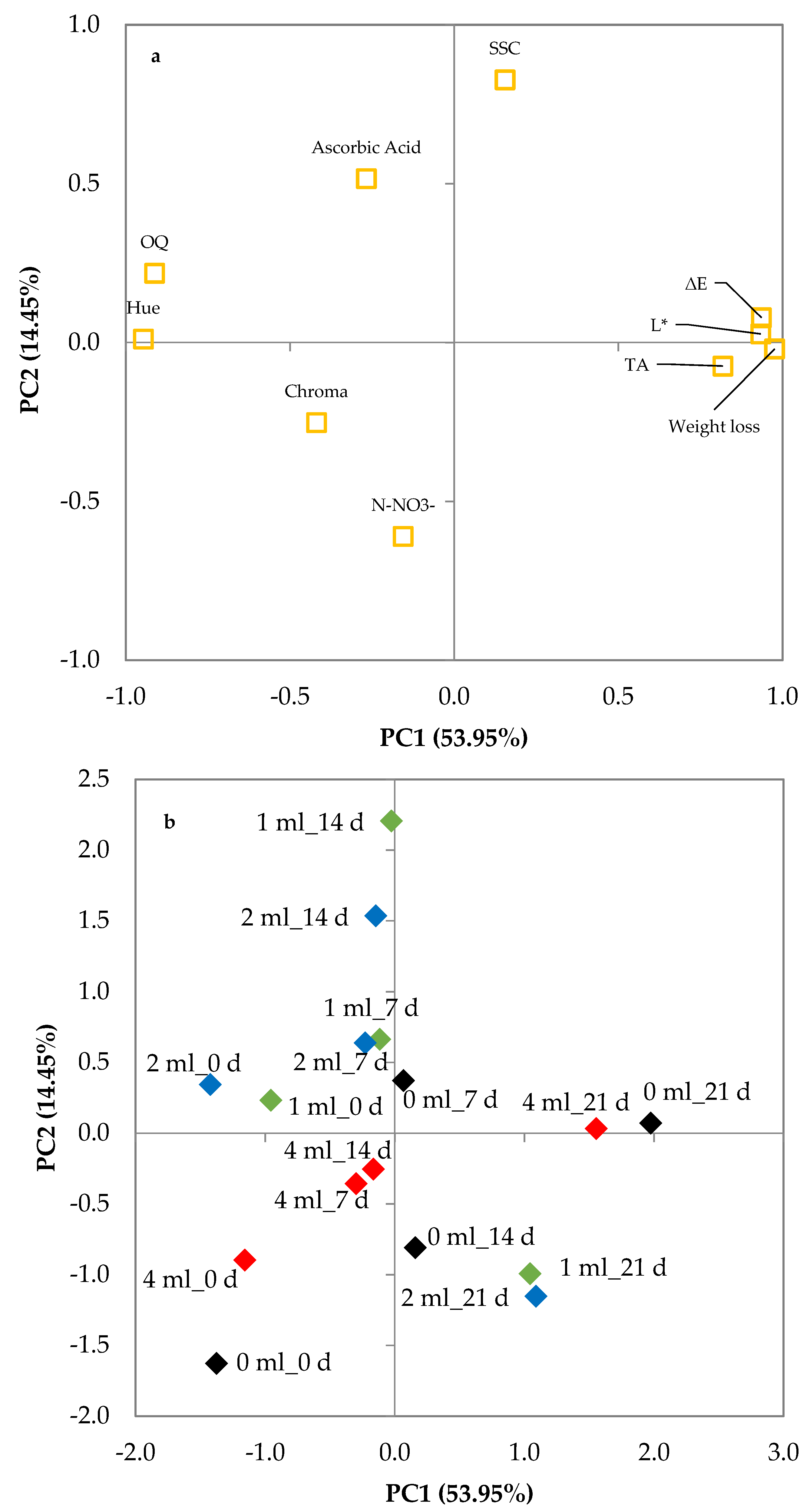
The plot of loadings shows the initial 10 variables projected on the plane of the two PCs confirming such a correlation (Figure 7a). The discrimination of the various combinations of preharvest seaweed extract treatments × storage time of fresh-cut leaf lettuce is shown in the plot of scores (Figure 7b). The score of control samples at the beginning of the storage period was located in quadrant III (−, −) whereas it shifted toward the positive part of the PC1 axis during storage ending in quadrant I (+, +) after 21 days of storage. The samples treated with seaweed extract maintained negative values of PC1 but increased PC2 values until 14 days of storage. During this storage period, the scores of the samples treated with 4 mL L−1 of seaweed extract were all in quadrant III (−, −), whereas the samples treated with 1 or 2 mL L−1 were located in quadrant II (−, +). At the end of the storage period, the samples treated with 1 or 2 mL L−1 of seaweed extract had the lowest value of PC1 and PC2 and were closer to the score of control samples at day 14 than those at day 21. Combining the information from the plots, it can be inferred that E. maxima extract levels affected leaf lettuce in different ways (Figure 7a,b). Storage time was positively related to PC1 (weight loss, ∆E, L*, and OQ). During the first 14 days of cold storage, the samples treated with 4 mL L−1 of seaweed extract showed, generally, lower variations of PC1 and PC2 values than the others, but during the last week of storage, the samples treated with 1 or 2 mL L−1 showed a lower increase of PC1 values.
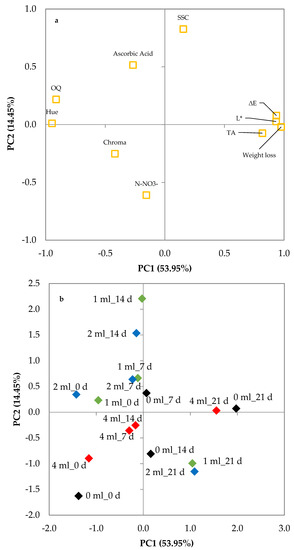
Figure 7.
Plot of (a) loadings (quality characteristics of minimally processed leaf lettuce during 21 d of storage at 4 °C) and (b) scores (trials) formed by the two principal components from the Principal Component Analysis. Leaf lettuce cultivated in nutrient solutions with 0 (black rhombuses), 1 (green rhombuses), 2 (blue rhombuses), and 4 mL L−1 (red rhombuses) of Ecklonia maxima extract; _0d, _7d, _14d, and_21d: days of storage at 4 °C.
4. Discussion
Seaweed extracts can improve the morpho-physiological and yield characteristics of many vegetable crops [35]. They can be applied in various ways like seed treatment, soil application, and foliar spray. Foliar application is the most common way of supplementation and has been shown to exert biostimulant effects on many crops [51,52] and product quality and shelf life [35]. Nevertheless, this kind of application can leave residues or spots on the leaves that could negatively affect the appearance of leafy vegetables or the processing of fresh-cut leafy vegetables. To overcome this problem, in this experiment we tested the possibility to supply different levels of an Ecklonia maxima commercial extract to leaf lettuce grown in a hydroponic floating system through the mineral nutrient solution and estimated their effects on growth and quality at harvest and during cold storage. This is one of the first experiments that has explored this technique for supplying seaweed extract through the mineral nutrient solution of a hydroponic cultivation system under production condition.
The supplementation of the E. maxima extract exerted positive effects on leaf lettuce growth even if with various responses to tested concentrations. Leaf lettuce reacted to the increase of E. maxima extract concentration in the nutrient solution following a quadratic trend for most of the morphological and growth parameters. Seaweed extracts can improve plant growth at low concentrations or reduce it at high concentrations [53]. Finnie and Van Staden [54] reported that a commercial extract from E. maxima promoted the growth of tomato roots when applied at low concentrations while it strongly inhibited root growth at a high concentration.
From the curves of the estimated response to seaweed extract supplementation, it could be found that the concentration with the highest effect on plants falls between 2 and 4 mL L−1. In this range, plant growth and fresh and dry matter accumulation increased significantly compared to untreated plants, especially considering leaf biomass. Similarly, Di Mola et al. [55,56] found that weekly foliar application with 3 mL L−1 of a seaweed extract from E. maxima to a baby lettuce crop grown in soil under greenhouse elicited significant increases in plant growth and fresh yield. They recorded an increase of marketable fresh yield that reached 14–17% at most, whereas in our experiment the supplementation of the seaweed extract through the mineral nutrient solution had a similar extent with the lowest dose (1 mL L−1), and increased by about 35% leaf fresh and dry weight and yields when the two highest levels were supplied. This could indicate a crop-specific differential response to E. maxima treatments and thus they need specific studies to optimize biostimulant use based on crops and application systems.
E. maxima extracts have exerted a positive influence on vigor, yield quality and quantity of other crops such as tomato, cucumbers, beans, spinach, zucchini, okra, etc. [36,57,58,59,60,61]. Tomato growth was significantly improved in plants treated with a seaweed extract of E. maxima regardless of whether it was supplied as a foliar spray at regular intervals, or as one initial soil drench [62], confirming that this seaweed extract can have a growth-promoting effect even when supplemented at root level as found in our work.
The improvements recorded in terms of biomass accumulation, vegetative growth, and yield could be related to the variations in stomatal conductance measured in the plants supplemented with the seaweed extract from E. maxima that recorded increases up to 50% with the highest concentration compared to the untreated plants. Similarly, Rouphael et al. [57] found that the application of E. maxima seaweed extract could reduce the stomatal resistance of zucchini leaves even under saline stress conditions.
A higher stomatal conductance may determine the rise of transpiration rates and water consumption, but also stimulates gas exchange and CO2 assimilation through photosynthesis resulting in increased dry matter accumulation and WUE. A high transpiration rate is not problematic when plants are grown on panels floating on the nutrient solution. Thus, the higher stomatal conductance determined by E. maxima seaweed treatments could be beneficial for this cultivation technique.
In our work, we also found an increase in nitrogen use efficiency in seaweed-treated plants (+18.4%, +22.5%, and +30.3%, respectively, with 1, 2, and 4 mL L−1). The enhanced growth stimulated by the seaweed extract increased the nitrogen needs of the plants. Seaweed extracts have been shown to influence the regulation of genes that have a significant role in nutrient uptake such as a nitrate transporter gene (NRT1.1) responsible for nitrogen sensing and auxin. The upregulated expression of this gene may result in improved nitrogen assimilation, enhanced growth, and increased yield [35,63,64].
The growth improvements recorded in our experiment in terms of fresh and dry biomass accumulation when plants were grown in a nutrient solution supplemented with the seaweed extract-based biostimulant were associated with modifications of leaf number and leaf morphology. Leafiness and leaf characteristics are very important in leafy vegetables as they can affect yield and marketability.
Leaf number is determined by shoot meristem size and leaf initiation rate [65]. Leaf initiation is thought to be regulated by the positive feedback loop between auxin and its transporter PIN1. Moreover, the increase of auxin level in the vegetative meristem might lead to leaf initiation [66]. The sites of leaf primordium initiation are induced in the meristem by auxin peaks and by auxin-induced repression of the so-called KNOXI gene [67]; this repression enhances the effects of gibberellins, which stimulate leaflet growth and differentiation. Thus, auxins play an important role in stimulating leaflet initiation and growth [68] and have been shown to exert this role both if they come from plant biosynthesis or from exogenous applications [69,70,71,72]. This process involves also other hormones such as cytokinins. Immanem et al. [73] showed that auxins and cytokinins can exert distinct but interconnected stimulation to cambium activity.
The extracts of brown seaweeds contain auxin, cytokinin, gibberellic acid, and other phytohormones [35]. The growth-promoting effects of seaweed extracts are often linked to the direct or indirect effect of their content in plant growth regulators.
The biostimulant used in our experiment (Basfoliar Kelp SL Compo Expert, Münster, Germany) is produced from E. maxima collected on the South African coastline and mechanically cold-processed to obtain a natural solution of phytohormones particularly rich in auxins (11 mg L−1 of auxins) and containing amino acids, carbohydrates, proteins, vitamins, and traces of cytokinins (0.04%). Thus, the lettuce plants grown in nutrient solution containing the commercial seaweed extract were supplemented with 11–44 μg L−1 of exogenous auxins according to the biostimulant concentration used.
Exogenous auxins may variously affect the morphological parameters of treated plants. The supplementation of high concentrations of auxin in maize roots produced shorter plants [41], while the application of NAA to tomato plants increased their height [74], confirming that the exogenous application of auxins can determine various phenotypic manifestations according to the origin and concentration of the auxin and plant species. Moreover, the effects of auxin rely on its concentration but also carbohydrate content in the tissues and light conditions may have a role [75].
As reported above, seaweed extracts from E. maxima contain cytokinins that can also play an important role in plant development. Cytokinins contained in the seaweed extracts can promote cell multiplication, resulting in increased leaf area [53,76], as also found in the present study.
The growth-promoting effect of seaweed extract produced from E. maxima could also be due to other mechanisms of action. The phytohormone-like effect of seaweed extracts could also be determined by other chemical substances in the extract other than phytohormones themselves [35]. E. maxima extracts contain several bioactive compounds such as amino acids, polysaccharides, phenolic compounds, osmolytes, etc., that could induce physiological responses reminiscent of phytohormones [35,56]. The phenolic compounds present in E. maxima extract could influence the metabolism and level of auxins in plants [77,78,79]. Some phenolic compounds such as chlorogenic acid and rutin can increase auxin activity as they can act as alternative substrates for oxidative enzymes (auxin-oxidase) and therefore protect auxins from oxidative breakdown [80]. Moreover, some phenolic compounds may avoid auxin decarboxylation thus increasing the level of active forms of this hormone [81]. This was confirmed by Aremu et al. [19] who found a higher content of endogenous auxin in plants treated with E. maxima extract. It has been also suggested that the bioactive compounds in the seaweed extract might trigger a signal transduction pathway throughout elicitation of intrinsic phytohormone synthesis [55].
Another possible mechanism behind the growth-promoting effect of seaweed extract could be found in the stimulation effect on the development of roots system in terms of length, volume, and biomass accumulation that determine an increase of the uptake and translocation of nutrients and their assimilation in the biomass [82]. It was reported that lettuce plants treated with a commercial extract of E. maxima grown under optimal conditions increased yield and the content of Ca, K, and Mg in the leaves [83]. E. maxima-based biostimulants applied as root application or foliar spray also improved root growth, increased P content, but decreased N content of nutrient-stressed cucumbers, [84]. In our work, we confirmed that lettuce plants recorded an increase in root biomass and yield even when the seaweed extract from E. maxima is added to the nutrient solution of a hydroponic floating system.
Seaweed extracts may affect the physiology of treated plants as well, affecting global transcriptome profiles and determining changes in their metabolome [85]. Many metabolic regulatory pathways of plants could be modified by the chemical components of seaweed extracts, thus increasing the transcripts of regulatory enzymes involved in nitrogen and antioxidant metabolisms [83]. A more efficient nitrogen metabolism could explain the lower content of nitrates recorded in our experiment in the leaves of leaf lettuce supplemented with E. maxima extract through the nutrient solution. Nevertheless, we did not record any effect on other quality parameters (soluble solid and ascorbic acid content, and titratable acidity).
Preharvest environmental conditions and agronomic practices may influence product characteristics and physiology also after harvesting [86]. To investigate the effects of preharvest treatments with E. maxima extract on leaf lettuce shelf life, soon after harvest, the plants were minimally processed and cold-stored as fresh-cut produce for 21 days. Minimally processed leafy vegetables are very perishable products that can suffer fast quality degradation during storage, resulting in loss of commercial value [48]. At harvest, vegetables are detached from the plant losing the source of water, nutrients, and organic molecules including hormones. This can trigger physiological disorders that can be enhanced by preharvest environmental conditions or mineral imbalance arising during growth.
Weight loss is one of the major causes of deterioration occurring during the storage of vegetables as it can strongly affect the appearance and quality of vegetables, especially those minimally processed. It occurs as a consequence of water loss through transpiration or evaporation, but it can be also determined by respiration that degrades carbohydrate reserves [87,88]. Even a little weight loss could adversely affect appearance, salable weight, and texture quality [89]. The weight losses of fresh-cut leaf lettuce leaves were comparable on average during the first two weeks of storage but at the end of storage, the preharvest application of the E. maxima extract showed to be effective in limiting the total weight loss compared to control. Processing and leaf tissue deterioration increase the transpiration through the outer periderm or cuticle ending to be the main responsible for water loss [90]. Leaf senescence could be regulated and retarded by cytokinins which can influence different metabolic processes [91]. A decline in cytokinin level has been recorded in senescent leaves, whereas exogenous application of cytokinin can delay leaf senescence in many crops [92]. The effects of exogenous cytokinins on leaf senescence through foliar or root-zone application have been related to increased chlorophyll synthesis, reduced chlorophyll degradation, and decreased lipase, lipoxygenase, and proteolytic activities which are involved in membrane breakdown [92,93]. Membrane integrity has an important role in delaying senescence as it maintains compartmentation and avoids nutrient and enzyme leakage, and water loss [93].
Seaweed extracts contain substances with cytokinin-like activity and may also induce endogenous cytokinin synthesis in the treated plants [92,94,95]. Along with other plant growth regulators such as gibberellins, auxins, brassinosteroids abscisic acid, and polyamines [23,25,37], many cytokinin derivates have been identified in E. maxima extracts [37] confirming their cytokinin-like activity. Moreover, spinach plants treated with E. maxima extract recorded a significant increase in the levels of primary endogenous cytokinins compared to the control plants [60]. The higher endogenous concentration was related to a higher number of leaves and higher yield in seaweed-treated spinach plants and could have also affected leaf senescence and membrane integrity of the leaf of lettuce in our experiment explaining the lower weight loss recorded in treated samples at the end of the storage period.
The perceived quality of leafy vegetables is greatly determined by appearance and color as they affect food choice and satisfactoriness and could also impact the consumer’s perception of sensory quality. Color characteristics and the way they change during storage can be influenced by preharvest [12,15,48,96,97] or postharvest [98,99,100,101,102] factors. Leafy vegetable color can be altered by browning and yellowing (chlorophyll degradation) phenomena that could negatively affect marketability [48]. In this study, we recorded lower saturation and a slower decrease of hue angle during storage in the samples from plants treated with E. maxima extract, which showed a less vivid and more greenish color and consequently a lower chlorophyll degradation [103,104], and smaller variation of ∆E. Color variations recorded in treated leaves during cold storage could be also related to an increase in cytokinin leaf content as it is well known that cytokinins delay leaf senescence through the promotion of chlorophyll synthesis or preservation of the chloroplast structure [93]. Moreover, chlorophyll degradation in harvested leaves is ascribed to the shortage of enough endogenous hormones [105,106], thus, the content of various plant growth regulators identified in E. maxima extracts could have influenced phytohormones levels and their homeostasis during storage.
The human eye can record even small variations of color changes that can influence the acceptability of leafy vegetable products. The quality perception of the minimally processed leaf lettuce, assessed by evaluating the overall visual quality (OQ) of each sample, appeared to be related to color variations during cold storage. The scores for leaf lettuce OQ assigned by the panel decreased early (after the first week of cold storage) and significantly in the untreated samples whereas they had a significant drop only at the end of the storage period (21 days at 4 °C) in the sample from plants grown with 2 and 4 mL L−1 of E. maxima extract in the nutrient solution. Cold-stored leafy vegetables packed in sealed plastic bags can effectively slow down the occurrence of alterations and decay [86,107,108], but the delayed senescence of the samples treated with the E. maxima extract was effective in retaining the overall visual quality of these samples over the threshold of marketability for up to 21 d of cold storage, especially supplementing 2 mL L−1 of E. maxima extract.
Principal component analyses can be effective in unraveling the effects of several experimental factors (genetic materials, pre- and postharvest management) on yield and quality parameters of many vegetable crops, as reported in many experiments carried out on different species and growing and storage conditions [12,86,96,97,109,110,111]. This was also the case in the present experiment since the different effects determined by the treatments with E. maxima extract on leaf lettuce plants were clearly shown by the Principal Component Analyses that outlined the various responses of leaf lettuce during growth and storage to the supplementation of different levels of seaweed extract through the nutrient solution.
The PCA analysis performed on morpho-physiological and quality parameters of leaf lettuce plants at harvest showed that lettuce was positively affected by seaweed extract treatments even if to a different extent for each parameter. Furthermore, plant response to E. maxima treatment was shown to be dose-dependent, hence upholding that seaweed extract application needs specific research to optimize biostimulants use based on crops and application systems. Postharvest responses to preharvest E. maxima treatments were also dose-dependent, thus confirming that seaweed extract concentrations should be verified according to yield, quality, and storability goals for different species.
5. Conclusions
Growth and biomass accumulation of leaf lettuce plants were affected by Ecklonia maxima extract concentration in the nutrient solution of a hydroponic floating system. The presence of the seaweed extract in the mineral nutrient solution was effective as a plant biostimulant and yield enhancer, especially between 2 and 4 mL L−1. The growth-promoting effect was evident on various traits such as biomass accumulation, leaf expansion, stomatal conductance, WUE, NUE, etc. Preharvest treatments with E. maxima extract was also effective in delaying senescence and enhancing the shelf-life of minimally processed leaf lettuce. The delay in leaf senescence of the samples treated with E. maxima extract retained an overall quality over the limit of marketability for up to 21 d of cold storage, especially using 2 mL L−1 of extract.
Author Contributions
Conceptualization, A.M. (Alessandro Miceli), A.M. (Alessandra Moncada) and F.V.; data curation, A.M. (Alessandro Miceli) and F.V.; formal analysis, A.M. (Alessandro Miceli), A.M. (Alessandra Moncada) and F.V.; investigation, A.M. (Alessandro Miceli), A.M. (Alessandra Moncada) and F.V.; methodology, A.M. (Alessandro Miceli), A.M. (Alessandra Moncada) and F.V.; supervision, A.M. (Alessandro Miceli), A.M. (Alessandra Moncada) and F.V.; validation, A.M. (Alessandro Miceli) and A.M. (Alessandra Moncada); writing—original draft, A.M. (Alessandro Miceli), A.M. (Alessandra Moncada) and F.V.; writing—review and editing, A.M. (Alessandro Miceli) and F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corbo, M.; Campaniello, D.; Speranza, B.; Bevilacqua, A.; Sinigaglia, M. Non-conventional tools to preserve and prolong the quality of minimally-processed fruits and vegetables. Coatings 2015, 5, 931–961. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, I.; Chattopadhyay, A. Pre- and post-harvest losses in vegetables. In Advances in Postharvest Technologies of Vegetable Crops; Postharvest Biology and Technology Series; Apple Academic Press: Waretown, NJ, USA, 2018; pp. 25–87. [Google Scholar]
- Miceli, A.; Settanni, L. Influence of agronomic practices and pre-harvest conditions on the attachment and development of Listeria monocytogenes in vegetables. Ann. Microbiol. 2019, 69, 185–199. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M.; Dar, B.N.; Greiner, R.; Roohinejad, S. Microbiological contamination of ready-to-eat vegetable salads in developing countries and potential solutions in the supply chain to control microbial pathogens. Food Control 2018, 85, 235–244. [Google Scholar] [CrossRef]
- Al-Chalabi, M. Vertical farming: Skyscraper sustainability? Sustain. Cities Soc. 2015, 18, 74–77. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Despommier, D. The vertical farm: Controlled environment agriculture carried out in tall buildings would create greater food safety and security for large urban populations. J. Verbrauch. Lebensm. 2011, 6, 233–236. [Google Scholar] [CrossRef]
- Kalantari, F.; Tahir, O.M.; Joni, R.A.; Fatemi, E. Opportunities and challenges in sustainability of vertical farming: A review. J. Landsc. Ecol. 2018, 11, 35–60. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Moncada, A.; D’Anna, F. Effect of salt stress in lettuce cultivation. Acta Hortic. 2003, 609, 371–375. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; D’Anna, F. Evaluation of strawberry cultivars in soilless cultivation in sicily. Acta Hortic. 2008, 801, 1121–1127. [Google Scholar] [CrossRef]
- Settanni, L.; Miceli, A.; Francesca, N.; Cruciata, M.; Moschetti, G. Microbiological investigation of Raphanus sativus L. grown hydroponically in nutrient solutions contaminated with spoilage and pathogenic bacteria. Int. J. Food Microbiol. 2013, 160, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncada, A.; Miceli, A.; Sabatino, L.; Iapichino, G.; D’Anna, F.; Vetrano, F. Effect of molybdenum rate on yield and quality of lettuce, escarole, and curly endive grown in a floating system. Agronomy 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- D’Anna, F.; Miceli, A.; Vetrano, F. First results of floating system cultivation of Eruca sativa L. Acta Hortic. 2003, 609, 361–364. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F.; D’Anna, F. First results on yield and quality response of Basil (Ocimum basilicum L.) grown in a floating system. Acta Hortic. 2003, 609, 377–381. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Zandi, P.; Basu, S.K. Role of plant growth-promoting rhizobacteria (PGPR) as Biofertilizers in stabilizing agricultural ecosystems. In Organic Farming for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 71–87. [Google Scholar]
- Aremu, A.O.; Masondo, N.A.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Bíba, O.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K.; et al. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta 2015, 241, 1313–1324. [Google Scholar] [CrossRef]
- Kavipriya, R.; Dhanalakshmi, P.K.; Jayashree, S.; Thangaraju, N. Seaweed extract as a biostimulant for legume crop, green gram. J. Ecobiotechnol. 2011, 3, 16–19. [Google Scholar]
- Verkleij, F.N. Seaweed extracts in agriculture and horticulture: A review. Biol. Agric. Hortic. 1992, 8, 309–324. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Seasonal variation in the polyamines of Ecklonia maxima. Bot. Mar. 2012, 55, 539–546. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Comparison of cytokinin-and auxin-like activity in some commercially used seaweed extracts. J. Appl. Phycol. 1996, 8, 503–508. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Turečová, V.; Strnad, M.; Van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- Khan, W.; Zhai, R.; Souleimanov, A.; Critchley, A.T.; Smith, D.L.; Prithiviraj, B. Commercial extract of Ascophyllum nodosum improves root colonization of alfalfa by its bacterial symbiont Sinorhizobium meliloti. Commun. Soil Sci. Plant Anal. 2012, 43, 2425–2436. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Mooney, P.A.; Van Staden, J. Effect of seaweed concentrate on the growth of wheat under condition of water fern. S. Afr. J. Sci. 1985, 8, 632–633. [Google Scholar]
- Featonby-Smith, B.C.; Van Staden, J. The effect of seaweed concentrate and fertilizer on growth and the endogenous cytokinin content of Phaseolus vulgaris. S. Afr. J. Bot. 1984, 3, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Crouch, I.J.; Van Staden, J. Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- El Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Ghaderiardakani, F.; Collas, E.; Damiano, D.K.; Tagg, K.; Graham, N.S.; Coates, J.C. Effects of green seaweed extract on Arabidopsis early development suggest roles for hormone signalling in plant responses to algal fertilisers. Sci. Rep. 2019, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Bolton, J.J.; Molloy, F.J.; Rotmann, K.W.G. Commercial seaweeds in southern Africa. In Proceedings of the 17th International Seaweed Symposium, Cape Town, South Africa, 28 January–2 February 2001; Oxford University Press: Oxford, UK, 2003; pp. 1–12. [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Kulkarni, M.G.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Effect of a commercial seaweed extract (Kelpak®) and polyamines on nutrient-deprived (N, P and K) okra seedlings. Sci. Hortic. 2013, 151, 142–146. [Google Scholar] [CrossRef]
- Stirk, W.A.; Arthur, G.D.; Lourens, A.F.; Novák, O.; Strnad, M.; van Staden, J. Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J. Appl. Phycol. 2004, 16, 31–39. [Google Scholar] [CrossRef]
- Pitts, R.J.; Cernac, A.; Estelle, M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998, 16, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Basra, A. Plant Growth Regulators in Agriculture and Horticulture: Their Role and Commercial Uses; CRC Press: Boca Raton, FL, USA, 2000; ISBN 1560228911. [Google Scholar]
- De Smet, I.; Lau, S.; Voß, U.; Vanneste, S.; Benjamins, R.; Rademacher, E.H.; Schlereth, A.; De Rybel, B.; Vassileva, V.; Grunewald, W.; et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 2705–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 16, 86–99. [Google Scholar] [CrossRef]
- Ko, D.; Helariutta, Y. Shoot–root communication in flowering plants. Curr. Biol. 2017, 27, R973–R978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-90-481-2531-9. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; van den Boom, T.; Weber, E. Phanologische Entwicklungsstadien von Gemusepflanzen I. Zwiebel-, Wurzel-, Knollen-und Blattgemuse. Nachr. Dtsch. Pflanzenschutzd. 1995, 47, 193–205. [Google Scholar]
- Goto, E.; Both, A.-J.; Albright, L.D.; Langhans, R.W.; Leed, A.R. Effect of dissolved oxygen concentration on lettuce growth in floating hydroponics. In Proceedings of the International Symposium on Plant Production in Closed Ecosystems (ISHS Acta Horticulturae 440), Narita, Japan, 26–29 August 1996; pp. 205–210. [Google Scholar]
- Baligar, V.C.; Fageria, N.K. Nutrient use efficiency in plants: An overview. Nutr. Use Effic. Basics Adv. 2015, 32, 921–950. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy leaf area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef] [PubMed]
- Miceli, A.; Miceli, C. Effect of nitrogen fertilization on the quality of Swiss chard at harvest and during storage as minimally processed produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]
- Rodrigo, M.C.; Ramos, C. Nitrate sap analysis as a tool to assess nitrogen nutrition in artichoke. In Proceedings of the VI International Symposium on Artichoke, Cardoon and Their Wild Relatives 730, Lorca, Spain, 28–31 March 2006; pp. 251–256. [Google Scholar]
- Caracciolo, G.; D’Anna, E.; Moncada, A.; D’Anna, F. Evaluation of the quality and antioxidant capacity of woodland strawberry biotypes in Sicily. J. Food Agric. Environ. 2013, 11, 522–525. [Google Scholar]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2019, 17, 553–558. [Google Scholar] [CrossRef]
- Begum, M.; Bordoloi, B.C.; Singha, D.D.; Ojha, N.J. Role of seaweed extract on growth, yield and quality of some agricultural crops: A review. Agric. Rev. 2018, 39, 321–326. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Finnie, J.F.; van Staden, J. Effect of seaweed concentrate and applied hormones on in vitro cultured tomato roots. J. Plant Physiol. 1985, 120, 215–222. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of vegetal- and seaweed extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel-grown baby lettuce under four regimes of nitrogen fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; El-Nakhel, C.; Leone, V.; Mori, M. Effect of seaweed (Ecklonia maxima) extract and legume-derived protein hydrolysate biostimulants on baby leaf lettuce grown on optimal doses of nitrogen under greenhouse conditions. Aust. J. Crop Sci. 2020, 14, 1456–1464. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Rengasamy, K.R.R.; Pendota, S.C.; Gruz, J.; Plačková, L.; Novák, O.; Doležal, K.; Van Staden, J. Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. New Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Effect of seaweed concentrate from Ecklonia maxima (Osbeck) Papenfuss on Meloidogyne incognita infestation on tomato. J. Appl. Phycol. 1993, 5, 37–43. [Google Scholar] [CrossRef]
- Featonby-Smith, B.C.; Van Staden, J. The effect of seaweed concentrate on the growth of tomato plants in nematode-infested soil. Sci. Hortic. 1983, 20, 137–146. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Castaings, L.; Marchive, C.; Meyer, C.; Krapp, A. Nitrogen signalling in Arabidopsis: How to obtain insights into a complex signalling network. J. Exp. Bot. 2011, 62, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Pitorre, D.; Poretska, O.; Marizzi, C.; Winter, N.; Poppenberger, B.; Sieberer, T. Altered meristem program1 suppresses ectopic stem cell niche formation in the shoot apical meristem in a largely cytokinin-independent manner. Plant Physiol. 2015, 167, 1471–1486. [Google Scholar] [CrossRef] [Green Version]
- Guenot, B.; Bayer, E.; Kierzkowski, D.; Smith, R.S.; Mandel, T.; Žádníková, P.; Benková, E.; Kuhlemeier, C. Pin1-independent leaf initiation in Arabidopsis. Plant Physiol. 2012, 159, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Blein, T.; Hasson, A.; Laufs, P. Leaf development: What it needs to be complex. Curr. Opin. Plant Biol. 2010, 13, 75–82. [Google Scholar] [CrossRef]
- Gonzalez, N.; Vanhaeren, H.; Inzé, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012, 17, 332–340. [Google Scholar] [CrossRef]
- DeMason, D.A.; Chawla, R. Roles for auxin during morphogenesis of the compound leaves of pea (Pisum sativum). Planta 2004, 218, 435–448. [Google Scholar] [CrossRef]
- Wang, S.; Tiwari, S.B.; Hagen, G.; Guilfoyle, T.J. Auxin response factor7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell 2005, 17, 1979–1993. [Google Scholar] [CrossRef] [Green Version]
- Barkoulas, M.; Hay, A.; Kougioumoutzi, E.; Tsiantis, M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 2008, 40, 1136–1141. [Google Scholar] [CrossRef]
- Koenig, D.; Bayer, E.; Kang, J.; Kuhlemeier, C.; Sinha, N. Auxin patterns Solanum lycopersicum leaf morphogenesis. Development 2009, 136, 2997–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Immanen, J.; Nieminen, K.; Smolander, O.P.; Kojima, M.; Alonso Serra, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mähönen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.S.; Sitapara, H.H.; Patel, K.A. Influence of plant growth regulators on growth, yield and quality of tomato and brinjal. Int. J. For. Crop Improv. 2012, 3, 116–118. [Google Scholar]
- Kolachevskaya, O.O.; Lomin, S.N.; Arkhipov, D.V.; Romanov, G.A. Auxins in potato: Molecular aspects and emerging roles in tuber formation and stress resistance. Plant Cell Rep. 2019, 38, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Wadas, W.; Dziugieł, T. Changes in assimilation area and chlorophyll content of very early potato (Solanum tuberosum L.) cultivars as influenced by biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- De Klerk, G.-J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.A.T.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. Vitr. Cell. Dev. Biol. 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell. Dev. Biol. 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Wilson, P.; van Staden, J. Rhizocaline, rooting co-factors, and the concept of promoters and inhibitors of adventitious rooting—A review. Ann. Bot. 1990, 66, 479–490. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Evidence for rooting factors in a seaweed concentrate prepared from Ecklonia maxima. J. Plant Physiol. 1991, 137, 319–322. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A commercial extract of brown macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of spinach in vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Nelson, W.R.; Van Staden, J. The effect of seaweed concentrate on growth of nutrient-stressed, greenhouse cucumbers. HortScience 1984, 19, 81–82. [Google Scholar]
- Nair, P.; Kandasamy, S.; Zhang, J.; Ji, X.; Kirby, C.; Benkel, B.; Hodges, M.D.; Critchley, A.T.; Hiltz, D.; Prithiviraj, B. Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012, 13, 643. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Vetrano, F.; Sabatino, L.; D’Anna, F.; Moncada, A. Influence of preharvest gibberellic acid treatments on postharvest quality of minimally processed leaf lettuce and rocket. Horticulturae 2019, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Roura, S.I.; Davidovich, L.A.; Del Valle, C.E. Quality loss in minimally processed Swiss chard related to amount of damaged area. LWT-Food Sci. Technol. 2000, 33, 53–59. [Google Scholar] [CrossRef]
- Roura, S.I.; Davidovich, L.A.; Del Valle, C.E. Postharvest changes in fresh Swiss chard (Beta vulgaris, type cycla) under different storage conditions. J. Food Qual. 2000, 23, 137–147. [Google Scholar] [CrossRef]
- Hodges, D.M.; Toivonen, P.M.A. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol. Technol. 2008, 48, 155–162. [Google Scholar] [CrossRef]
- Watada, A.E.; Qi, L. Quality of fresh-cut produce. Postharvest Biol. Technol. 1999, 15, 201–205. [Google Scholar] [CrossRef]
- Hare, P.D.; Van Staden, J. The molecular basis of cytokinin action. Plant Growth Regul. 1997, 23, 41–78. [Google Scholar] [CrossRef]
- Veerasamy, M.; He, Y.; Huang, B. Leaf senescence and protein metabolism in creeping bentgrass exposed to heat stress and treated with cytokinins. J. Am. Soc. Hortic. Sci. 2007, 132, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Mok, M.C. Cytokinins: Chemistry, activity, and function. In Cytokinins: Chemistry, Activity and Function; Mok, W.D., Mok, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 155–166. ISBN 978-1315892184. [Google Scholar]
- Zhang, X.; Ervin, E.H. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Wally, O.S.D.; Critchley, A.T.; Hiltz, D.; Craigie, J.S.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithiviraj, B. Regulation of phytohormone biosynthesis and accumulation in arabidopsis following treatment with commercial extract from the marine macroalga ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Vetrano, F.; Moncada, A. Effects of foliar application of gibberellic acid on the salt tolerance of tomato and sweet pepper transplants. Horticulturae 2020, 6, 93. [Google Scholar] [CrossRef]
- Alfonzo, A.; Gaglio, R.; Miceli, A.; Francesca, N.; Di Gerlando, R.; Moschetti, G.; Settanni, L. Shelf life evaluation of fresh-cut red chicory subjected to different minimal processes. Food Microbiol. 2018, 73, 298–304. [Google Scholar] [CrossRef]
- Miceli, A.; Gaglio, R.; Francesca, N.; Ciminata, A.; Moschetti, G.; Settanni, L. Evolution of shelf life parameters of ready-to-eat escarole (Cichorium endivia var. latifolium) subjected to different cutting operations. Sci. Hortic. 2019, 247, 175–183. [Google Scholar] [CrossRef]
- Miceli, A.; Romano, C.; Moncada, A.; D’Anna, F.; Vetrano, F. Effect of cold storage on the quality of minimally processed cauliflower. Carpathian J. Food Sci. Technol. 2015, 7, 70–74. [Google Scholar]
- La Scalia, G.; Aiello, G.; Miceli, A.; Nasca, A.; Alfonzo, A.; Settanni, L. Effect of vibration on the quality of strawberry fruits caused by simulated transport. J. Food Process Eng. 2016, 39, 140–156. [Google Scholar] [CrossRef]
- Miceli, C.; Moncada, A.; Vetrano, F.; D’Anna, F.; Miceli, A. Suitability of borago officinalis for minimal processing as fresh-cut produce. Horticulturae 2019, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Ihl, M.; Shene, C.; Scheuermann, E.; Bifani, V. Correlation for pigment content through colour determination using tristimulus values in a green leafy vegetable, Swiss chard. J. Sci. Food Agric. 1994, 66, 527–531. [Google Scholar] [CrossRef]
- Madeira, A.C.; Ferreira, A.; de Varennes, A.; Vieira, M.I. SPAD Meter versus tristimulus colorimeter to estimate chlorophyll content and leaf color in sweet pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Aharoni, N. Interrelationship between ethylene and growth regulators in the senescence of lettuce leaf discs. J. Plant Growth Regul. 1989, 8, 309–317. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Gaglio, R.; Miceli, A.; Sardina, M.T.; Francesca, N.; Moschetti, G.; Settanni, L. Evaluation of microbiological and physico-chemical parameters of retail ready-to-eat mono-varietal salads. J. Food Process. Preserv. 2019, 43, 13955. [Google Scholar] [CrossRef]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio (Cichorium intybus L.): Nutrient value in fresh vs. stored leaves. J. Food Compos. Anal. 2016, 51, 76–84. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Vetrano, F.; Moncada, A.; Miceli, A. Use of gibberellic acid to increase the salt tolerance of leaf lettuce and rocket grown in a floating system. Agronomy 2020, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of salt stress by plant growth-promoting bacteria in hydroponic leaf lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).