Molecular Insights into the Effects of Rootstocks on Maturation of Blood Oranges
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Biochemical Analysis
2.3. Gene Expression Analysis
2.4. The qPCR Data and Statistical Analysis
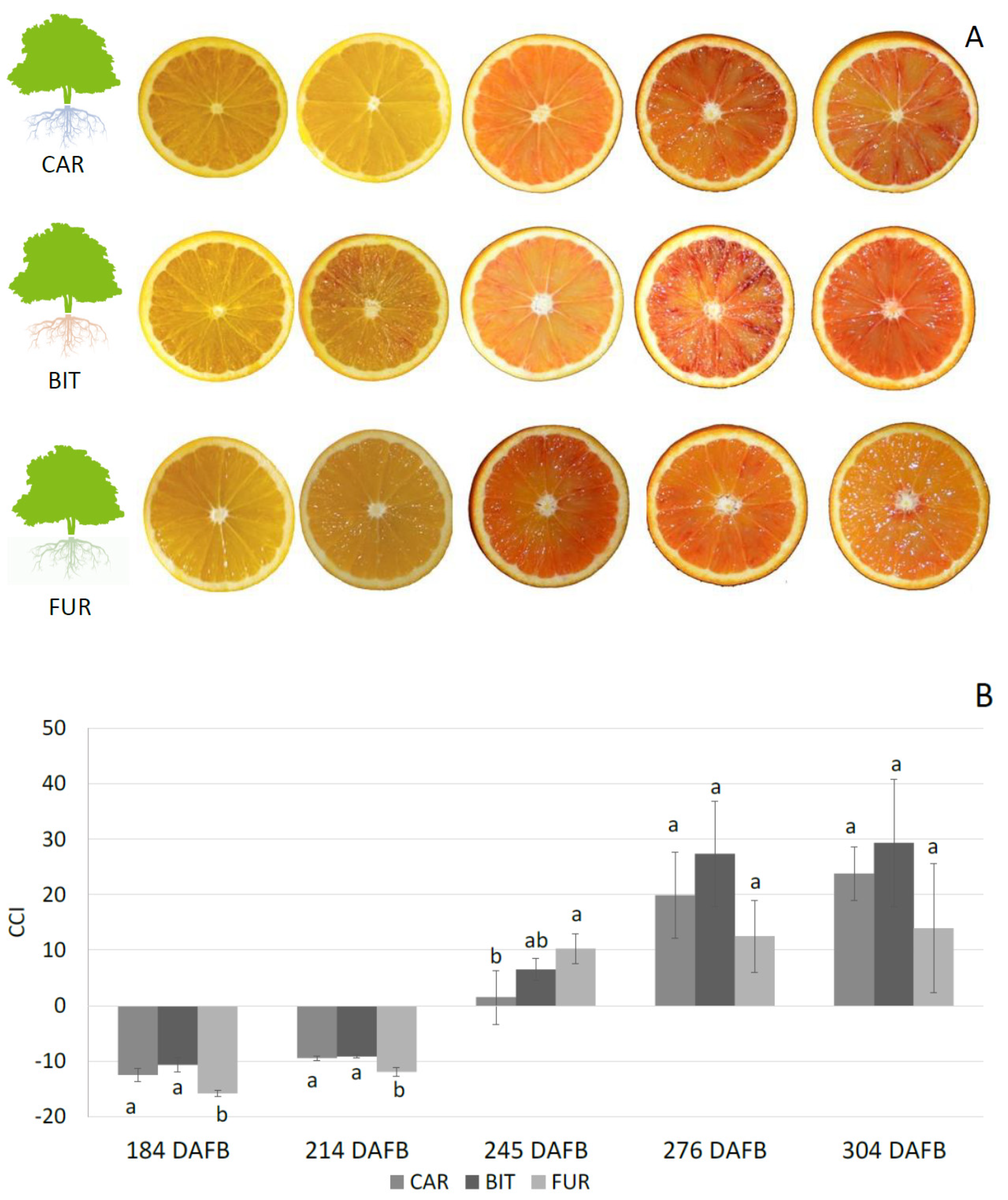
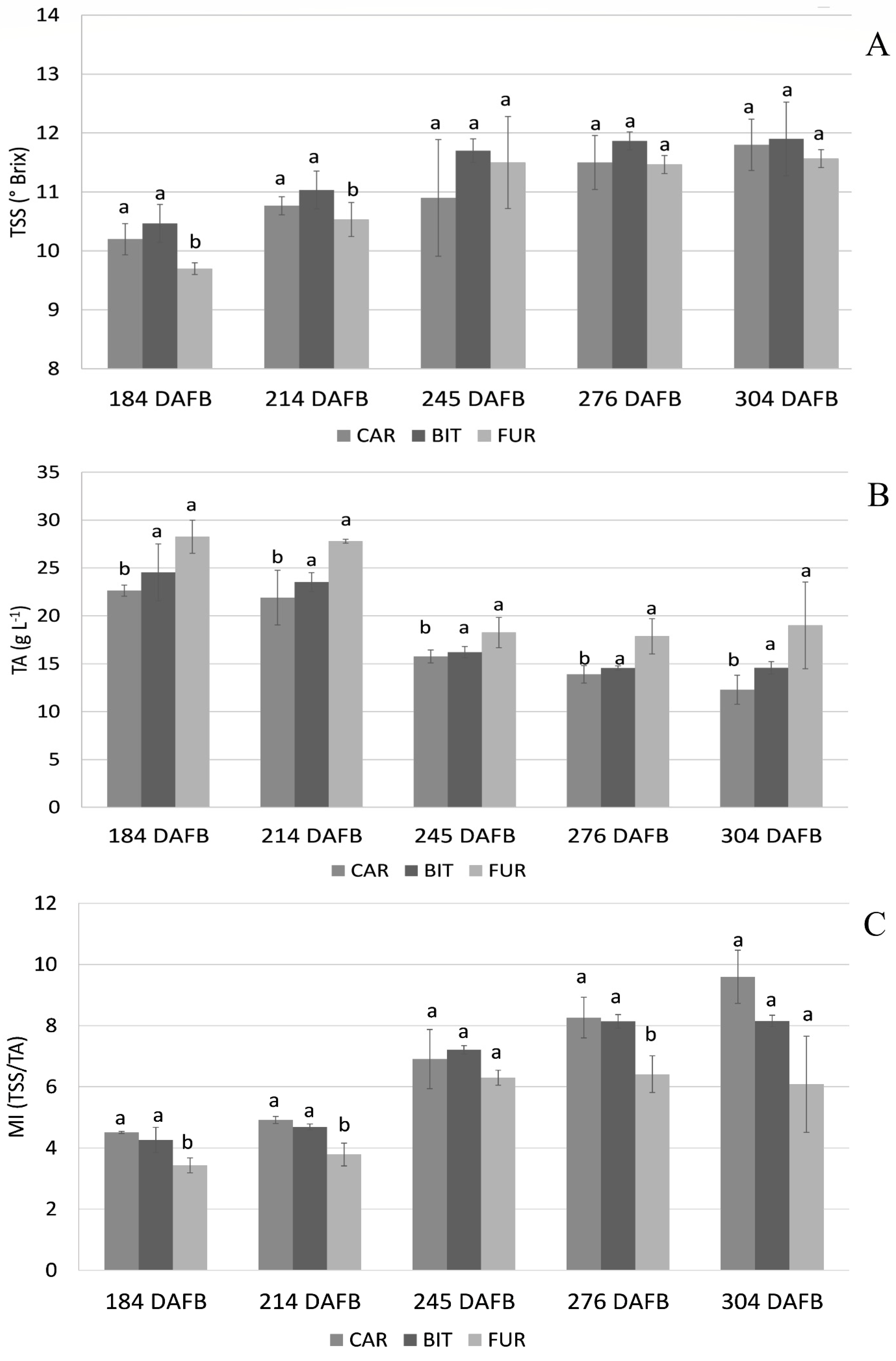
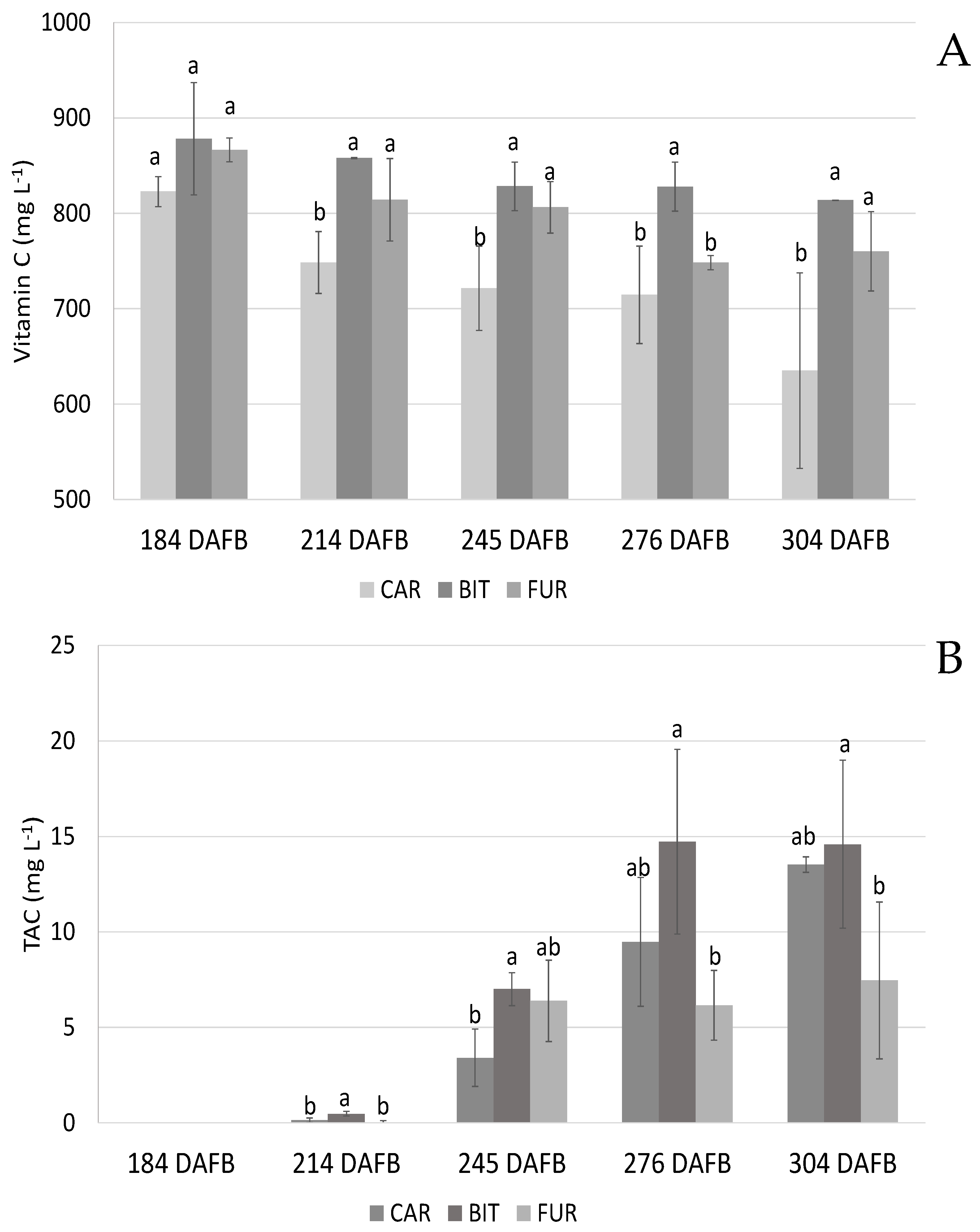
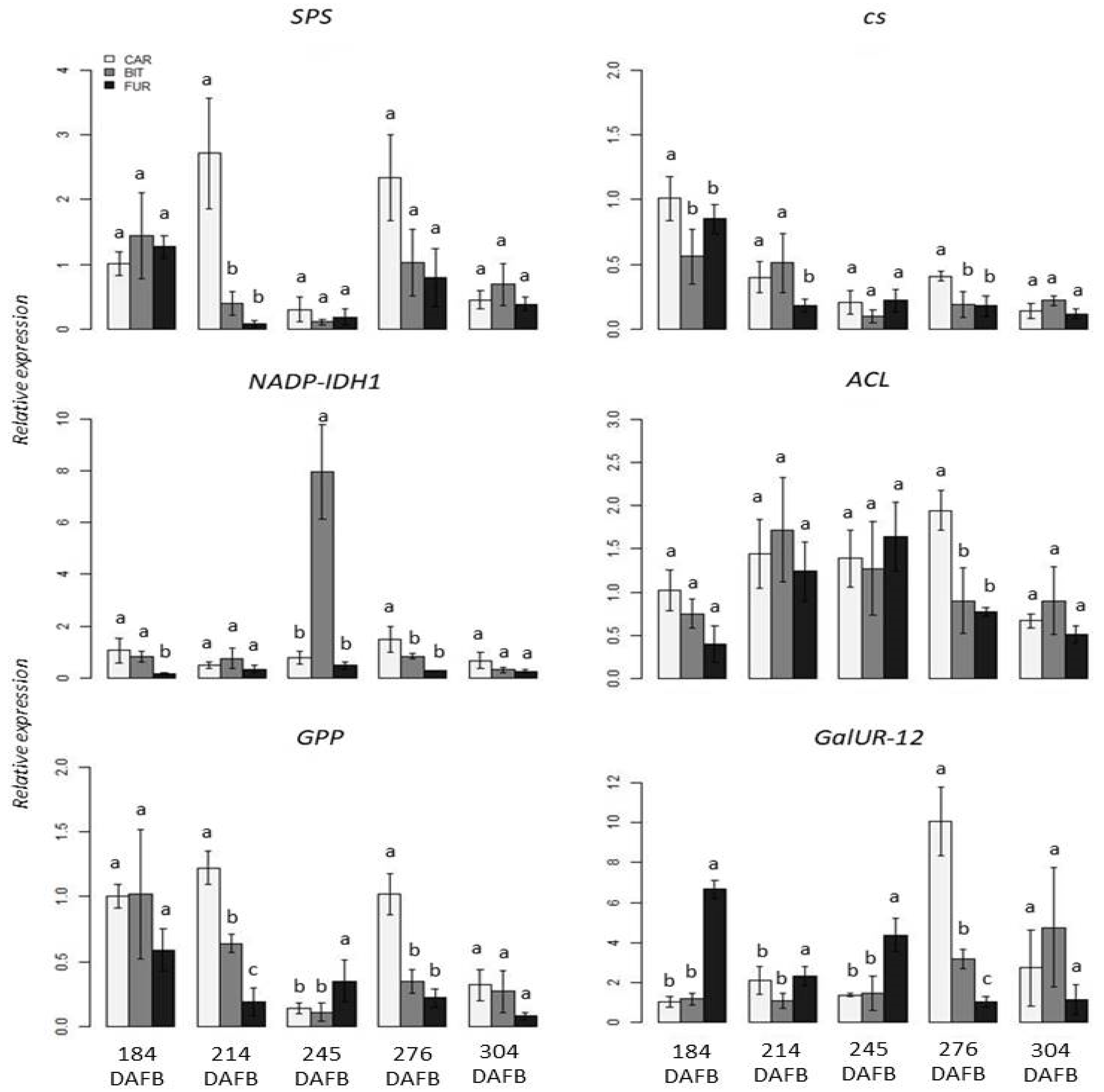
3. Results
3.1. Biochemical Analysis
3.2. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gainza, F.; Opazo, I.; Guajardo, V.; Meza, P.; Ortiz, M.; Pinochet, J.; Muñoz, C. Rootstock breeding in prunus species: Ongoing efforts and new challenges. Chil. J. Agric. Res. 2015, 75, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Forner-Giner, M.A.; Alcaide, A.; Primo-Millo, E.; Forner, J.B. Performance of “Navelina” orange on 14 rootstocks in Northern Valencia (Spain). Sci. Hortic. 2003, 98, 223–232. [Google Scholar] [CrossRef]
- Castle, W.S. Rootstock as a fruit quality factor in citrus and deciduous tree crops. New Zeal. J. Crop Hortic. Sci. 1995, 23, 383–394. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, C.; Dong, W.; Jiang, Q.; Wang, D.; Li, S.; Chen, M.; Liu, C.; Sun, C.; Chen, K. Transcriptome and metabolome analyses of sugar and organic acid metabolism in Ponkan (Citrus reticulata) fruit during fruit maturation. Gene 2015, 554, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Forner, J.B.; Hernández, F.; Forner-Giner, M.A. Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. ex Tan.): Variation from rootstock. Sci. Hortic. 2014, 174, 60–64. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lipan, L.; Hernández, F.; Martínez, J.J.; Legua, P.; Carbonell-Barrachina, Á.A.; Melgarejo, P. Quality Parameters, Volatile Composition, and Sensory Profiles of Highly Endangered Spanish Citrus Fruits. J. Food Qual. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Forner-Giner, M.A.; Continella, A.; Grosser, J. Citrus Rootstock Breeding and Selection. In The Citrus Genome; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Abouzari, A.; Nezhad, N.M. The investigation of citrus fruit quality. Popular characteristic and breeding. Acta Univ. Agric. Silvic. Mendelianae Brun. 2016, 64, 725–740. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Medina, V.; Carmona, L.; Bruno, M.; Al-Babili, S.; Zacarías, L. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J. Exp. Bot. 2013, 64, 4461–4478. [Google Scholar] [CrossRef]
- Lo Piero, A.R. The state of the art in biosynthesis of anthocyanins and its regulation in pigmented sweet oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem 2015, 63, 4031–4041. [Google Scholar] [CrossRef]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.Á.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2020, 342, 128305. [Google Scholar] [CrossRef]
- Carmona, L.; Alquézar, B.; Diretto, G.; Sevi, F.; Malara, T.; Lafuente, M.T.; Peña, L. Curing and low-temperature combined post-harvest storage enhances anthocyanin biosynthesis in blood oranges. Food Chem. 2020, 128334. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Mistretta, A.; Marventano, S.; Nolfo, F.; Calabrese, G.; Buscemi, S.; Drago, F.; Veronesi, U.; Scuderi, A. Red orange: Experimental models and epidemiological evidence of its benefits on human health. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Caruso, M.; Continella, A.; Modica, G.; Pannitteri, C.; Russo, R.; Salonia, F.; Arlotta, C.; Gentile, A.; Russo, G. Rootstocks influence yield precocity, productivity, and pre-harvest fruit drop of mandared pigmented mandarin. Agronomy 2020, 10, 1305. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Hervalejo, A.; Pereira-Caro, G.; Muñoz-Redondo, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J.; Moreno-Rojas, J.M. Effect of Rootstock and Harvesting Period on the Bioactive Compounds and Antioxidant Activity of Two Orange Cultivars (‘Salustiana’ and ‘Sanguinelli’) Widely Used in Juice Industry. Processes 2020, 8, 1212. [Google Scholar] [CrossRef]
- Cebadera-Miranda, L.; Domínguez, L.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Igual, M.; Martínez-Navarrete, N.; Fernández-Ruiz, V.; Morales, P.; Cámara, M. Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): Bioactive compounds and colour appearance of blood oranges. Food Chem. 2019, 270, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, G.; Tietel, Z.; Porat, R. Effects of rootstock/scion combinations on the flavor of citrus fruit. J. Agric. Food Chem. 2013, 61, 11286–11294. [Google Scholar] [CrossRef] [PubMed]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. (Amsterdam) 2018, 233, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Terol, J.; Soler, G.; Talon, M.; Cercos, M. The aconitate hydratase family from Citrus. BMC Plant Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.X.; Shi, C.Y.; Liu, X.; Ning, D.Y.; Jing, L.F.; Yang, H.; Liu, Y.Z. Citrate accumulation-related gene expression and/or enzyme activity analysis combined with metabolomics provide a novel insight for an orange mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, A.; Takanokura, Y.; Moriguchi, T.; Omura, M.; Akihama, T. Differential expression of three sucrose-phosphate synthase isoforms during sucrose accumulation in citrus fruits (Citrus unshiu Marc.). Plant Sci. 1999, 140, 169–178. [Google Scholar] [CrossRef]
- Langenkamper, G.; Fung, R.W.M.; Newcomb, R.; Atkinson, R.G.; Gardner, R.C.; MacRae, E. Sucrose Phosphate Synthase Genes in Plants Belong to Three Different Families. J. Mol. Evol. 2002, 54, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Completing a pathway to plant vitamin C synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 9109–9110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellidou, I.; Kanellis, A.K. Genetic control of ascorbic acid biosynthesis and recycling in horticultural crops. Front. Chem. 2017, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arena, E.; Fallico, B.; Maccarone, E. Evaluation of antioxidant capacity of blood orange juices as influenced by constituents, concentration process and storage. Food Chem. 2001, 74, 423–427. [Google Scholar] [CrossRef]
- Rapisarda, P.; Lo Bianco, M.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Rapisarda, P.; Tomaino, A.; Lo Cascio, R.; Bonina, F.; De Pasquale, A.; Saija, A. Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. J. Agric. Food Chem. 1999, 47, 4718–4723. [Google Scholar] [CrossRef] [PubMed]
- Pannitteri, C.; Continella, A.; Lo Cicero, L.; Gentile, A.; La Malfa, S.; Sperlinga, E.; Napoli, E.M.; Strano, T.; Ruberto, G.; Siracusa, L. Influence of postharvest treatments on qualitative and chemical parameters of Tarocco blood orange fruits to be used for fresh chilled juice. Food Chem. 2017, 230, 441–447. [Google Scholar] [CrossRef]
- DOGV. Diari Oficial de la Comunitat Valenciana 5346. 2006, pp. 30321–30328. Available online: https://dogv.gva.es/datos/2006/09/14/pdf/dogv_5346A.pdf (accessed on 8 August 2021).
- Butelli, E.; Licciardello, C.; Ramadugu, C.; Durand-Hulak, M.; Celant, A.; Reforgiato Recupero, G.; Froelicher, Y.; Martin, C. Noemi Controls Production of Flavonoid Pigments and Fruit Acidity and Illustrates the Domestication Routes of Modern Citrus Varieties. Curr. Biol. 2019, 29, 158–164.e2. [Google Scholar] [CrossRef] [Green Version]
- Lo Piero, A.R.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef]
- Alós, E.; Rodrigo, M.J.; Zacarías, L. Differential transcriptional regulation of l-ascorbic acid content in peel and pulp of citrus fruits during development and maturation. Planta 2014, 239, 1113–1128. [Google Scholar] [CrossRef]
- Dvinge, H.; Bertone, P. HTqPCR: High-throughput analysis and visualization of quantitative real-time PCR data in R. Bioinformatics 2009, 25, 3325–3326. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Guo, Y.; Sheng, Q.; Shyr, Y. Heatmap3: An improved heatmap package with more powerful and convenient features. BMC Bioinform. 2014, 15, 15–16. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, R.N.; Palmeri, R.; Fabiano, S.; Rapisarda, P.; Spagna, G. Characteristic of β-glucosidase from Sicilian blood oranges in relation to anthocyanin degradation. Enzyme Microb. Technol. 2007, 41, 570–575. [Google Scholar] [CrossRef]
- Continella, A.; Pannitteri, C.; La Malfa, S.; Legua, P.; Distefano, G.; Nicolosi, E.; Gentile, A. Influence of different rootstocks on yield precocity and fruit quality of ‘Tarocco Scirè’ pigmented sweet orange. Sci. Hortic. 2018, 230, 62–67. [Google Scholar] [CrossRef]
- Nadernejad, N.; Ahmadimoghadam, A.; Hossyinifard, J.; Poorseyedi, S. Effect of different rootstocks on PAL activity and phenolic compounds in flowers, leaves, hulls and kernels of three pistachio (Pistacia vera L.) cultivars. Trees-Struct. Funct. 2013, 27, 1681–1689. [Google Scholar] [CrossRef]
- Shirley, B.W.; Kubasek, W.L.; Storz, G.; Bruggemann, E.; Koornneef, M.; Ausubel, F.M.; Goodman, H.M. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995, 8, 659–671. [Google Scholar] [CrossRef]
- Lo Piero, A.R.; Puglisi, I.; Petrone, G. Gene characterization, analysis of expression and in vitro synthesis of dihydroflavonol 4-reductase from Citrus sinensis (L.) Osbeck. Phytochemistry 2006, 67, 684–695. [Google Scholar] [CrossRef]
- Catalano, C.; Ciacciulli, A.; Salonia, F.; Russo, M.P.; Caruso, P.; Caruso, M.; Russo, G.; Distefano, G.; Licciardello, C. Target-genes reveal species and genotypic specificity of anthocyanin pigmentation in citrus and related genera. Genes 2020, 11, 807. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Cotroneo, P.S.; Russo, M.P.; Ciuni, M.; Recupero, G.R.; Lo Piero, A.R. Quantitative real-time reverse transcriptase-PCR profiling of anthocyanin biosynthetic genes during orange fruit ripening. J. Am. Soc. Hortic. Sci. 2006, 131, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin biosynthesis and accumulation in blood oranges during postharvest storage at different low temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef]
- Di Matteo, A.; Di Rauso Simeone, G.; Cirillo, A.; Rao, M.A.; Di Vaio, C. Morphological characteristics, ascorbic acid and antioxidant activity during fruit ripening of four lemon (Citrus limon (L.) Burm. F.) cultivars. Sci. Hortic. 2021, 276, 109741. [Google Scholar] [CrossRef]
- Domingues, A.R.; Marcolini, C.D.M.; da Gonçalves, C.H.S.; Gonçalves, L.S.A.; Roberto, S.R.; Carlos, E.F. Fruit ripening development of ‘Valencia’ orange trees grafted on different ‘Trifoliata’ hybrid rootstocks. Horticulturae 2021, 7, 3. [Google Scholar] [CrossRef]
- Ben Abdelaali, S.; Rodrigo, M.J.; Saddoud, O.; Zacarías, L.; Hajlaoui, M.R.; Mars, M. Carotenoids and colour diversity of traditional and emerging Tunisian orange cultivars (Citrus sinensis (L.) Osbeck). Sci. Hortic. 2018, 227, 296–304. [Google Scholar] [CrossRef]
- Cercós, M.; Soler, G.; Iglesias, D.J.; Gadea, J.; Forment, J.; Talón, M. Global analysis of gene expression during development and ripening of citrus fruit flesh. A proposed mechanism for citric acid utilization. Plant Mol. Biol. 2006, 62, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, Q.; Yin, X.R.; Lin, Q.; Chen, J.Y.; Allan, A.C.; Xu, C.J.; Chen, K.S. Effect of hot air treatment on organic acid- and sugar-metabolism in Ponkan (Citrus reticulata) fruit. Sci. Hortic. 2012, 147, 118–125. [Google Scholar] [CrossRef]
- Chen, M.; Xie, X.; Lin, Q.; Chen, J.; Grierson, D.; Yin, X.; Sun, C.; Chen, K. Differential Expression of Organic Acid Degradation-Related Genes During Fruit Development of Navel Oranges (Citrus sinensis) in Two Habitats. Plant Mol. Biol. Report. 2013, 31, 1131–1140. [Google Scholar] [CrossRef]
- Hu, X.M.; Shi, C.Y.; Liu, X.; Jin, L.F.; Liu, Y.Z.; Peng, S.A. Genome-wide identification of citrus ATP-citrate lyase genes and their transcript analysis in fruits reveals their possible role in citrate utilization. Mol. Genet. Genomics 2014, 290, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Salvador, A.; Besada, C.; Navarro, P.; Bermejo, A. Physico-chemical, sensorial and nutritional quality during the harvest season of ‘Tango’ mandarins grafted onto Carrizo Citrange and Forner-Alcaide no. 5. Food Chem. 2021, 339, 127781. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bellomo, S.E.; Intelisano, S. Storage Temperature Effects on Blood Orange Fruit Quality. J. Agric. Food Chem. 2001, 49, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Mondello, L.; Cotroneo, A.; Errante, G.; Dugo, G.; Dugo, P. Determination of anthocyanins in blood orange juices by HPLC analysis. J. Pharm. Biomed. Anal. 2000, 23, 191–195. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef]
- Suriano, S.; Alba, V.; Di Gennaro, D.; Suriano, M.S.; Savino, M.; Tarricone, L. Genotype/rootstocks effect on the expression of anthocyanins and flavans in grapes and wines of Greco Nero n. (Vitis vinifera L.). Sci. Hortic. 2016, 209, 309–315. [Google Scholar] [CrossRef]
- Font, I.; Forcada, C.; Guajardo, V.; Chin-Wo, S.R.; Moreno, M.Á. Association mapping analysis for fruit quality traits in prunus persica using SNP markers. Front. Plant Sci. 2019, 9, 2005. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.K.; Sharma, R.M. Effect of rootstocks on tree growth, yield, quality and leaf mineral composition of lemon (Citrus limon (L.) Burm.). Sci. Hortic. 2016, 200, 131–136. [Google Scholar] [CrossRef]
- Sharma, R.M.; Dubey, A.K.; Awasthi, O.P.; Kaur, C. Growth, yield, fruit quality and leaf nutrient status of grapefruit (Citrus paradisi Macf.): Variation from rootstocks. Sci. Hortic. 2016, 210, 41–48. [Google Scholar] [CrossRef]
- Sharma, R.M.; Dubey, A.K.; Awasthi, O.P. Physiology of grapefruit (Citrus paradisi Macf.) cultivars as affected by rootstock. J. Hortic. Sci. Biotechnol. 2015, 90, 325–331. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.M.; Jin, L.F.; Shi, C.Y.; Liu, Y.Z.; Peng, S.A. Identification and transcript analysis of two glutamate decarboxylase genes, CsGAD1 and CsGAD2, reveal the strong relationship between CsGAD1 and citrate utilization in citrus fruit. Mol. Biol. Rep. 2014, 41, 6253–6262. [Google Scholar] [CrossRef]
- Bennici, S.; Las Casas, G.; Distefano, G.; Gentile, A.; Lana, G.; Di Guardo, M.; Nicolosi, E.; La Malfa, S.; Continella, A. Rootstock affects floral induction in citrus engaging the expression of the flowering locus t (Cift). Agriculture 2021, 11, 140. [Google Scholar] [CrossRef]
- Tzarfati, R.; Ben-Dor, S.; Sela, I.; Goldschmidt, E.E. Graft-induced Changes in MicroRNA Expression Patterns in Citrus Leaf Petioles. Open Plant Sci. J. 2013, 7, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Cao, J.; Su, M.; Feng, G.; Xu, Y.; Yi, H. Genome-wide comprehensive analysis of transcriptomes and small RNAs offers insights into the molecular mechanism of alkaline stress tolerance in a citrus rootstock. Hortic. Res. 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Accession Number | Gene Name | Forward 5′-3′ | Reverse 5′-3′ | Reference |
|---|---|---|---|---|
| XM_006481431.3 | PAL | GATTTGAGACATTTGGAGGA | ATGGATGAAGCTCTCCACTA | |
| XM_006420545.2 | CHS | TCTATCGACGGGCATCTTC | TGCCTCGGTTAGGCTTTT | [34] |
| NM_001288931.1 | DFR | GCTGTTCGTGCTACTGTTC | GGCTAAATCGGCTTTCCATA | |
| XM_025097974.1 | ANS | GGGTGACTGCTAAATGTGTT | CAAGTCCCCTGTGAAGAATA | |
| NM_001320060.1 | UFGT | TCTTCAGCACTCCGCAATC | TCCATCGGATACGTCGTAAG | |
| NM_001288889.1 | R2R3Myb | ACAATCCACCCCGTCTGATC | CTGGCCTGCTTCAATGACTC | |
| XM_006483335.2 | SPS | TTGTAACTAGCACCCGACAGG | CAACCATACGAGGCATAAACC | [4] |
| XM_006480234.2 | ACL | GATACTGTTGGAGACTTGGG | GCTCTCTTACGACCATCAGG | [23] |
| XM_006494513.2 | NADP-ID184 DAFB | GAAAATTGGGGATTGGGATT | CAACAGAGGTGCAGCTCAAA | [23] |
| XM_006482744.2 | CS | GGTGCCCCCAATATTAACAA | AGAGCTCGGTCCCATATCAA | [23] |
| XM_006474957.2 | GGP | TACCAAAGTGGGGCAAGAAG | TGGCAACAACACTTGGAGAA | [35] |
| XM_006492225.2 | GalUR-12 | CCCAGGTTTCTTTGAGGTGGGTTTATC | TACTGTGGAATTTGTTCGATCTTTTGCAGC | [35] |
| AY498567 | EF | CACCACCCCCAAGTACTC | GTTGTCACCCTCGAAACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lana, G.; Modica, G.; Las Casas, G.; Siracusa, L.; La Malfa, S.; Gentile, A.; Sicilia, A.; Distefano, G.; Continella, A. Molecular Insights into the Effects of Rootstocks on Maturation of Blood Oranges. Horticulturae 2021, 7, 468. https://doi.org/10.3390/horticulturae7110468
Lana G, Modica G, Las Casas G, Siracusa L, La Malfa S, Gentile A, Sicilia A, Distefano G, Continella A. Molecular Insights into the Effects of Rootstocks on Maturation of Blood Oranges. Horticulturae. 2021; 7(11):468. https://doi.org/10.3390/horticulturae7110468
Chicago/Turabian StyleLana, Giuseppe, Giulia Modica, Giuseppina Las Casas, Laura Siracusa, Stefano La Malfa, Alessandra Gentile, Angelo Sicilia, Gaetano Distefano, and Alberto Continella. 2021. "Molecular Insights into the Effects of Rootstocks on Maturation of Blood Oranges" Horticulturae 7, no. 11: 468. https://doi.org/10.3390/horticulturae7110468
APA StyleLana, G., Modica, G., Las Casas, G., Siracusa, L., La Malfa, S., Gentile, A., Sicilia, A., Distefano, G., & Continella, A. (2021). Molecular Insights into the Effects of Rootstocks on Maturation of Blood Oranges. Horticulturae, 7(11), 468. https://doi.org/10.3390/horticulturae7110468









