Control of Penicillium expansum by an Epiphytic Basidiomycetous Yeast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Putative Biocontrol Strains
2.2. Biocontrol Agent and Pathogen Inoculum
2.3. Biocontrol Agent Identification
2.4. Biocontrol Assays
2.5. Biocontrol Mode of Action
2.6. Data Analysis
3. Results
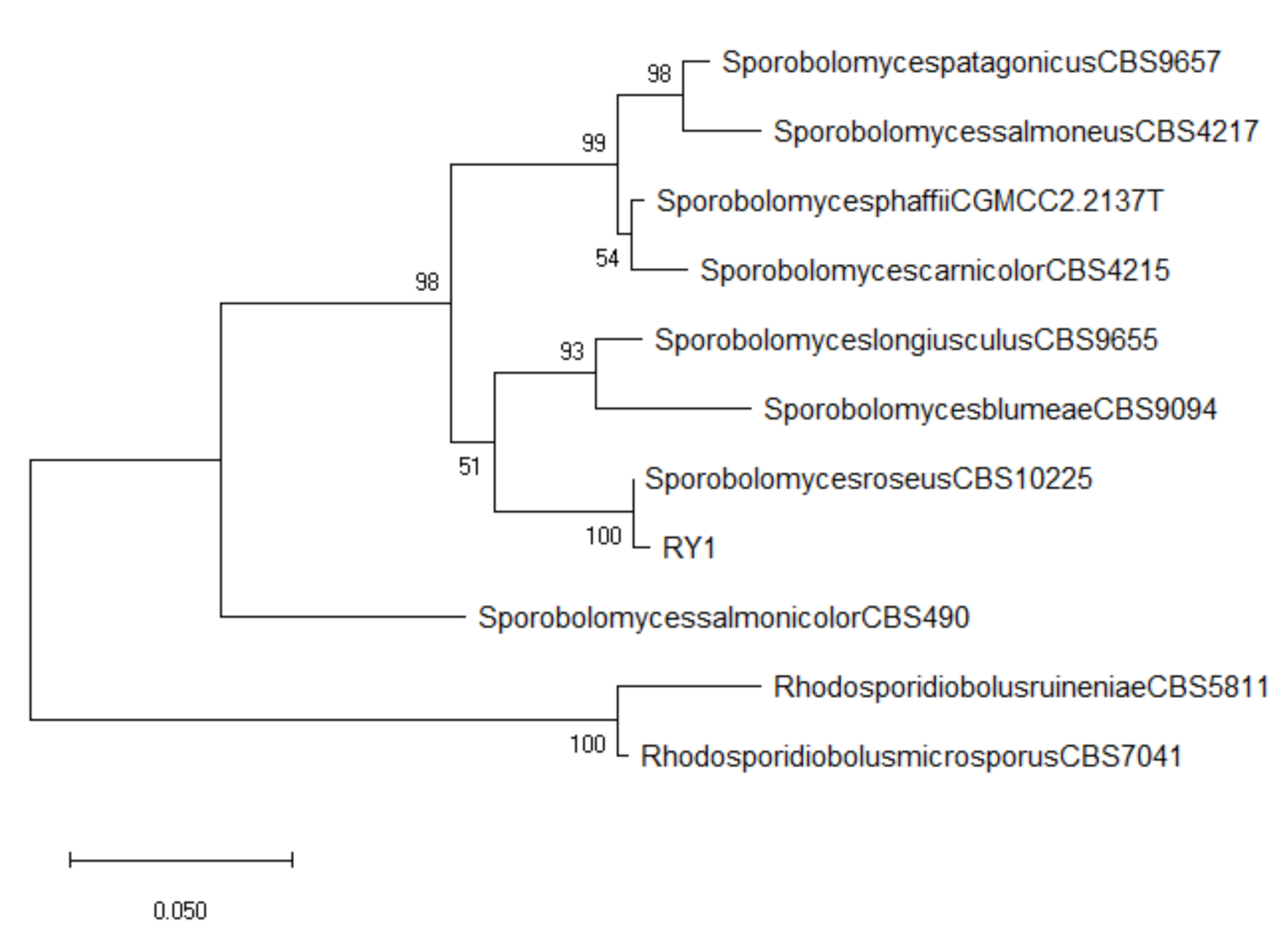
3.1. Biocontrol Agent Isolation and Identification
3.2. Biocontrol Assays
3.3. Evaluation of the Mode of Action
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 2002, 24, 123–134. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Teixidó, N.; Usall, J.; Magan, N.; Medina, A. Predicted ecological niches and environmental resilience of different formulations of the biocontrol yeast Candida sake CPA-1 using the Bioscreen C. BioControl 2018, 63, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, A.; Schena, L.; Pentimone, I.; Nigro, F. Control of postharvest rots of sweet cherries by pre-and postharvest applications of Aureobasidium pullulans in combination with calcium chloride or sodium bicarbonate. Postharvest Biol. Technol. 2005, 36, 245–252. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Spadaro, D.; Ciavorella, A.; Zhang, D.; Garibaldi, A.; Gullino, M.L. Effect of culture media and pH on the biomass production and biocontrol efficacy of a Metschnikowia pulcherrima strain to be used as a biofungicide for postharvest disease control. Can. J. Microbiol. 2010, 56, 128–137. [Google Scholar] [CrossRef]
- European Commission, Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 365, 5–24.
- Lorenzini, M.; Zapparoli, G.; Azzolini, M.; Carvalho, C.; Sampaio, J.P. Sporobolomyces agrorum sp. nov. and Sporobolomyces sucorum sp. nov., two novel basidiomycetous yeast species isolated from grape and apple must in Italy. Int. J. Syst. Evol. Microbiol. 2019, 69, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Peterson, D.L.; Bors, R. Control of storage decay of apples with Sporobolomyces roseus. Plant Dis. 1994, 78, 466–470. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; De Cicco, V.; Ippolito, A. Early detection of Botrytis cinerea latent infections as a tool to improve postharvest quality of table grapes. Postharvest Biol. Technol. 2012, 68, 64–71. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogeneties. In PCR Protocols. A guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, J.W., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; De Girolamo, A.; Schena, L.; Solfrizzo, M.; Ippolito, A.; Visconti, A. Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone. Eur. Food Res. Technol. 2009, 228, 381–389. [Google Scholar] [CrossRef]
- Mari, M.; Bertolini, P.; Pratella, G.C. Non-conventional methods for the control of post-harvest pear diseases. J. Appl. Microbiol. 2003, 94, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.M.; Groenewald, M.; Takashima, M.; Theelen, B.; Han, P.J.; Liu, X.-Z.; Boekhout, T.; Bai, F.-Y. Phylogeny of yeasts and related filamentous fungi within Pucciniomycotina determined from multigene sequence analyses. Stud. Mycol. 2015, 81, 27–53. [Google Scholar] [CrossRef] [Green Version]
- Dik, A.J.; Fokkema, N.J.; van Pelt, J.A. Influence of climatic and nutritional factors on yeast population dynamics in the phyllosphere of wheat. Micro. Ecol. 1992, 23, 41–52. [Google Scholar] [CrossRef]
- Fokkema, N.J.; Den Houter, J.G.; Kosterman, Y.J.C.; Nelis, A.L. Manipulation of yeasts on field-grown wheat leaves and their antagonistic effect on Cochliobolus sativus and Septoria nodorum. Trans. Br. Mycol. Soc. 1979, 72, 19–29. [Google Scholar] [CrossRef]
- Tyagi, S.; Dube, V.P.; Charaya, M.U. Biological control of the purple blotch of onion caused by Alternaria porri (Ellis) Ciferri. Int. J. Pest Manag. 1990, 36, 384–386. [Google Scholar]
- Davoli, P.; Mierau, V.; Weber, R.W.S. Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl. Biochem. Microbiol. 2004, 40, 392–397. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Punelli, M.; Ippolito, A.; Fanelli, C. Study on the role of patulin on pathogenicity and virulence of Penicillium expansum. Int. J. Food Microbiol. 2012, 153, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ianiri, G.; Idnurm, A.; Wright, S.A.; Durán-Patrón, R.; Mannina, L.; Ferracane, R.; Ritieni, A.; Castoria, R. Searching for genes responsible for patulin degradation in a biocontrol yeast provides insight into the basis for resistance to this mycotoxin. Appl. Environ. Microbiol. 2013, 79, 3101–3115. [Google Scholar] [CrossRef] [Green Version]
- Fokkema, N.J. Competition for endogenous and exogenous nutrients between Sporobolomyces roseus and Cochliobolus sativus. Can. J. Bot. 1984, 62, 2463–2468. [Google Scholar] [CrossRef]
- Fredlund, E.; Druvefors, U.; Lingsten, K.-J.; Boysen, M.E.; Schnürer, J. 2002. Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Res. 2002, 2, 395–402. [Google Scholar] [PubMed]
- Strobel, G.A.; Kluck, K.; Hess, W.M.; Sears, J.; Ezra, D.; Vargas, P.N. Muscodor albus E-6, an endophyte of Guazuma ulmifolia making volatile antibiotics: Isolation, characterization and experimental establishment in the host plant. Microbiology 2007, 153, 2613–2620. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, I.S.; Goelen, T.; Herrera-Malaver, B.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Associative learning and memory retention of nectar yeast volatiles in a generalist parasitoid. Anim. Behav. 2019, 153, 137–146. [Google Scholar] [CrossRef]
- Chauhan, N.M.; Raut, J.S.; Karuppayil, S.M. Acetaldehyde inhibits the yeast-to-hypha conversion and biofilm formation in Candida albicans. Mycoscience 2011, 52, 356–360. [Google Scholar] [CrossRef]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Uittamo, J.; Siikala, E.; Kaihovaara, P.; Salaspuro, M.; Rautemaa, R. Chronic candidosis and oral cancer in APECED-patients: Production of carcinogenic acetaldehyde from glucose and ethanol by Candida albicans. Int. J. Cancer 2009, 124, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; McSpadden Gardener, B. Biological Control of Plant Pathogens. The Plant Health Instructor. Available online: https://www.apsnet.org/edcenter/disimpactmngmnt/topc/Documents/PHI-BiologicalControl.pdf (accessed on 20 September 2021).
- Avissar, I.; Droby, S.; Pesis, E. Characterisation of acetaldehyde effects on Rhizopus stolonifer and Botrytis cinerea. Ann. Appl. Biol. 1990, 116, 213–220. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Białkowska, A.M.; Krysiak, J.; Florczak, T.; Szulczewska, K.M.; Wanarska, M.; Turkiewicz, M. The psychrotrophic yeast Sporobolomyces roseus LOCK 1119 as a source of a highly active aspartic protease for the in vitro production of antioxidant peptides. Biotechnol. Appl. Biochem. 2018, 65, 726–738. [Google Scholar] [CrossRef] [PubMed]




| Species | Isolate | Genbank Accession No. |
|---|---|---|
| Sporobolomyces patagonicus | CBS9657 | NR_137666 |
| Sporobolomyces phaffii | CGMCC2.2137 T | NR_137660 |
| Sporobolomyces salmoneus | CBS4217 | KY105531 |
| Sporobolomyces carnicolor | CBS4215 | NR_137659 |
| Sporobolomyces longiusculus | CBS9655 | JN246566 |
| Sporobolomyces blumeae | CBS9094 | KY105507 |
| Sporobolomyces salmonicolor | CBS490 | NR_149325 |
| Sporobolomyces roseus | CBS10225 | NR_137659 |
| Rhodosporidiobolus ruineniae | CBS5811 | AF444491 |
| Rhodosporidiobolus microsporus | CBS7041 | NR_073290 |
| Production of Volatiles | Antibiosis | |
|---|---|---|
| Control | 27.0 ± 4.0 a | 26.0 ± 1.0 a |
| S. roseus | 17.5 ± 2.0 b | 24.0 ± 3.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanzani, S.M.; Sgaramella, M.; Mosca, S.; Solfrizzo, M.; Ippolito, A. Control of Penicillium expansum by an Epiphytic Basidiomycetous Yeast. Horticulturae 2021, 7, 473. https://doi.org/10.3390/horticulturae7110473
Sanzani SM, Sgaramella M, Mosca S, Solfrizzo M, Ippolito A. Control of Penicillium expansum by an Epiphytic Basidiomycetous Yeast. Horticulturae. 2021; 7(11):473. https://doi.org/10.3390/horticulturae7110473
Chicago/Turabian StyleSanzani, Simona Marianna, Michele Sgaramella, Saveria Mosca, Michele Solfrizzo, and Antonio Ippolito. 2021. "Control of Penicillium expansum by an Epiphytic Basidiomycetous Yeast" Horticulturae 7, no. 11: 473. https://doi.org/10.3390/horticulturae7110473
APA StyleSanzani, S. M., Sgaramella, M., Mosca, S., Solfrizzo, M., & Ippolito, A. (2021). Control of Penicillium expansum by an Epiphytic Basidiomycetous Yeast. Horticulturae, 7(11), 473. https://doi.org/10.3390/horticulturae7110473







