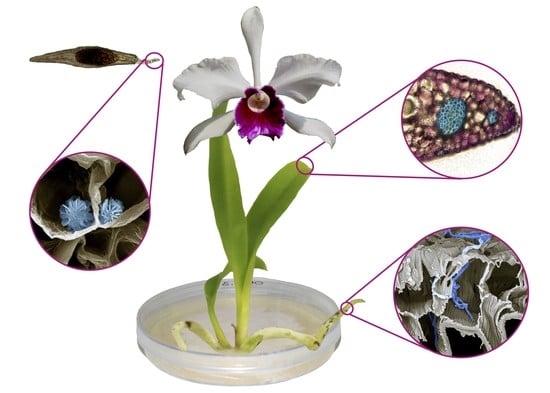
Seed Micromorphology, In Vitro Germination, and Early-Stage Seedling Morphological Traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flower Hand Pollination and Seed Collection
2.2. Seed Sowing
2.3. Microscopy
2.3.1. Plant Material
2.3.2. Light Microscopy
2.3.3. Scanning Electron Microscopy
2.4. Data Analysis
3. Results
3.1. Seed Germination
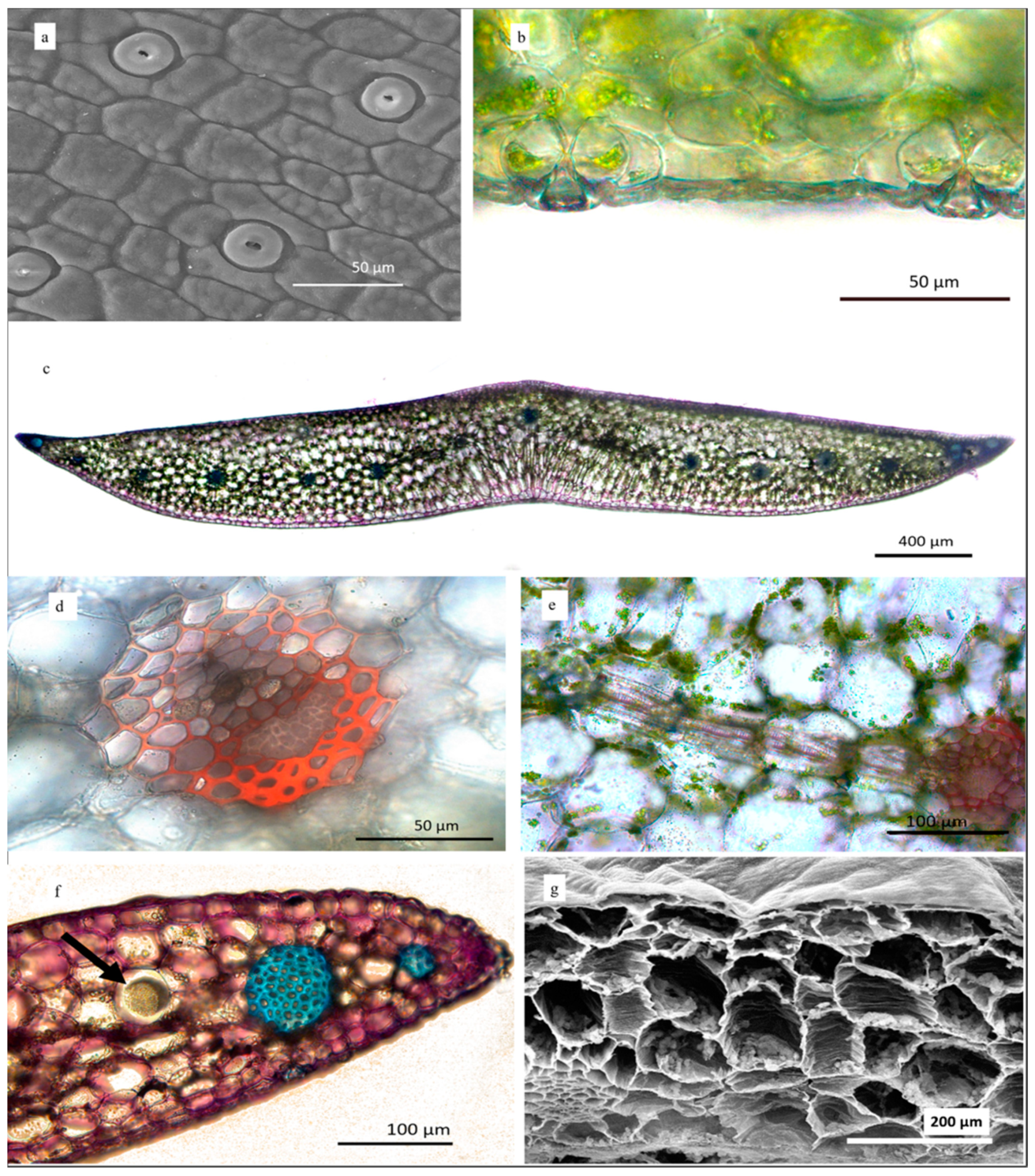
3.2. Morphology
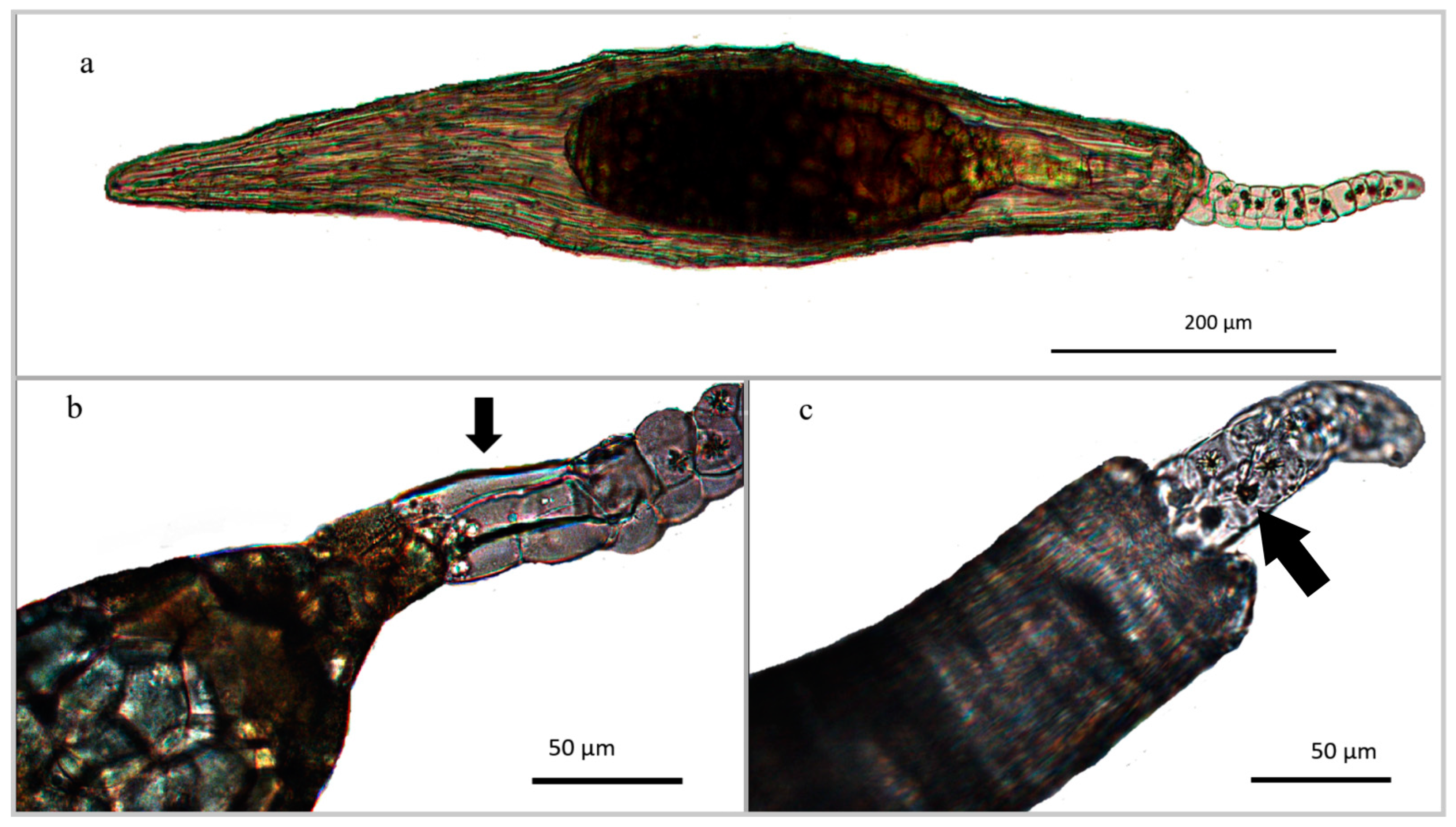
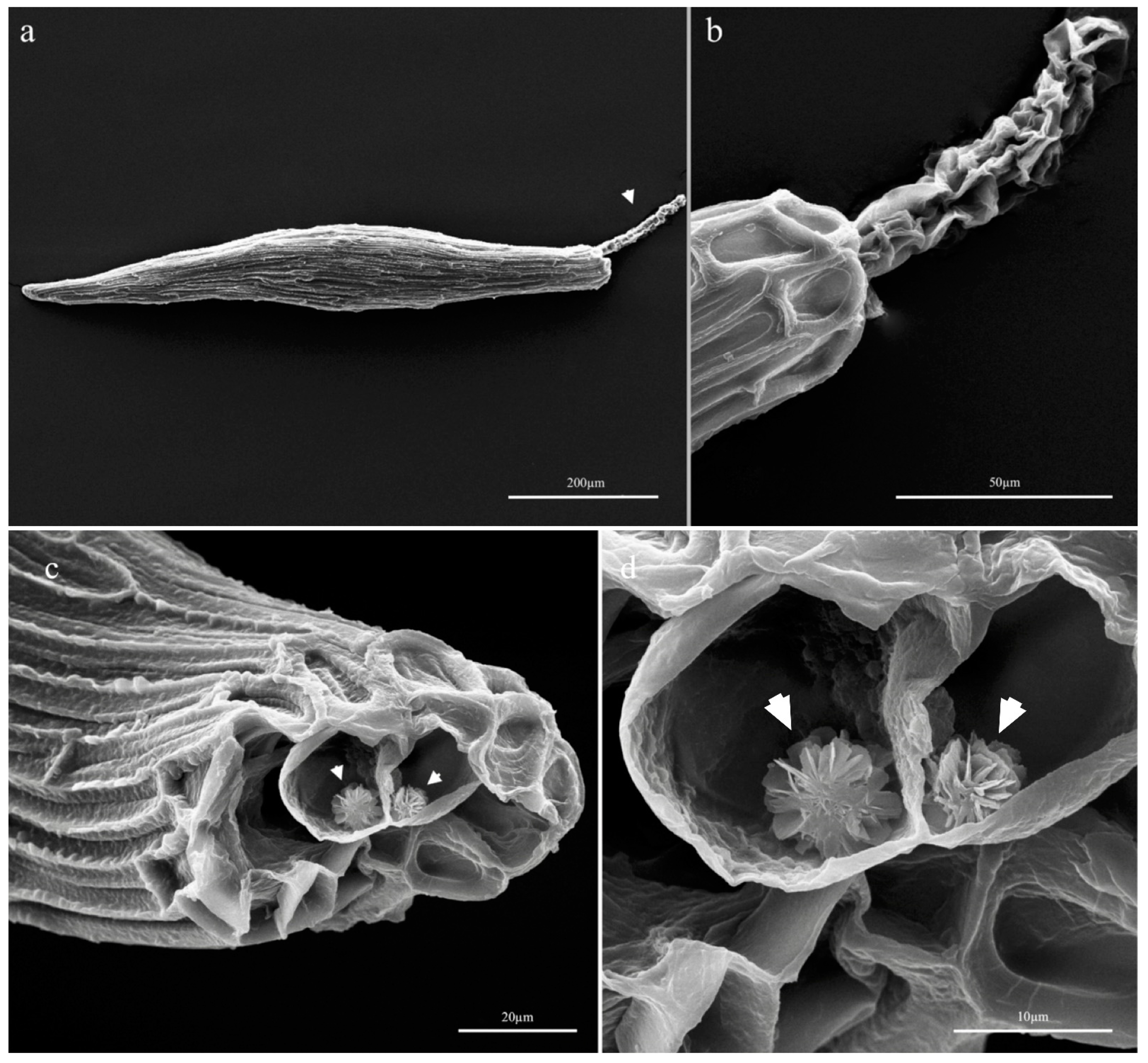
3.2.1. Seed Morphology
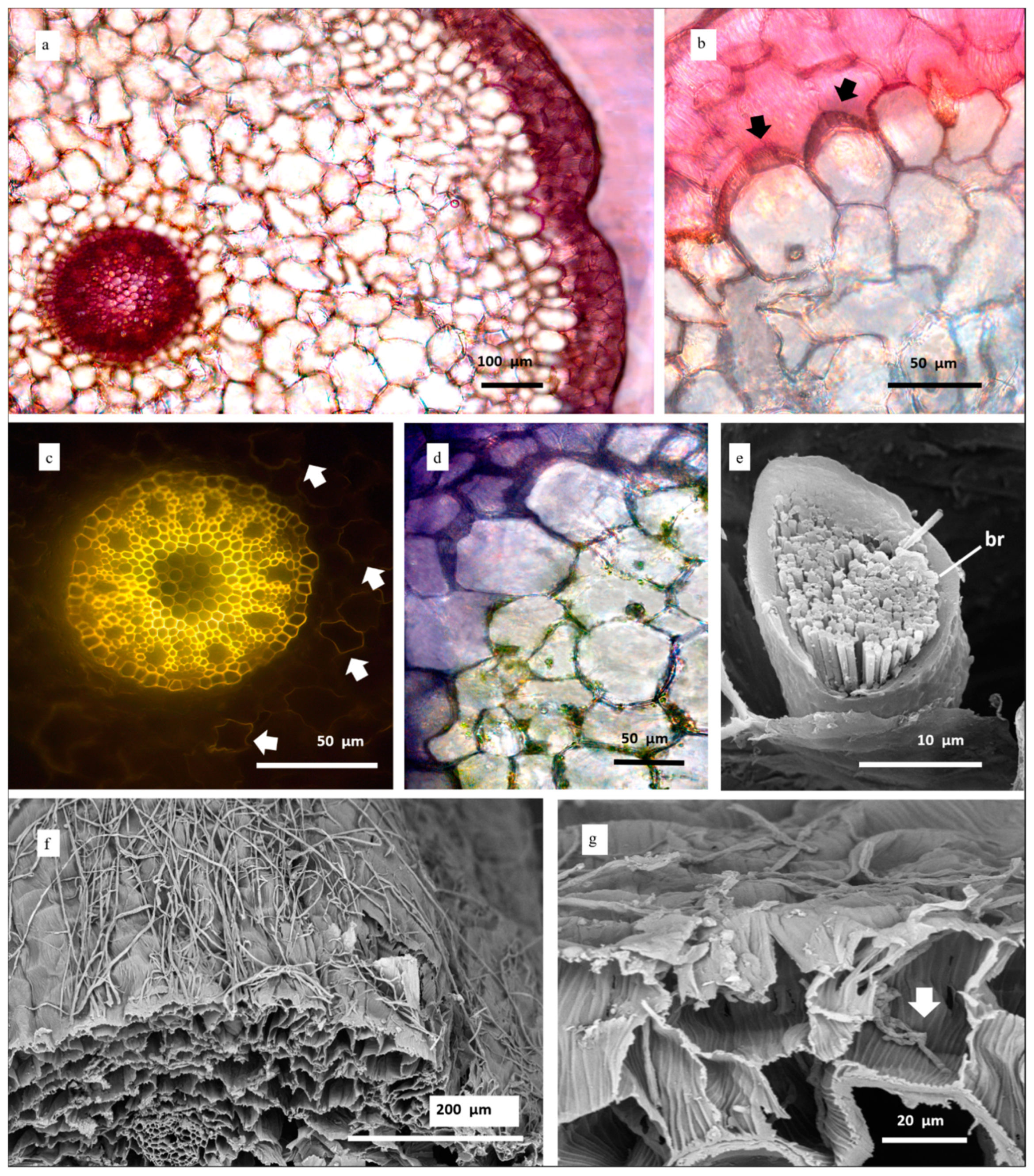
3.2.2. Root Sections
3.2.3. Leaf Surface and Transversal Sections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, C.C.; Bales, J.W.; Palmer-Fortune, J.E.; Nicholson, R.G. Darwin’s bee-trap: The kinetics of Catasetum, a new world orchid. Plant Signal. Behav. 2008, 3, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, H.N. Cell differentiation and mycorrhizal infection in Dactylorhiza majalis (Rchb. F.) Hunt & Summerh. (Orchidaceae) during germination in vitro. New Phytol. 1990, 116, 137–147. [Google Scholar]
- Leake, J.R. The biology of myco-heterotrophic (‘saprotrophic’) plants. New Phytol. 1994, 127, 171–216. [Google Scholar] [CrossRef] [PubMed]
- Girlanda, M.; Selosse, M.A.; Cafasso, D.; Brilli, F.; Delfine, S.; Fabbian, R.; Ghignone, S.; Pinelli, P.; Segreto, R.; Loreto, F.; et al. Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol. Ecol. 2006, 15, 491–504. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef] [Green Version]
- Dressler, R.L. The Orchids. Natural History and Classification, 2nd ed.; Harvard University Press: Cambridge, UK, 1981; p. 334. [Google Scholar]
- Dressler, R.L. Phylogeny and Classification of the Orchid Family, 1st ed.; Dioscorides Press: Portland, OR, USA, 1993; p. 330. [Google Scholar]
- Van den Berg, C.; Higgins, W.E.; Dressler, R.L.; Whitten, W.M.; Soto-Arenas, M.A.; Chase, M.W. A phylogenetic study of Laeliinae (Orchidaceae) based on combined nuclear and plastid DNA sequences. Ann. Bot. 2009, 104, 417–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, C.; Goldman, D.H.; Freudenstein, J.V.; Pridgeon, A.M.; Cameron, K.M.; Chase, M.W. An overview of the phylogenetic relationships within Epidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae). Am. J. Bot. 2005, 92, 613–624. [Google Scholar] [CrossRef]
- Chiron, G.R.; Castro Neto, V.P. Révision des espèces brésiliennes du genre Laelia Lindley. Richardiana 2002, 2, 4–28. [Google Scholar]
- Van den Berg, C. New combinations in the genus Cattleya Lindl. Neodiversity 2008, 3, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Rio Grande do Sul. Decreto 52.109, de 2 de Dezembro de 2014. Declara as Espécies da Flora Nativa Ameaçadas de Extinção do Estado do Rio Grande do Sul; Diário Oficial do Estado do Rio Grande do Sul: Porto Alegre, Brazil, 2014; Volume 72, pp. 2–11. [Google Scholar]
- Hosomi, S.T.; Custódio, C.C.; Seaton, P.T.; Marks, T.R.; Barbosa Machado-Neto, N. Improved assessment of viability and germination of Cattleya (Orchidaceae) seeds following storage. Vitr. Cell Dev. Biol.-Plant 2012, 48, 127–136. [Google Scholar] [CrossRef]
- Seidel Junior, D.; Venturieri, G.A. Ex vitro acclimatization of Cattleya forbesii and Laelia purpurata seedlings in a selection of substrates. Acta Sci. Agron. 2011, 33, 97–103. [Google Scholar]
- Caballero-Villalobos, L.; Silva-Arias, G.A.; Buzatto, C.R.; Nervo, M.H.; Singer, R.B. Generalized food-deceptive pollination in four Cattleya (Orchidaceae: Laeliinae) species from Southern Brazil. Flora 2017, 234, 195–206. [Google Scholar] [CrossRef]
- Silva Júnior, J.M.; da Rodrigues, M.; de Castro, E.M.; Bertolucci, S.K.V.; Pasqual, M. Changes in anatomy and chlorophyll synthesis in orchids propagated in vitro in the presence of urea. Acta Sci. Agron. 2013, 35, 65–72. [Google Scholar] [CrossRef]
- Gallo, F.R.; Souza, L.A.; Milaneze-Gutierre, M.A.; Almeida, O.J.G. Seed structure and in vitro seedling development of certain Laeliinae species (Orchidaceae). Rev. Mex. Biodivers. 2016, 87, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Kowsalya, A.; Rojamala, K.; Thangavelu, M. Comparative vegetative anatomy of South Indian Vandas (Orchidaceae). Flora 2017, 235, 59–75. [Google Scholar] [CrossRef]
- Stancato, G.C.; Chagas, E.P.; Mazzafera, P. Development and germination of seeds of Laelia purpurata (Orchidaceae). Lindleyana 1998, 13, 97–100. [Google Scholar]
- Gonçalves, L.D.M.; Prizão, E.C.; Milaneze Gutierre, M.A.; Mangolin, C.A.; da Silva Machado, M.d.F.P. Use of complex supplements and light-differential effects for micropropagation of Hadrolaelia purpurata (=Laelia purpurata) and Encyclia randii orchids. Acta Sci. Agron. 2012, 34, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Dearnaley, J.D.W.; Martos, F.; Selosse, M.A. Orchid mycorrhizas: Molecular ecology, physiology, evolution, and conservation aspects. In Fungal Associations, 2nd ed.; Hock, B., Ed.; Springer: Berlin, Germany, 2012; pp. 207–230. [Google Scholar]
- Rasmussen, H.N.; Rasmussen, F.N. Seedling mycorrhiza: A discussion of origin and evolution in Orchidaceae. Bot. J. Linn. Soc. 2014, 175, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Withner, C.L. The Cattleyas and Their Relatives. Vol. VI. The South American Encyclia Species; Timber Press: Portland, OR, USA, 2000. [Google Scholar]
- Almeida, P.R.M.; Van den Berg, C.; Goes-Neto, A. Morphological and molecular characterization of species of Tulasnella (Homobasidiomycetes) associated with neotropical plants of Laeliinae (Orchidaceae) occurring in Brazil. Lankasteriana 2007, 7, 22–27. [Google Scholar]
- Adamo, M.; Chialva, M.; Calevo, J.; De Rose, S.; Girlanda, M.; Perotto, S.; Balestrini, R. The Dark Side of orchid symbiosis: Can Tulasnella calospora decompose host tissues? Int. J. Mol. Sci. 2020, 21, 3139. [Google Scholar] [CrossRef] [PubMed]
- Calevo, J.; Giovannini, A.; Cornara, L.; Peccenini, S. Asymbiotic seed germination of hand-pollinated terrestrial orchids. Acta Hortic. 2017, 1155, 415–418. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Morel, G. Clonal propagation of orchids by meristem culture. Cymbidium Soc. News 1965, 20, 3–11. [Google Scholar]
- Malmgren, S. Orchid propagation: Theory and practice. In North American Native Terrestrial Orchids: Propagation and Production, Proceedings of the North American Native Terrestrial Orchid Conference, Germantown, MD, USA, 16–17 March 1996; Allen, C., Ed.; pp. 63–71.
- Calevo, J.; Copetta, A.; Marchioni, I.; Bazzicalupo, M.; Pianta, M.; Shirmohammadi, N.; Cornara, L.; Giovannini, A. The use of a new culture medium and organic supplement to improve in vitro early stage development of five orchid species. Plant Biosyst. 2020. [Google Scholar] [CrossRef]
- Ercole, E.; Rodda, M.; Girlanda, M.; Perotto, S. Establishment of a symbiotic in vitro system between a green meadow orchid and a Rhizoctonia-like fungus. Bio-Protocol 2015, 5, e1482. [Google Scholar] [CrossRef]
- Girlanda, M.; Segreto, R.; Cafasso, D.; Liebel, H.T.; Rodda, M.; Ercole, E.; Cozzolino, S.; Gebauer, G.; Perotto, S. Photosynthetic mediterranean meadow orchids feature partial mycoheterotrphy and specific mycorrhyzal associations. Am. J. Bot. 2011, 98, 1148–1163. [Google Scholar] [CrossRef] [Green Version]
- Barthlott, W.; Große-Veldmann, B.; Korotkova, N. Orchid Seed Diversity: A Scanning Electron Microscopy Survey, 1st ed.; Englera. Botanischer Garten und Botanisches Museum: Berlin, Germany, 2014. [Google Scholar]
- Calevo, J.; Giovannini, A.; Cornara, L.; Peccenini, S.; Monroy, F. Orchis patens Desf.: Seed morphology of an endangered Mediterranean orchid. Plant Biosyst. 2017, 151, 761–765. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef] [Green Version]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 16 January 2021).
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The upper tail probabilities of Spearman’s rho. J. R. Stat. Soc. 1975, 24, 377–379. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A. Nonparametric Statistical Methods; John Wiley & Sons: New York, NY, USA, 1973; pp. 185–194. [Google Scholar]
- Pridgeon, A.M.; Stern, W.L.; Benzing, D.H. Tilosomes in roots of Orchidaceae: Morphology and systematic occurrence. Am. J. Bot. 1983, 70, 1365–1377. [Google Scholar] [CrossRef]
- Barthlott, W. Morphologie der Samen von orchideen in Hinblick auf taxonomische und functionelle Aspekte. In Proceedings of the 8th World Orchid Conference, Frankfurt, Germany, 10–17 April 1975; Senghas, K., Ed.; German Orchid Society Inc.: Frankfurt, Germany, 1976; pp. 444–455. [Google Scholar]
- Prychid, C.J.; Rudall, P.J. Calcium oxalate crystals in Monocotyledons: A review of their structure and systematics. Ann. Bot. 1999, 84, 725–739. [Google Scholar] [CrossRef]
- Solereder, H.; Meyer, F. Systematische Anatomie der Monokotyledonen. VI. Microspermae; Gebrüder Borntraeger: Berlin, Germany, 1930. [Google Scholar]
- Tominski, P. Die Anatomie des Orchideenblattes in ihrer Abhängigkeit von Klima und Standort. Ph.D. Thesis, Universität Berlin, Berlin, Germany, 1905. [Google Scholar]
- Sandoval-Zapotitla, E.; Terrazas, T.; Villaseñor, J. Diversity of mineral inclusions in the subtribe Oncidiinae (Orchidaceae). Rev. Biol. Trop. 2010, 58, 733–755. [Google Scholar] [PubMed]
- Lee, Y.; Yeung, E.C.; Lee, N.; Chung, M. Embryo development in the Lady’s Slipper orchid, Paphiopedilum delenatii, with emphasis on the ultrastructure of the suspensor. Ann. Bot. 2006, 98, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yeung, E.C. The osmotic property and fluorescent tracer movement of developing orchid embryos of Phaius tankervilliae (Aiton) BI. Sex. Plant Reprod. 2010, 23, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volk, G.M.; Lynch-Holm, V.J.; Kostman, T.A.; Goss, L.J.; Franceschi, V.R. The Role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratiotes leaves. Plant Biol. 2002, 4, 34–45. [Google Scholar] [CrossRef]
- Schwarze, F.W. Wood decay under the microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Gadd, G.M.; Bahri-Esfahani, J.; Li, Q.; Rhee, Y.J.; Wei, Z.; Fomina, M.; Liang, X. Oxalate production by fungi: Significance in geomycology, biodeterioration and bioremediation. Fungal Biol. Rev. 2014, 28, 36–55. [Google Scholar] [CrossRef]
- Dutton, M.V.; Evans, C.S. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 1996, 42, 881–895. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying enzymes in filamentous basidiomycetes–ecological, functional and phylogenetic review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Peterson, R.L.; Currah, R.S. Synthesis of mycorrhizae between protocorms of Goodyera repens (Orchidaceae) and Ceratobasidium cereale. Can. J. Bot. 1990, 68, 1117–1125. [Google Scholar] [CrossRef]
- Richardson, K.A.; Peterson, R.L.; Currah, R.S. Seed reserves and early symbiotic protocorm development of Platanthera hyperborea (Orchidaceae). Can. J. Bot. 1992, 70, 291–300. [Google Scholar] [CrossRef]
- Wright, M.; Guest, D. Development of mycorrhizal associations in Caladenia tentaculata. Selbyana 2004, 25, 114–124. [Google Scholar]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Barsberg, S.; Lee, Y.; Rasmussen, H. Development of C-lignin with G/S-lignin and lipids in orchid seed coats—An unexpected diversity exposed by ATR-FT-IR spectroscopy. Seed Sci. Res. 2018, 28, 41–51. [Google Scholar] [CrossRef]
- Perotto, S.; Rodda, M.; Benetti, A.; Sillo, F.; Ercole, E.; Rodda, M.; Girlanda, M.; Murat, C.; Balestrini, R. Gene expression in mycorrhizal orchid protocorms suggests a friendly plant–fungus relationship. Planta 2014, 239, 1337–1349. [Google Scholar] [CrossRef]
- Fochi, V.; Chitarra, W.; Kohler, A.; Voyron, S.; Singan, V.R.; Lindquist, E.A.; Barry, K.B.; Girlanda, M.; Grigoriev, I.V.; Martin, F.; et al. Fungal and plant gene expression in the Tulasnella calospora–Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytol. 2017, 213, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Miura, C.; Saisho, M.; Yagame, T.; Yamato, M.; Kaminaka, H. Bletilla striata (Orchidaceae) seed coat restricts the invasion of fungal hyphae at the initial stage of fungal colonization. Plants 2019, 8, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, W.L. Anatomy of Monocotyledons. Volume X: Orchidaceae; Gregory, M., Cutler, D.F., Eds.; Oxford University Press: Oxford, UK, 2014; p. 288. [Google Scholar]
- Stern, W.L.; Carlsward, B.S. Comparative vegetative anatomy and systematics of Laeliinae (Orchidaceae). Bot. J. Linn. Soc. 2009, 160, 21–41. [Google Scholar] [CrossRef] [Green Version]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Alarm photosynthesis: Calcium oxalate crystals as an internal CO2 source in plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulyananda, T.; Nilsen, E.T. The role of idioblasts in leaf water relations of tropical Rhododendron. Am. J. Bot. 2017, 104, 828–839. [Google Scholar] [CrossRef] [Green Version]
- Konno, K.; Inoue, T.A.; Nakamura, M. Synergistic defensive function of raphides and protease through the needle effect. PLoS ONE 2014, 9, e91341. [Google Scholar] [CrossRef] [Green Version]
- Nurfadilah, S.; Yulia, N.D.; Ariyanti, E.E. Morphology, anatomy, and mycorrhizal fungi colonization in roots of epiphytic orchids of Sempu Island, East Java, Indonesia. Biodiversitas 2016, 17, 592–603. [Google Scholar] [CrossRef]
- Chomicki, G.; Bidel, L.; Ming, F.; Coiro, M.; Zhang, X.; Wang, Y.; Baissac, Y.; Jay-Allemand, C.; Renner, S. The velamen protects photosynthetic orchid roots against UV-B damage, and a large dated phylogeny implies multiple gains and losses of this function during the Cenozoic. New Phytol. 2015, 205, 1330–1341. [Google Scholar] [CrossRef]
- Muthukumar, T.; Kowsalya, A. Comparative anatomy of aerial and substrate roots of Acampe praemorsa (Rox.) Blatt. & MC. Cann. Flora 2017, 266, 17–28. [Google Scholar]
- Idris, N.; Collings, D. Cell wall development in the velamen layer of the orchid Miltoniopsis investigated by confocal microscopy. Malays. J. Microsc. 2015, 10, 20–26. [Google Scholar]
- Peterson, C.A.; Enstone, D.E. Functions of passage cells in the endodermis and exodermis of roots. Physiol. Plant. 2006, 97, 592–598. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Krishnamurthy, K.V.; Britto, S.J.; Arockiasamy, D.I. Visualization of orchid mycorrhizal fungal structures with fluorescence dye using epifluorescence microscopy. Curr. Sci. 2000, 79, 1527–1528. [Google Scholar]
- Porembski, S.; Barthlott, W. Velamen radicum micromorphology and classification of Orchidaceae. Nord. J. Bot. 1988, 8, 117–137. [Google Scholar] [CrossRef]
- Kedrovski, H.R.; Sajo, M.D.G. What are tilosomes? An update and new perspectives. Acta Bot. Bras. 2019, 33, 106–115. [Google Scholar] [CrossRef]
- Benzing, D.H.; Ott, D.W.; Friedman, W.E. Roots of Sobralia macrantha (Orchidaceae): Structure and Function of the Velamen-Exodermis Complex. Am. J. Bot. 1982, 69, 608–614. [Google Scholar] [CrossRef]
- Kwok-ki, H.; Hock-Hin, Y.; Hew, C. The presence of photosynthetic machinery in aerial roots of leafy orchids. Plant Cell Physiol. 1983, 24, 1317–1321. [Google Scholar]
- Scarpella, E.; Meijer, A.H. Pattern formation in the vascular system of monocot and dicot plant species. New Phytol. 2004, 164, 209–242. [Google Scholar] [CrossRef] [PubMed]
- Carlsward, B.S.; Stern, W.L.; Bytebier, B. Comparative vegetative anatomy and systematics of the angraecoids (Vandeae, Orchidaceae) with an emphasis on the leafless habit. Bot. J. Linn. Soc. 2006, 151, 165–218. [Google Scholar] [CrossRef]
- Moreira, A.S.F.P.; Filho, J.P.D.L.; Isaias, R.M.D.S. Structural adaptations of two sympatric epiphytic orchids (Orchidaceae) to a cloudy forest environment in rocky outcrops of Southeast Brazil. Rev. Bio. Trop. 2013, 61, 1053–1065. [Google Scholar]
- Guan, Z.J.; Zhang, S.B.; Guan, K.Y.; Li, S.Y.; Hong, H. Leaf anatomical structure of Paphiopedilum and Cypripedium and their adaptive significance. J. Plant Res. 2011, 124, 289–298. [Google Scholar] [CrossRef]
- Rindyastuti, R.; Nurfadilah, S.; Rahadiantoro, A.; Hapsari, L.; Abywijaya, I. Leaf anatomical characters of four epiphytic orchids of Sempu Island, East Java, Indonesia: The importance in identification and ecological adaptation. Biodiversitas 2018, 19, 1906–1918. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Uhl, D.; Mosbrugger, V.; Kerp, H. Evolution and function of leaf venation architecture: A review. Ann. Bot. 2011, 87, 553–566. [Google Scholar] [CrossRef]
- Zanenga-Godoy, R.; Costa Gonçalves, C. Foliar anatomy of four species of genus Cattleya Lindl. (Orchidaceae) of the Brazilian Central Planalt. Acta Bot. Bras. 2003, 17, 101–118. [Google Scholar] [CrossRef]
| Medium | Germination % |
|---|---|
| 1/2 MS | 46.5 ± 6.4 a |
| KC | 26.3 ± 4.3 b |
| M551 | 24.1 ± 2.6 b |
| CG0 | 17.1 ± 2.0 c |
| CG50 | 21.4 ± 2.9 bc |
| CG100 | 22.9 ± 5.3 bc |
| OA + MUT4178 | 18.3 ± 4.0 c |
| Seed Type | Elongated |
|---|---|
| Seed length (μm) | 800.47 ± 97.42 |
| Seed width (μm) | 154.74 ± 22.37 |
| Seed L/W ratio | 5.33 ± 0.62 |
| Embryo length (μm) | 331.59 ± 48.04 |
| Embryo width (μm) | 127.22 ± 19.97 |
| Suspensor length (μm) | 302.51 ± 64.53 |
| Seed testa cells | Without intercellular spaces |
| N° cells in longitudinal axis | >5 |
| Suspensor layer | 2–3 cell thick |
| Crystals | Calcium oxalate druses in the suspensor |
| Root | Characters |
|---|---|
| Hairs | From few to numerous if roots belonged to plants grown in CG50 and CG 100 |
| Velamen cells | Moderately large, polygonal shaped, arranged in 2–3 layers. Helical thickenings in cell walls |
| Exodermis cells | Large, polygonal-shaped, arranged in monolayer. ∩-thickened. |
| Cortex cells | Circular to polygonal shaped. Variable size. Presence of chloroplasts. Some elements show thickenings in the cell walls. |
| Central cylinder | Endodermis cell walls square to polygonal shaped, O-thickened; passage cells in correspondence of phloem; thin-walled pericycle cells alternating with protoxylematic elements. |
| Stele | Polyarch actynostele (10 arches) |
| Crystals | Raphides in the parenchymatic cortex cells |
| Other features | Tufted tilosomes near short thin-walled passage cells scattered in the exodermis |
| Leaf | |
| Cuticle | Granular ridged; 7–10 μm thick; |
| Stomata | Tetracytic apparatus. C-shaped guard cells. Present in the abaxial surface. Mean stomatal length: 23.5 ± 2.9; mean stomatal width: 26.5 ± 2.6. |
| Epidermis | Cells rectangular/hexagonal shaped. Generally, one-layered (two-layered in the midvein zone) |
| Hypodermis | Present in correspondence of the midvein |
| Mesophyll | Dorsiventral in correspondence of the midvein, homogeneous towards the margins. Seven vascular bundles for each side of the midvein, arranged in a row. Transverse veins connecting principal vascular bundles. Abaxial row of schlerenchymatic fibers. Banded mesophyll cells. |
| Crystals | Idioblasts containing raphides |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzicalupo, M.; Calevo, J.; Adamo, M.; Giovannini, A.; Copetta, A.; Cornara, L. Seed Micromorphology, In Vitro Germination, and Early-Stage Seedling Morphological Traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg. Horticulturae 2021, 7, 480. https://doi.org/10.3390/horticulturae7110480
Bazzicalupo M, Calevo J, Adamo M, Giovannini A, Copetta A, Cornara L. Seed Micromorphology, In Vitro Germination, and Early-Stage Seedling Morphological Traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg. Horticulturae. 2021; 7(11):480. https://doi.org/10.3390/horticulturae7110480
Chicago/Turabian StyleBazzicalupo, Miriam, Jacopo Calevo, Martino Adamo, Annalisa Giovannini, Andrea Copetta, and Laura Cornara. 2021. "Seed Micromorphology, In Vitro Germination, and Early-Stage Seedling Morphological Traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg" Horticulturae 7, no. 11: 480. https://doi.org/10.3390/horticulturae7110480
APA StyleBazzicalupo, M., Calevo, J., Adamo, M., Giovannini, A., Copetta, A., & Cornara, L. (2021). Seed Micromorphology, In Vitro Germination, and Early-Stage Seedling Morphological Traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg. Horticulturae, 7(11), 480. https://doi.org/10.3390/horticulturae7110480