Assessment of Plant Growth Regulators and Carbon Sources on the Germination and Growth Process of Dandelion (Taraxacum officinale G.H. Weber ex Wiggers) under In Vitro Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Seeds Germination
2.3. In Vitro Development Seedling
2.4. Data Analysis
3. Results
3.1. Germination Capacity
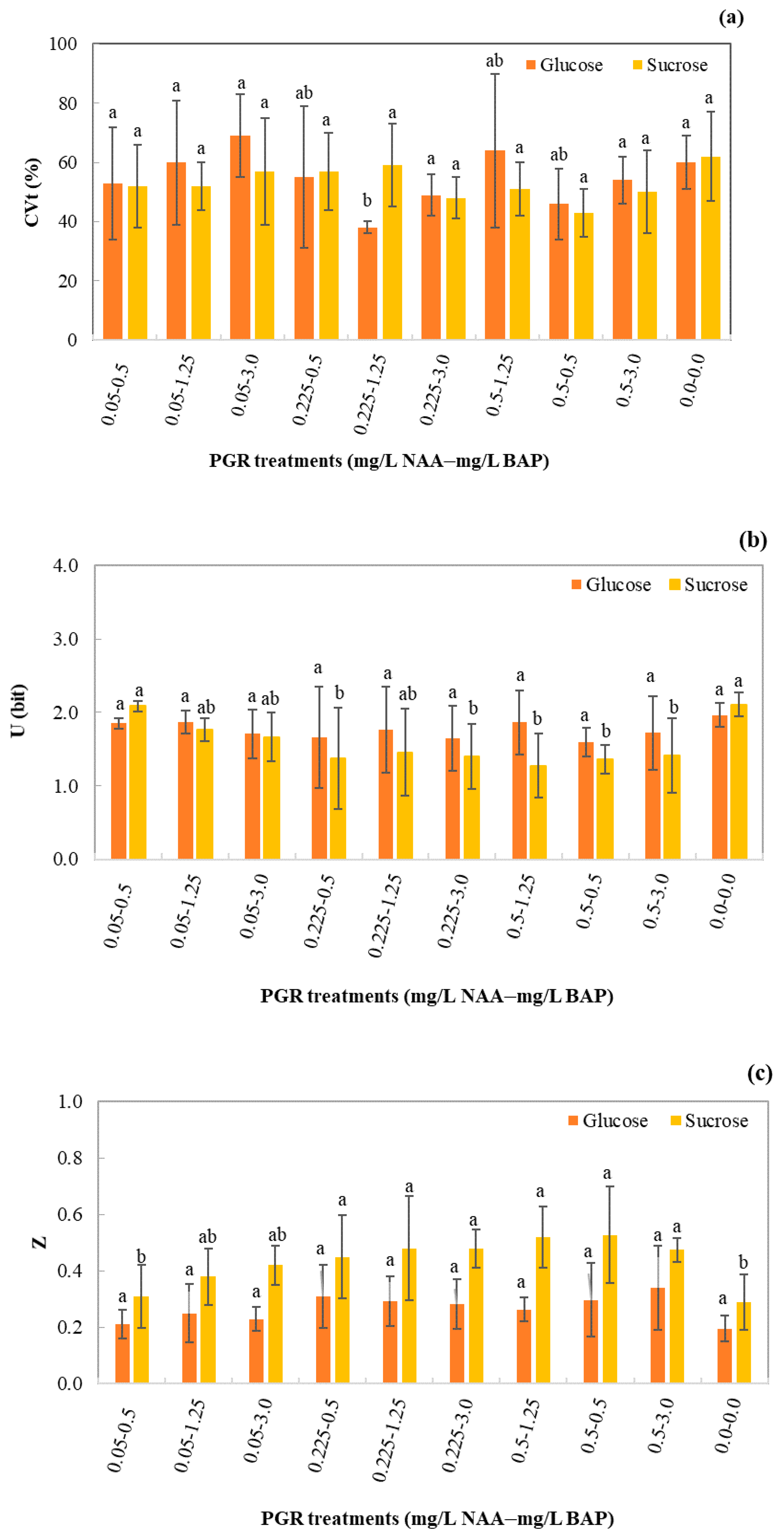
3.2. Germination Uniformity and Synchrony
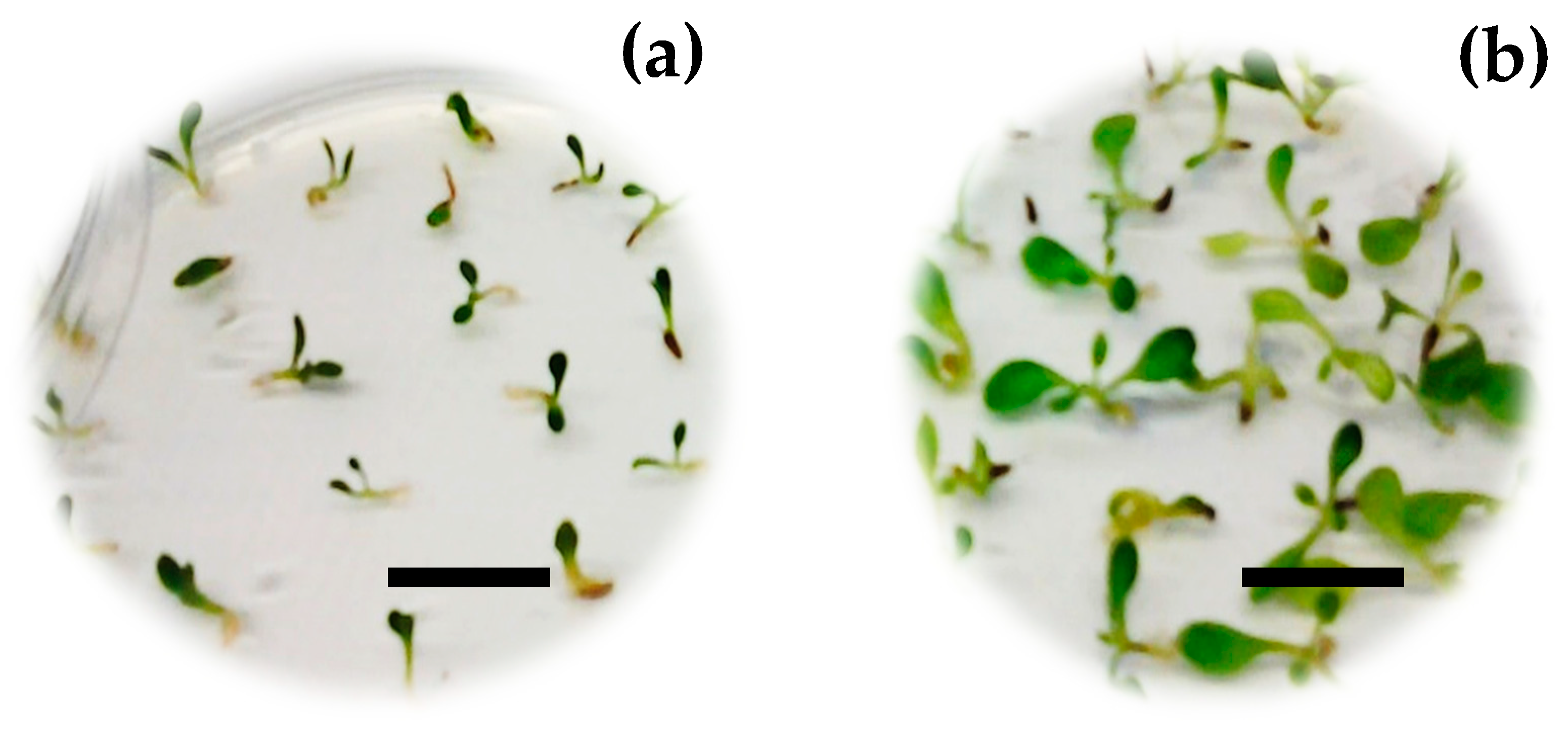
3.3. Seedling Development
4. Discussion
4.1. Germination Capacity, Uniformity, and Synchrony
4.2. Effect of Carbon Source and PGRs on Germination
4.3. Effect of Carbon Source and PGRs on Seedling Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and Identification of Compounds from Bioactive Extracts of Taraxacum officinale Weber ex F. H. Wigg. (Dandelion) as a Potential Source of Antibacterial Agents. Evid. Based Complement. Altern. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum-A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.; Oh, E.; Jung, K.; Ko, K. Optimization of in vitro seed germination of Taraxacum platycarpum. Korea J. Environ. Agric. 2009, 28, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Djingova, R.; Kuleff, I. Seasonal variations in the metal concentration of Taraxacum officinale, Plantago major and Plantago lanceolata. Chem. Ecol. 1999, 16, 37–41. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Z.; Tan, X.; Wang, Z. Effects of sucrose on germination and seedling development of Brassica napus. Int. J. Biol. 2010, 2, P150. [Google Scholar] [CrossRef] [Green Version]
- Rolland, F.; Moore, B.; Sheen, J. Sugar sensing and signaling in plants. Plant Cell 2002, 14, S185–S205. [Google Scholar] [CrossRef] [Green Version]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–112. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Hussian, I.; Ahmad, R.; Farooq, M.; Rehman, A.; Amin, M.; Bakar, M. Seed priming: A tool to invigorate the seeds. Sci. Agric. 2014, 7, 122–128. [Google Scholar]
- Windauer, L.; Martinez, J.; Rapoport, D.; Wassner, D.; Benech-Arnold, R. Germination responses to temperature and water potential in Jatropha curcas seeds: A hydrotime model explains the difference between dormancy expression and dormancy induction at different incubation temperatures. Ann. Bot. 2012, 109, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Minibayeva, F.; Beckett, R.; Seal, C. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Welbaum, G.; Bradford, K.; Yim, K.; Booth, D.; Oluoch, M. Biophysical, physiological and biochemical processes regulating seed germination. Seed Sci. Res. 1998, 8, 161–172. [Google Scholar] [CrossRef]
- Tian, Y.; Guan, B.; Zhou, D.; Yu, J.; Li, G.; Lou, Y. Responses of seed germination, seedling growth and seed yield traits to seed pretreatment in maize (Zea mays L.). Sci. World J. 2014, 2014, 834630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrakhteh, S.; Frahmandfar, E.; Hamidi, A.; Ramandi, H. Evaluation of Growth Characteristics and seedling vigor in two cultivars of soybean dried under different temperature and fluidized beddryer. Int. J. Agric. Crop Sci. 2013, 5, 1537–2544. [Google Scholar]
- Gamborg, O.L.; Murashige, T.; Thorpe, T.; Vasil, I. Plant tissue culture media. In Vitro 1976, 12, 473–478. [Google Scholar] [CrossRef]
- Ranal, M.; de Santana, D.; Ferreira, W.; Mendes-Rodrigues, C. Calculating germination measurements and organizing spreadsheets. Rev. Bras. Botânica 2009, 32, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Patil, J.G.; Ahire, L.; Nikam, T.D. Influence of plant growth regulators on in vitro seed germination and seedling development of Digitalis purpurea L. Asian Australas. J. Plant Sci. Biotechnol. 2012, 6, 12–18. [Google Scholar]
- Martinez, M.E.; Jorquera, L.; Poirrier, P.; Díaz, K.; Chamy, R. Effect of the Carbon Source and Plant Growth Regulators (PGRs) in the Induction and Maintenance of an in vitro Callus Culture of Taraxacum officinale (L) Weber Ex F.H. Wigg. Agronomy 2021, 11, 1181. [Google Scholar] [CrossRef]
- Levitt, J.; Hamm, P. A method of increasing the rate of seed germination of Taraxacum kok-saghyz. Plant Physiol. 1943, 18, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Uteulin, K.; Mukhambetzhanov, S.; Rakhimbaev, I. Recovering Taraxacum kok-saghyz Rodin. via Seed and Callus Culture. WASET. Int. J. Biol. Biomol. Agric. Food Biotech. Eng. 2014, 8, 385–387. [Google Scholar]
- Hoya, A.; Shibaike, H.; Morita, T.; Ito, M. Germination and seedling survivorship characteristics of hybrids between native and alien species of dandelion (Taraxacum). Plant Species Biol. 2004, 19, 81–90. [Google Scholar] [CrossRef]
- Luo, J.; Cardina, J. Germination patterns and implications for invasiveness in three Taraxacum (Asteraceae) species. Weed Res. 2012, 52, 112–121. [Google Scholar] [CrossRef]
- Washitani, I. Germination responses of a seed population of Taraxacum officinale Weber to constant temperatures including the supra-optimal range. Plant Cell Environ. 1984, 7, 655–659. [Google Scholar] [CrossRef]
- Ryu, J.; Seo, K.; Choi, G.; Rha, E.; Lee, S. Effects of LED light illumination on germination, growth and anthocyanin content of dandelion (Taraxacum officinale). Korean J. Plant Resour. 2012, 25, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Františáková, L.; Winkler, J. Assessment of Achenes Germination of Species Taraxacum officinale and Tussilago farfara. 2010. MendelNet 34–47. Available online: https://docplayer.cz/16940333-Assessment-of-achenes-germination-of-species-taraxacum-officinale-and-tussilago-farfara.html (accessed on 27 May 2019).
- Letchamo, W.; Gosselin, A. Light, Temperature and duration of storage govern the germination and emergence of Taraxacum officinale seed. J. Hortic. Sci. 1996, 71, 373–377. [Google Scholar] [CrossRef]
- Hyo-Sik, Y.; Yang, H. Variation in germination and seedling growth of Taraxacum officinale seeds harvested from different seasons. Korean J. Ecol. 2001, 24, 353–357. [Google Scholar]
- Kozlowski, T. Volume II Germination Control. Metabolism, and Pathology; Department of Forestry, University of Wisconsin, Madison, Wisconsin. Academic Press: New York, NY, USA; London, UK, 1972; ISBN 9780323149488. [Google Scholar]
- Dekkers, B.; Schuurmans, J.; Smeekens, S. Glucose delays seed germination in Arabidopsis thaliana. Planta 2004, 218, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Price, J.; Li, T.; Kang, S.; Na, J.; Jang, J. Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol. 2003, 132, 1424–1438. [Google Scholar] [CrossRef] [Green Version]
- Eveland, A.; Jackson, D. Sugars, signaling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, R. Abscisic acid inhibition of radicle emergence but not seedling growth is suppressed by sugars. Plant Physiol. 2000, 122, 1179–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenas-Huertero, F.; Arroyo, A.; Zhou, L.; Sheen, J.; León, P. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 2000, 14, 2085–2096. [Google Scholar] [PubMed]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, I. Influence of plant growth regulator application on seed germination of dandelion (Taraxacum officinale). Weed Turfgrass Sci. 2013, 2, 152–158. [Google Scholar] [CrossRef]
- Pedroza-Manrique, J.; Fernandez-Lizarazo, C.; Suarez-Silva, A. Evaluation of the effect of three growth regulators in the germination of Comparettia falcata seeds under in vitro conditions. In Vitro Cell. Dev. Biol. Plant 2005, 41, 838–843. [Google Scholar] [CrossRef]
- Pierik, R.; Sprenkels, P.; van Der Harst, B.; van Der Meys, Q.G. Seed germination and further development of plantlets of Paphiopedilum ciliolare Pfitz. in vitro. Sci. Hortic. 1988, 34, 139–153. [Google Scholar] [CrossRef]
- Stevenson, C.C.; Harrington, G.N. The impact of supplemental carbon sources on Arabidopsis thaliana growth, chlorophyll content and anthocyanin accumulation. Plant Growth Regul. 2009, 59, 255–271. [Google Scholar] [CrossRef]
- Yang, G.; Lu, Z. Balanced seedling development of Alexandrian laurel under different in vitro PGR treatments. In Proceedings of the 38th Annual Meeting of the Plant Growth Regulation Society of America, Chicago, IL, USA, 24–28 July 2011; pp. 158–160. Available online: https://www.cabdirect.org/cabdirect/abstract/20173267856 (accessed on 12 August 2019).
- Su, Y.; Liu, Y.; Zhang, X. Auxin-cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Bangerth, F.; Li, C.J.; Gruber, J. Mutual interaction of auxin and cytokinins in regulating correlative dominance. Plant Growth Regul. 2000, 32, 205–217. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef]


| PGRs a (NAA mg/L–BAP mg/L) | CS Type | CS Concentration (%) |
|---|---|---|
| 0.05–0.5 | GLU | 1.0 |
| 0.05–1.25 | 2.3 | |
| 0.05–3.0 | 3.2 | |
| 0.225–0.5 | 5.5 | |
| 0.225–1.25 | SUC | 1.0 |
| 0.225–3.0 | 2.3 | |
| 0.5–0.5 | 3.2 | |
| 0.5–1.25 | 5.5 | |
| 0.5–3.0 | ||
| 0.0–0.0 |
| PGR Treatments | FGP (%) | |||
|---|---|---|---|---|
| (NAA mg/L–BAP mg/L) | GLU 1.0% | GLU 2.3% | GLU 3.2% | GLU 5.5% |
| 0.05–0.5 | 70 ± 5.7 b | 79 ± 8.3 a,b,c 79 ± 5.6 a,b,c 86 ± 8.4 a 61 ± 8.2 d,e 81 ± 6.6 a,b 57 ± 3.8 e 87 ± 6.0 a,b,c,d 71 ± 7.8 b,c 67 ± 11 c,d,e 58 ± 8.8 e | 51 ± 4.8 a 52 ± 6.9 a 50 ± 5.3 a 39 ± 8.7 b 46 ± 4.1 a,b 53 ± 10 a 55 ± 5.3 a 37 ± 4.8 b 54 ± 6.4 a 47 ± 6.7 a | 31 ± 5.7 c,d |
| 0.05–1.25 | 58 ± 5.7 c,d | 45 ± 6.9 a,b | ||
| 0.05–3.0 | 91 ± 6.1 a | 40 ± 5.7 a,b,c | ||
| 0.225–0.5 | 50 ± 4.0 d | 48 ± 4.4 a,b | ||
| 0.225–1.25 | 67 ± 6.4 b,c | 49 ± 8.0 a | ||
| 0.225–3.0 | 66 ± 7.0 b,c | 46 ± 4.9 a,b | ||
| 0.5–0.5 | 88 ± 4.7 a | 24 ± 6.8 d | ||
| 0.5–1.25 | 72 ± 7.9 b | 28 ± 8.2 d | ||
| 0.5–3.0 | 71 ± 8.6 b | 47 ± 5.0 a,b | ||
| 0–0 (control) | 68 ± 4.2 b | 40 ± 8.7 b,c | ||
| PGR Treatments | FGP (%) | |||
|---|---|---|---|---|
| (NAA mg/L–BAP mg/L) | SUC 1.0% | SUC 2.3% | SUC 3.2% | SUC 5.5% |
| 0.05–0.5 | 71 ± 7.0 a 69 ± 3.4 a 53 ± 5.0 b,c 50 ± 5.6 c 51 ± 13 c 48 ± 7.8 c 74 ± 9.8 a 65 ± 9.6 a,b 46 ± 8.1 c 53 ± 7.4 b.c | 71 ± 7.0 a,b 69 ± 5.0 a,b,c 77 ± 4.8 a,b 54 ± 6.4 d,e 65 ± 6.4 a,b,c,d 64 ± 14 b,c,d,e 80 ± 6.0 a 55 ± 11 c,d,e 75 ± 7.4 b,c 50 ± 15 e | 68 ± 7.5 a,b | 30 ±5.9 a |
| 0.05–1.25 | 70 ± 10 a | 32 ±7.6 a | ||
| 0.05–3.0 | 68 ± 5.3 a,b | 33 ±4.4 a | ||
| 0.225–0.5 | 46 ± 5.2 d,e | 27 ±7.5 a | ||
| 0.225–1.25 | 44 ± 5.2 d,e | 29 ±5.9 a | ||
| 0.225–3.0 | 62 ± 6.4 a,b,c | 30 ±6.6 a | ||
| 0.5–0.5 | 65 ± 14 a,b | 32 ±10 a | ||
| 0.5–1.25 | 39 ± 7.9 e | 25 ±4.5 a | ||
| 0.5–3.0 | 48 ± 8.0 c,d,e | 34 ±3.3 a | ||
| 0–0 b (control) | 55 ± 10 b,c,d | 29 ±19 a | ||
| PGR Treatments | CS 2.3% SUC | ||
|---|---|---|---|
| (NAA mg/L–BAP mg/L) | Number of Leaves per Seedling | Leaf Lengh (cm) | SVI (%× cm) × 100 |
| 0.05–0.5 | 5.7 ±0.4 a,b | 3.5 ±0.2 a,b | 2.5 ±0.2 b |
| 0.05–1.25 | 4.7 ±0.3 b,c | 3.1 ±0.4 a,b,c | 2.4 ±0.3 b,c |
| 0.05–3.0 | 4.7 ±0.8 b,c | 2.6 ±0.2 b.e | 2.0 ±0.3 b,c,d |
| 0.225–0.5 | 5.7 ±1.6 a,b | 3.8 ±0.8 a | 2.0 ±0.3 b,c,d |
| 0.225–1.25 | 6.6 ±0.1 a | 2.2 ±0.7 d | 2.6 ±0.6 b |
| 0.225–3.0 | 5.0 ±0.4 b,c | 2.7 ±0.6 b,c | 1.7 ±0.3 c,d |
| 0.5–0.5 | 4.6 ±1.7 b,c | 3.5 ±0.5 a,b | 1.4 ±0.7 d |
| 0.5–1.25 | 4.7 ±0.6 b,c | 2.9 ±0.1 b,c | 1.6 ±0.4 d |
| 0.5–3.0 | 4.2 ±0.9 c,d | 2.4 ±0.8 e | 1.8 ±0.2 c,d |
| 0–0 | 4.1 ±0.8 c | 2.8 ±0.8 b,c | 5.8 ±1.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, M.E.; Jorquera, L.; Poirrier, P.; Díaz, K.; Chamy, R. Assessment of Plant Growth Regulators and Carbon Sources on the Germination and Growth Process of Dandelion (Taraxacum officinale G.H. Weber ex Wiggers) under In Vitro Conditions. Horticulturae 2021, 7, 486. https://doi.org/10.3390/horticulturae7110486
Martínez ME, Jorquera L, Poirrier P, Díaz K, Chamy R. Assessment of Plant Growth Regulators and Carbon Sources on the Germination and Growth Process of Dandelion (Taraxacum officinale G.H. Weber ex Wiggers) under In Vitro Conditions. Horticulturae. 2021; 7(11):486. https://doi.org/10.3390/horticulturae7110486
Chicago/Turabian StyleMartínez, María Eugenia, Lorena Jorquera, Paola Poirrier, Katy Díaz, and Rolando Chamy. 2021. "Assessment of Plant Growth Regulators and Carbon Sources on the Germination and Growth Process of Dandelion (Taraxacum officinale G.H. Weber ex Wiggers) under In Vitro Conditions" Horticulturae 7, no. 11: 486. https://doi.org/10.3390/horticulturae7110486
APA StyleMartínez, M. E., Jorquera, L., Poirrier, P., Díaz, K., & Chamy, R. (2021). Assessment of Plant Growth Regulators and Carbon Sources on the Germination and Growth Process of Dandelion (Taraxacum officinale G.H. Weber ex Wiggers) under In Vitro Conditions. Horticulturae, 7(11), 486. https://doi.org/10.3390/horticulturae7110486








