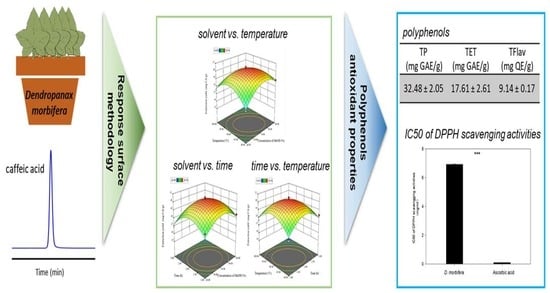
Optimization of Caffeic Acid Extraction from Dendropanax morbifera Leaves Using Response Surface Methodology and Determination of Polyphenols and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of D. morbifera Leaves
2.3. HPLC Analysis
2.4. Response Surface Methodology
2.5. Determination of Polyphenol Content and Antioxidant Capacity
2.5.1. Determination of the Content of Polyphenols
2.5.2. Scavenging Effect on DPPH
3. Results and Discussion
3.1. Box–Behnken Center Combination of RSM
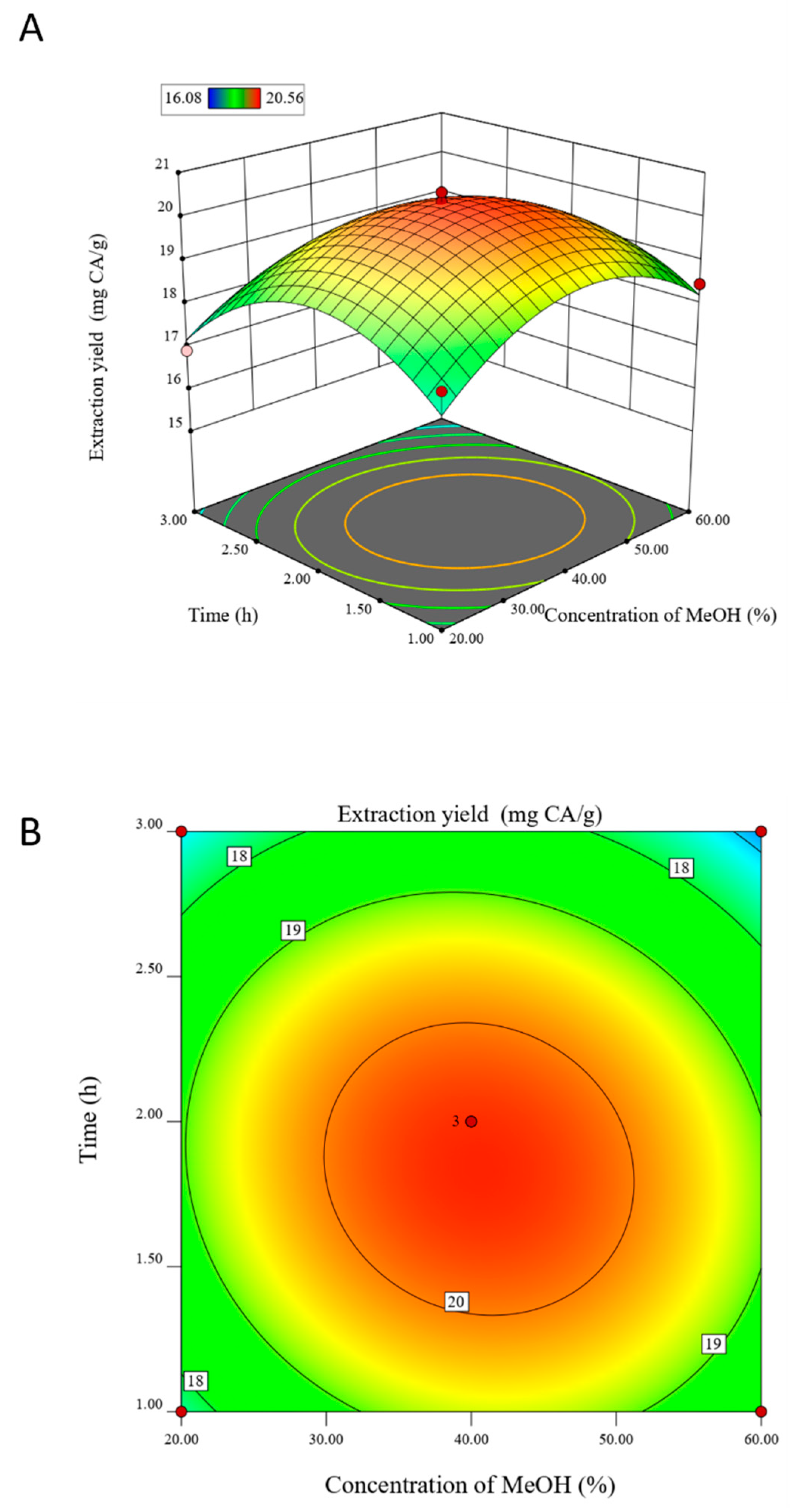
3.2. Analysis of Response Surface Test Results
3.3. Antioxidant Capacities of D. morbifera
3.4. Content of Polyphenols
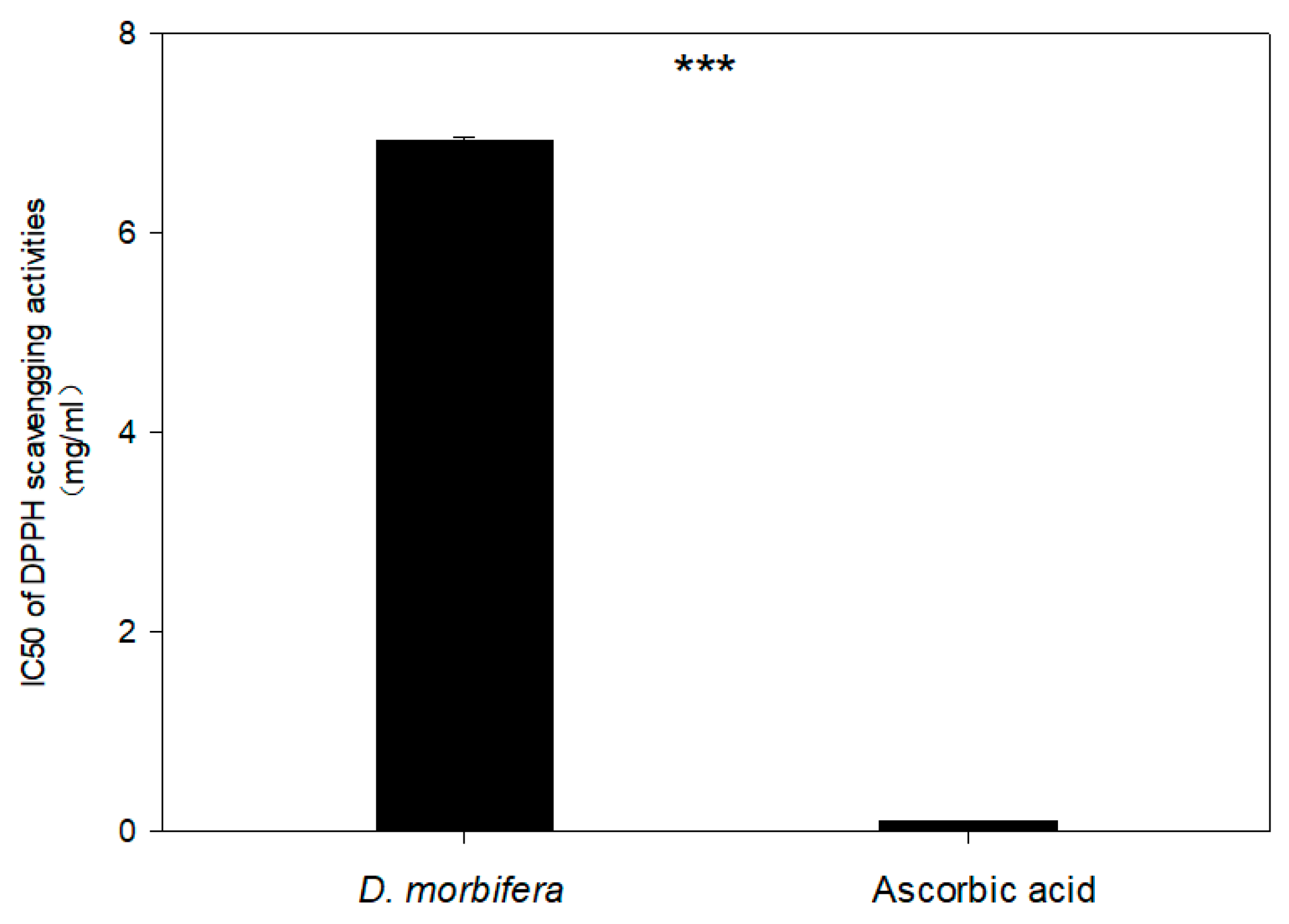
3.5. DPPH Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Im, K.-J.; Jang, S.-B.; Yoo, D.-Y. Anti-cancer effects of Dendropanax morbifera extract in MCF-7 and MDA-MB-231 cells. J. Korean Obstet. Gynecol. 2015, 28, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.; Lyu, J. A study of the application of hwangchil tree (Dendropanax morbiferus h. Lév.) in East Asia. J. Korean Med. Hist. 2020, 33, 43–57. [Google Scholar] [CrossRef]
- Jo, Y.-B.; Lee, J.-H. A Study on the effect of the Dendropanax mobifera extract on anti-hypertensive. J. Korea Acad. Coop. Soc. 2016, 17, 708–715. [Google Scholar] [CrossRef]
- Youn, J.S.; Kim, M.S.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Screening test for Dendropanax morbifera Leveille extracts: In vitro comparison to ox-LDL-induced lipid accumulation, ethanol-induced fatty liver and HMG-CoA reductase inhibition. J. Appl. Biol. Chem. 2018, 61, 1–8. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, M.-O.; Lee, H.; Kim, Y.; Kim, E.; Kim, J.S. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem. 2013, 141, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, D.W.; Yoo, D.Y.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Hong, S.M.; Kim, D.W.; Choi, J.H.; Moon, S.M.; et al. Dendropanax morbifera Léveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement. Altern. Med. 2014, 14, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Bae, D.H.; Lee, U.; Heo, H.J. Ameliorating effect of water extract from Dendropanax morbifera Lev. on memory dysfunction in streptozotocin-induced diabetic rats. Korean J. Food Sci. Technol. 2016, 48, 275–283. [Google Scholar] [CrossRef]
- Park, S.N.; Kim, K.J.; Ha, J.H.; Lee, H.M.; Jeon, S.H.; Kim, Y.H.; Hwang, Y.C.; Jie, Y.J.; Park, C.I.; Park, J.; et al. Cellular antioxidant activity and whitening effects of Dendropanax morbifera leaf extracts. Microbiol. Biotechnol. Lett. 2013, 41, 407–415. [Google Scholar] [CrossRef]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as natural antioxidants: Sources, Extraction and applications in food, cosmetics and drugs. In Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 197–235. [Google Scholar] [CrossRef]
- Eom, T.; Ko, G.; Kim, K.C.; Kim, J.S.; Unno, T. Dendropanax morbifera leaf extracts improved alcohol liver injury in association with changes in the gut microbiota of rats. Antioxidants 2020, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Klimek-Szczykutowicz, M.; Jafernik, K.; Ekiert, H.; Mahmoud, E.A.; Barakat, A.A.; El-Ansary, D.O. Mammillaria species—Polyphenols studies and anti-cancer, anti-oxidant, and anti-bacterial activities. Molecules 2019, 25, 131. [Google Scholar] [CrossRef] [Green Version]
- Jeong, C.H.; Jeong, H.R.; Choi, G.N.; Kim, D.O.; Lee, U.; Heo, H.J. Neuroprotective and anti-oxidant effects of caffeic acid isolated from Erigeron annuus leaf. Chin. Med. 2011, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: In vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555. [Google Scholar] [CrossRef]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W.; et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.-C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Jean-Francois, J.; Doiron, J. Caffeic acid, a versatile pharmacophore: An overview. Mini Rev. Med. Chem. 2011, 11, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, G.; Ginis, Z.; Akyol, S.; Erden, G.; Gurel, A.; Akyol, O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): Review of melanomas, lung and prostate cancers. Eur. Rev. Med Pharmacol. Sci. 2012, 16, 2064–2068. [Google Scholar]
- Tolba, M.; Azab, S.S.; Khalifa, A.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Lukitasari, M.; Nugroho, D.A.; Widodo, N. Chlorogenic Acid: The conceivable chemosensitizer leading to cancer growth suppression. J. Evid. Based Integr. Med. 2018, 23, 2515690x18789628. [Google Scholar] [CrossRef] [Green Version]
- Karunanidhi, A.; Thomas, R.; Van Belkum, A.; Neela, V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. BioMed Res. Int. 2012, 2013, 392058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eom, T.; Kim, K.C.; Kim, J.S. Dendropanax morbifera leaf polyphenolic compounds: Optimal extraction using the response surface method and their protective effects against alcohol-induced liver damage. Antioxidants 2020, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Chelladurai, S.J.S.; Murugan, K.; Ray, A.P.; Upadhyaya, M.; Narasimharaj, V.; Gnanasekaran, S. Optimization of process parameters using response surface methodology: A review. Mater. Today Proc. 2021, 37, 1301–1304. [Google Scholar] [CrossRef]
- Yolmeh, M.; Habibi Najafi, M.B.; Farhoosh, R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM). Food Chem. 2014, 155, 319–324. [Google Scholar] [CrossRef]
- Hani, N.M.; Torkamani, A.E.; Abidin, S.; Mahmood, W.A.K.; Juliano, P. The effects of ultrasound assisted extraction on antioxidative activity of polyphenolics obtained from Momordica charantia fruit using response surface approach. Food Biosci. 2017, 17, 7–16. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Vining, G.G.; Borror, C.M.; Kowalski, S.M. Response surface methodology: A retrospective and literature survey. J. Qual. Technol. 2018, 36, 53–77. [Google Scholar] [CrossRef]
- Dinh, C.D.; Nguyen Thi Ngoc, Y.; Lam Tri, D.; Nguyen Huu Khanh, Q.; Chinh Nguyen, D.; Giang Bach, L. Extraction conditions of polyphenol, flavonoid compounds with antioxidant activity from Veronia amygdalina Del. leaves: Modeling and optimization of the process using the response surface methodology RSM. Mater. Today Proc. 2019, 18, 4004–4010. [Google Scholar] [CrossRef]
- Shamsiev, A.; Park, J.; Olawuyi, I.F.; Odey, G.; Lee, W. Optimization of ultrasonic-assisted extraction of polyphenols and antioxidants from cumin (Cuminum cyminum L.). Korean J. Food Preserv. 2021, 28, 510–521. [Google Scholar] [CrossRef]
- Yoo, G.; Lee, I.K.; Park, S.; Kim, N.; Park, J.H.; Kim, S.H. Optimization of extraction conditions for phenolic acids from the leaves of Melissa officinalis L. using response surface methodology. Pharmacogn. Mag. 2018, 14, 155–161. [Google Scholar] [CrossRef]
- Kim, S.; Yun, E.J.; Bak, J.S.; Lee, H.; Lee, S.J.; Kim, C.T.; Lee, J.-H.; Kim, K.H. Response surface optimised extraction and chromatographic purification of rosmarinic acid from Melissa officinalis leaves. Food Chem. 2010, 121, 521–526. [Google Scholar] [CrossRef]
- Beringhs, A.O.; Dalmina, M.; Pasa, T.B.; Sonaglio, D. Response Surface Methodology IV-Optimal design applied to the performance improvement of an RP-HPLC-UV method for the quantification of phenolic acids in Cecropia glaziovii products. Rev. Bras. Farmacogn. 2015, 25, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Madureira, J.; Melgar, B.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Barros, L.; Cabo Verde, S. Phenolic compounds from irradiated olive wastes: Optimization of the heat-assisted extraction using response surface methodology. Chemosensors 2021, 9, 231. [Google Scholar] [CrossRef]
- Wang, J.; Lu, D.; Zhao, H.; Ling, X.; Jiang, B.; Ouyang, P. Application of response surface methodology optimization for the production of caffeic acid from tobacco waste. Afr. J. Biotechnol. 2009, 8, 1416–1424. [Google Scholar]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Royer, M.; Diouf, P.N.; Stevanovic, T. Polyphenol contents and radical scavenging capacities of red maple (Acer rubrum L.) extracts. Food Chem. Toxicol. 2011, 49, 2180–2188. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.; Dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Sharifi, H.; Zabihzadeh, S.M.; Ghorbani, M. The application of response surface methodology on the synthesis of conductive polyaniline/cellulosic fiber nanocomposites. Carbohydr. Polym. 2018, 194, 384–394. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Pan, Z.; Chen, M.; Ying, P.; Jiang, Y.; Lu, G. Extraction optimization of polyphenols from red giant amaranth by response surface methodology and its antioxidant activity. Cereal. Feed Ind. 2019, 7, 40–46. [Google Scholar] [CrossRef]
- Jiang, M.; Teruyuki, K.; Wang, Z.; Guo, J. Optimization of ultrasonic-assisted extraction of chlorogenic acid from lonicera ja-ponica by response surface methodology. Chem. Bioeng. 2017, 34, 35–39. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Andueza, S.; Manzocco, L.; Paz de Peña, M.; Cid, C.; Nicoli, C. Caffeic acid decomposition products: Antioxidants or pro-oxidants? Food Res. Int. 2009, 42, 51–55. [Google Scholar] [CrossRef]
- Wang, H. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of caffeic acid in peanut sprouts and evaluation of its in vitro effectiveness against oxidative stress-induced erythrocyte hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef]
- Park, S.Y.; Karthivashan, G.; Ko, H.M.; Cho, D.Y.; Kim, J.; Cho, D.J.; Ganesan, P.; Su-Kim, I.; Choi, D.K. Aqueous extract of Dendropanax morbiferus leaves effectively alleviated neuroinflammation and behavioral impediments in MPTP-induced Parkinson’s mouse model. Oxidative Med. Cell. Longev. 2018, 2018, 3175214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Li, T.; Jin, L.; Piao, Z.H.; Liu, B.; Ryu, Y.; Choi, S.Y.; Kim, G.R.; Jeong, J.E.; Wi, A.J.; et al. Dendropanax morbifera prevents cardiomyocyte hypertrophy by inhibiting the Sp1/GATA4 pathway. Am. J. Chin. Med. 2018, 46, 1021–1044. [Google Scholar] [CrossRef]
- Gibson, L.; Rupasinghe, H.P.; Forney, C.F.; Eaton, L. Characterization of changes in polyphenols, antioxidant capacity and physico-chemical parameters during lowbush blueberry fruit ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Torabian, S.; Haddad, E.; Rajaram, S.; Banta, J.; Sabate, J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J. Hum. Nutr. Diet. 2009, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.H.; Ho, C.-T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sasadara, M.M.V.; Wirawan, I.G.P. Effect of extraction solvent on total phenolic content, total flavonoid content, and antioxidant activity of Bulung Sangu (Gracilaria sp.) Seaweed. IOP Conf. Ser. Earth Environ. Sci. 2021, 712, 012005. [Google Scholar] [CrossRef]
- Ezez, D.; Tefera, M. Effects of solvents on total phenolic content and antioxidant activity of ginger extracts. J. Chem. 2021, 2021, 6635199. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sağdıç, O.; Albayrak, S. Antioxidant and antimicrobial activities of different extracts of some medicinal herbs consumed as tea and spices in Turkey. J. Food Biochem. 2012, 36, 547–554. [Google Scholar] [CrossRef]
- Mailoa, M.N.; Mahendradatta, M.; Laga, A.; Djide, N. Antimicrobial activities of tannins extract from guava leaves (Psidium guajava L.) on pathogens microbial. Int. J. Sci. Technol. Res. 2014, 3, 236–241. [Google Scholar]
- Ahmed, S.R.; Romi, I.; Ahmed, J.; Hasan, M.; Roy, R.; Khan, M. Phytochemical profiling and antioxidant potentiality of medicinal plants along with their antibacterial efficacy. J. Adv. Biotechnol. Exp. Ther. 2019, 2, 140–145. [Google Scholar] [CrossRef]
- Moyo, B.; Oyedemi, S.; Masika, P.; Muchenje, V. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 2012, 91, 441–447. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Badarinath, A.; Rao, K.M.; Chetty, C.M.S.; Ramkanth, S.; Rajan, T.; Gnanaprakash, K. A review on in-vitro antioxidant methods: Comparisions, correlations and considerations. Int. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]


| Independent Variable | Solvent Concentration (%) | Extraction Time (h) | Temperature (°C) |
|---|---|---|---|
| −1 | 20 | 1 | 80 |
| 0 | 40 | 2 | 90 |
| 1 | 60 | 3 | 100 |
| Run Order | Solvent Concentration | Extraction Time | Extraction Temperature |
|---|---|---|---|
| 1 | 0 | −1 | 1 |
| 2 | 1 | 0 | 1 |
| 3 | 1 | 1 | 0 |
| 4 | 1 | −1 | 0 |
| 5 | 0 | 0 | 0 |
| 6 | −1 | −1 | 0 |
| 7 | −1 | 0 | −1 |
| 8 | 0 | −1 | −1 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 |
| 11 | 0 | 1 | −1 |
| 12 | −1 | 0 | 1 |
| 13 | 0 | 1 | 1 |
| 14 | 1 | 0 | −1 |
| 15 | −1 | 1 | 0 |
| Run Order | Solvent Concentration (%, v/v) | Extraction Time (h) | Extraction Temperature (°C) | Actual Value (mg/g) | Predicted Value (mg/g) |
|---|---|---|---|---|---|
| 1 | 40 | 1 | 100 | 17.64 | 18.02 |
| 2 | 60 | 2 | 100 | 16.44 | 16.32 |
| 3 | 60 | 3 | 90 | 16.23 | 16.73 |
| 4 | 60 | 1 | 90 | 18.48 | 18.23 |
| 5 | 40 | 2 | 90 | 20.13 | 20.35 |
| 6 | 20 | 1 | 90 | 18.16 | 17.66 |
| 7 | 20 | 2 | 80 | 17.04 | 17.17 |
| 8 | 40 | 1 | 80 | 17.23 | 17.61 |
| 9 | 40 | 2 | 90 | 20.35 | 20.35 |
| 10 | 40 | 2 | 90 | 20.56 | 20.35 |
| 11 | 40 | 3 | 80 | 18.32 | 17.94 |
| 12 | 20 | 2 | 100 | 17.62 | 17.75 |
| 13 | 40 | 3 | 100 | 16.08 | 15.70 |
| 14 | 60 | 2 | 80 | 18.85 | 18.73 |
| 15 | 20 | 3 | 90 | 16.92 | 17.17 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value Prob > F | Significant |
|---|---|---|---|---|---|---|
| Model | 28.54 | 9 | 3.17 | 11.68 | 0.0073 | ** |
| A-Solvent | 0.00845 | 1 | 0.00845 | 0.031 | 0.8669 | |
| B-Time | 1.96 | 1 | 1.96 | 7.22 | 0.0435 | * |
| C-Temperature | 1.67 | 1 | 1.67 | 6.17 | 0.0556 | |
| AB | 0.26 | 1 | 0.26 | 0.94 | 0.3770 | |
| AC | 2.24 | 1 | 2.24 | 8.23 | 0.0350 | * |
| BC | 1.76 | 1 | 1.76 | 6.47 | 0.0517 | |
| A2 | 6.88 | 1 | 6.88 | 25.32 | 0.0040 | ** |
| B2 | 8.7 | 1 | 8.7 | 32.03 | 0.0024 | ** |
| C2 | 8.25 | 1 | 8.25 | 30.38 | 0.0027 | ** |
| Residual | 1.36 | 5 | 0.27 | |||
| Lack of Fit | 1.27 | 3 | 0.42 | 9.12 | 0.1004 | |
| Pure Error | 0.092 | 2 | 0.046 | |||
| Core Total | 29.9 | 14 | ||||
| R-Squared | value: 0.9546 | |||||
| TP (mg GAE/g) | TET (mg GAE/g) | TFlav (mg QE/g) |
|---|---|---|
| 32.48 ± 2.05 | 17.61 ± 2.61 | 9.14 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Bu, T.; Liu, S.; Kim, S. Optimization of Caffeic Acid Extraction from Dendropanax morbifera Leaves Using Response Surface Methodology and Determination of Polyphenols and Antioxidant Properties. Horticulturae 2021, 7, 491. https://doi.org/10.3390/horticulturae7110491
Zhang M, Bu T, Liu S, Kim S. Optimization of Caffeic Acid Extraction from Dendropanax morbifera Leaves Using Response Surface Methodology and Determination of Polyphenols and Antioxidant Properties. Horticulturae. 2021; 7(11):491. https://doi.org/10.3390/horticulturae7110491
Chicago/Turabian StyleZhang, Ming, Ting Bu, Shuilin Liu, and Sooah Kim. 2021. "Optimization of Caffeic Acid Extraction from Dendropanax morbifera Leaves Using Response Surface Methodology and Determination of Polyphenols and Antioxidant Properties" Horticulturae 7, no. 11: 491. https://doi.org/10.3390/horticulturae7110491
APA StyleZhang, M., Bu, T., Liu, S., & Kim, S. (2021). Optimization of Caffeic Acid Extraction from Dendropanax morbifera Leaves Using Response Surface Methodology and Determination of Polyphenols and Antioxidant Properties. Horticulturae, 7(11), 491. https://doi.org/10.3390/horticulturae7110491