Traditional Herbal Remedies Used for Managing Anxiety and Insomnia in Italy: An Ethnopharmacological Overview
Abstract
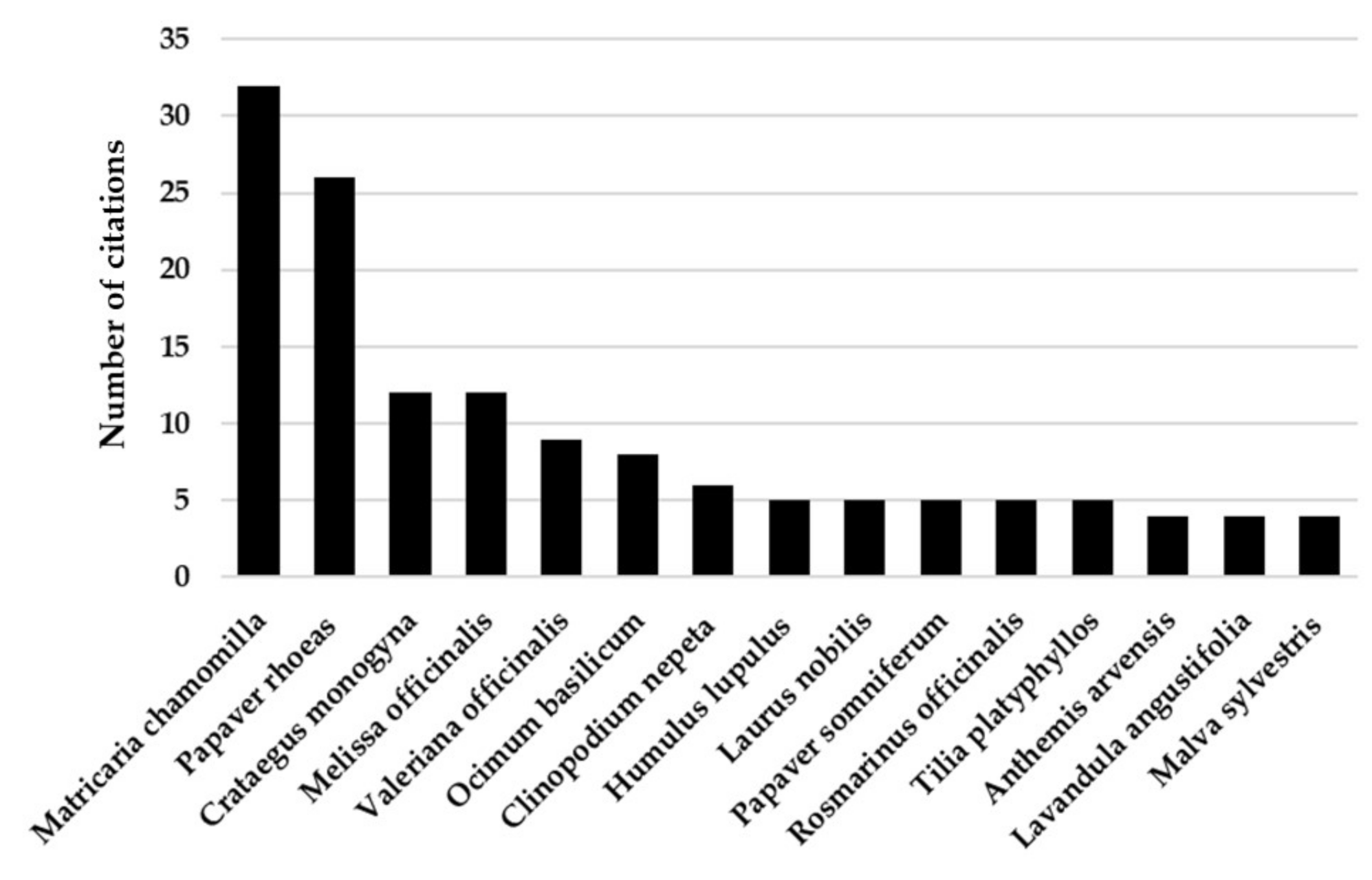
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Anthemis arvensis L.
3.2. Clinopodium nepeta L.
3.3. Crataegus monogyna Jacq.
3.4. Humulus lupulus L.
3.5. Laurus nobilis L.
3.6. Lavandula angustifolia Mill.
3.7. Malva sylvestris L.
3.8. Matricaria chamomilla L.
3.9. Melissa officinalis L.
3.10. Ocimum basilicum L.
3.11. Papaver rhoeas L.
3.12. Papaver somniferum L.
3.13. Rosmarinus officinalis L.
3.14. Tilia platyphyllos Scop.
3.15. Valeriana officinalis L.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ernst, E. Herbal remedies for anxiety—A systematic review of controlled clinical trials. Phytomedicine 2006, 13, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.G. Insomnia: Symptom or diagnosis? Clin. Psychol. Rev. 2001, 21, 1037–1059. [Google Scholar] [CrossRef]
- Kucharczyk, E.R.; Morgan, K.; Hall, A.P. The occupational impact of sleep quality and insomnia symptoms. Sleep Med. Rev. 2012, 16, 547–559. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, E.; Saliba, A.J.; Wiener, K.K.; Sarris, J. Prevalence and predictors of herbal medicine use in adults experiencing anxiety: A critical review of the literature. Adv. Integr. Med. 2015, 2, 38–48. [Google Scholar] [CrossRef]
- Kogadeeva, M.; Zamboni, N. SUMOFLUX: A Generalized Method for Targeted 13C Metabolic Flux Ratio Analysis. PLoS Comput. Biol. 2016, 12, e1005109. [Google Scholar] [CrossRef] [Green Version]
- Lydiard, R.B.; Rickels, K.; Herman, B.; Feltner, D.E. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int. J. Neuropsychopharmacol. 2010, 13, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Hoehn-Saric, R. Psychic and somatic anxiety: Worries, somatic symptoms and physiological changes. Acta Psychiatr. Scand. 1998, 393, 32–38. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Vieira, K.F. Nutritional and herbal supplements for anxiety and anxiety-related disorders: Systematic review. Nutr. J. 2010, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Lydiard, R.B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 2003, 64 (Suppl. 3), 21–27. [Google Scholar]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef]
- Koen, N.; Stein, D.J. Pharmacotherapy of anxiety disorders: A critical review. Dialogues Clin. Neurosci. 2011, 13, 423–437. [Google Scholar]
- Stewart, S.A. The effects of benzodiazepines on cognition. J. Clin. Psychiatry 2005, 66 (Suppl. 2), 9–13. [Google Scholar]
- Uzun, S.; Kozumplik, O.; Jakovljevic, M.; Sedic, B. Side effects of treatment with benzodiazepines. Psychiatr. Danub. 2010, 22, 90–93. [Google Scholar]
- Ji, X.; Ivers, H.; Beaulieu-Bonneau, S.; Morin, C.M. Complementary and alternative treatments for insomnia/insomnia-depression-anxiety symptom cluster: Meta-analysis of English and Chinese literature. Sleep Med. Rev. 2021, 58, 101445. [Google Scholar] [CrossRef]
- Tilburt, J.C.; Kaptchuk, T.J. Herbal medicine research and global health: An ethical analysis. Bull. World Health Organ. 2008, 86, 594–599. [Google Scholar] [CrossRef]
- Gasparini, M.; Aurilia, C.; Lubian, D.; Testa, M. Herbal remedies and the self-treatment of stress: An Italian survey. Eur. J. Integr. Med. 2016, 8, 465–470. [Google Scholar] [CrossRef]
- Kinrys, G.; Coleman, E.; Rothstein, E. Natural remedies for anxiety disorders: Potential use and clinical applications. Depress. Anxiety 2009, 26, 259–265. [Google Scholar] [CrossRef]
- Leach, M.J.; Page, A.T. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Heinrich, M. Ethnopharmacology: A Short History of A Multidisciplinary Field of Research; John Wiley & Sons Ltd.: Chichester, UK, 2015. [Google Scholar]
- Heinrich, M.; Edwards, S.; Moerman, D.E.; Leonti, M. Ethnopharmacological field studies: A critical assessment of their conceptual basis and methods. J. Ethnopharmacol. 2009, 124, 1–17. [Google Scholar] [CrossRef]
- Heinrich, M.; Gibbons, S. Ethnopharmacology in drug discovery: An analysis of its role and potential contribution. J Pharm. Pharmacol. 2001, 53, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Popovic, Z.; Matic, R.; Bojovic, S.; Stefanovic, M.; Vidakovic, V. Ethnobotany and herbal medicine in modern complementary and alternative medicine: An overview of publications in the field of I&C medicine 2001–2013. J. Ethnopharmacol. 2016, 181, 182–192. [Google Scholar]
- Pardo-de-Santayana, M.; Quave, C.L.; Sõukand, R.; Pieroni, A. Medical ethnobotany and ethnopharmacology of Europe. Ethnopharmacology 2015, 29, 343–355. [Google Scholar]
- Alarcomicronn, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and local food plants in the south of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224. [Google Scholar] [CrossRef] [Green Version]
- Gurdal, B.; Kultur, S. An ethnobotanical study of medicinal plants in Marmaris (Mugla, Turkey). J. Ethnopharmacol. 2013, 146, 113–126. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Mullalija, B.; Mustafa, B.; Hajdari, A.; Quave, C.L.; Pieroni, A. Ethnobotany of rural and urban Albanians and Serbs in the Anadrini region, Kosovo. Genet. Resour. Crop Evol. 2021, 68, 1825–1848. [Google Scholar] [CrossRef]
- Maruca, G.; Spampinato, G.; Turiano, D.; Laghetti, G.; Musarella, C.M. Ethnobotanical notes about medicinal and useful plants of the Reventino Massif tradition (Calabria region, Southern Italy). Genet. Resour. Crop Evol. 2019, 66, 1027–1040. [Google Scholar] [CrossRef]
- Mautone, M.; De Martino, L.; De Feo, V. Ethnobotanical research in Cava de’ Tirreni area, Southern Italy. J. Ethnobiol. Ethnomed. 2019, 15, 50. [Google Scholar] [CrossRef] [Green Version]
- Menale, B.; Castro, O.D.; Iorio, E.D.; Ranaldi, M.; Muoio, R. Discovering the ethnobotanical traditions of the island of Procida (Campania, southern Italy). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 1–19. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—An alpine ethnobotanical study. J. Ethnopharmacol. 2013, 145, 517–529. [Google Scholar] [CrossRef] [PubMed]
- WFO. World Flora Online. Available online: http://www.worldfloraonline.org (accessed on 1 September 2021).
- Stevens, P.F. Angiosperm Phylogeny Website, Version 14. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 18 April 2021).
- Brummitt, P.K.; Powell, C.E. Authors of Plant Names; Royal Botanic Gardens, Kew: Richmond, UK, 1992. [Google Scholar]
- Rivera, D.; Allkin, R.; Obon, C.; Alcaraz, F.; Verpoorte, R.; Heinrich, M. What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 2014, 152, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019, 1, 13. [Google Scholar]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef]
- Seaman, F.C. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot. Rev. 1982, 48, 121–594. [Google Scholar] [CrossRef]
- Shetty, K. Biotechnology to harness the benefits of dietary phenolics; focus on Lamiaceae. Asia Pac. J. Clin. Nutr. 1997, 6, 162–171. [Google Scholar]
- Bottoni, M.; Milani, F.; Colombo, L.; Nallio, K.; Colombo, P.S.; Giuliani, C.; Bruschi, P.; Fico, G. Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches. Molecules 2020, 25, 4144. [Google Scholar] [CrossRef]
- Vitalini, S.; Puricelli, C.; Mikerezi, I.; Iriti, M. Plants, people and traditions: Ethnobotanical survey in the Lombard Stelvio National Park and neighbouring areas (Central Alps, Italy). J. Ethnopharmacol. 2015, 173, 435–458. [Google Scholar] [CrossRef]
- Loi, M.C.; Maxia, L.; Maxia, A. Ethnobotanical Comparison Between the Villages of Escolca and Lotzorai (Sardinia, Italy). J. Herbs Spices Med. Plants 2005, 2005, 67–84. [Google Scholar] [CrossRef]
- Uncini Manganelli, R.E.; Camangi, F.; Tomei, P.E.; Oggiano, N. L’uso delle Erbe Nella Tradizione Rurale della Toscana. Voll. I–II; ARSIA: Firenze, Italy, 2002. [Google Scholar]
- Uncini Manganelli, R.E.; Tomei, P.E. Ethnopharmacobotanical studies of the Tuscan Archipelago. J. Ethnopharmacol. 1999, 65, 181–202. [Google Scholar] [CrossRef]
- Guarino, C.; De Simone, L.; Santoro, S. Ethnobotanical study of the Sannio area, Campania. South. Italy Ethnobot. Res. Appl. 2008, 6, 255–317. [Google Scholar] [CrossRef] [Green Version]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Bonsangue, G.; Letizia Gargano, M.; Venturella, G.; La Bella, S. Popular uses of wild plant species for medicinal purposes in the Nebrodi Regional Park (North-Eastern Sicily, Italy). J. Ethnopharmacol. 2014, 157, 21–37. [Google Scholar] [CrossRef]
- Camangi, F.; Stefani, A.; Tomei, P.E. Il casentino: Tradizioni etnofarmacobotaniche nei comuni di Poppi e Bibbiena (Arezzo, Toscana). Atti Soc. Tosc. Sci. Nat. Mem. 2003, 110, 55–69. [Google Scholar]
- Loi, M.C.; Poli, F.; Sacchetti, G.; Selenu, M.B.; Ballero, M. Ethnopharmacology of ogliastra (villagrande strisaili, sardinia, Italy). Fitoterapia 2004, 75, 277–295. [Google Scholar] [CrossRef]
- Idolo, M.; Motti, R.; Mazzoleni, S. Ethnobotanical and phytomedicinal knowledge in a long-history protected area, the Abruzzo, Lazio and Molise National Park (Italian Apennines). J. Ethnopharmacol. 2010, 127, 379–395. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Pavesi, A. New or uncommon uses of several medicinal plants in some areas of central Italy. J. Ethnopharmacol. 1990, 29, 213–223. [Google Scholar] [CrossRef]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Aleo, M.; Cambria, S.; Bazan, G. Tradizioni etnofarmacobotaniche in alcune comunità rurali dei Monti di Trapani (Sicilia occidentale). Quad. Bot. Amb. Appl. 2013, 24, 27–48. [Google Scholar]
- Passalacqua, N.G.; Guarrera, P.M.; De Fine, G. Contribution to the knowledge of the folk plant medicine in Calabria region (Southern Italy). Fitoterapia 2007, 78, 52–68. [Google Scholar] [CrossRef]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. Scholarly vs. traditional knowledge: Effects of sacred natural sites on ethnobotanical practices in Tuscany, Central Italy. Hum. Ecol. 2019, 47, 653–667. [Google Scholar] [CrossRef]
- Ballero, M.; Poli, F.; Sacchetti, G.; Loi, M.C. Ethnobotanical research in the territory of Fluminimaggiore (south-western Sardinia). Fitoterapia 2001, 72, 788–801. [Google Scholar] [CrossRef]
- Motti, R.; Antignani, V.; Idolo, M. Traditional plant use in the Phlegraean Fields Regional Park (Campania, Southern Italy). Hum. Ecol. 2009, 37, 775–782. [Google Scholar] [CrossRef]
- Savo, V.; Giulia, C.; Maria, G.P.; David, R. Folk phytotherapy of the Amalfi Coast (Campania, Southern Italy). J. Ethnopharmacol. 2011, 135, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Fortini, P.; Di Marzio, P.; Guarrera, P.M.; Iorizzi, M. Ethnobotanical study on the medicinal plants in the Mainarde Mountains (central-southern Apennine, Italy). J. Ethnopharmacol. 2016, 184, 208–218. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Corradi, L. Ethnopharmacobotanical remarks on the province of Chieti town (Abruzzo, central Italy). J. Ethnopharmacol. 2001, 74, 17–40. [Google Scholar] [CrossRef]
- Leto, C.; Tuttolomondo, T.; La Bella, S.; Licata, M. Ethnobotanical study in the Madonie Regional Park (Central Sicily, Italy)—Medicinal use of wild shrub and herbaceous plant species. J. Ethnopharmacol. 2013, 146, 90–112. [Google Scholar] [CrossRef]
- Lucchetti, L.; Zitti, S.; Taffetani, F. Ethnobotanical uses in the Ancona district (Marche region, Central Italy). J. Ethnobiol. Ethnomed. 2019, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Menale, B.; Amato, G.; Di Prisco, C.; Muoio, R. Traditional uses of plants in North-Western Molise (central Italy). Delpinoa 2006, 48, 29–36. [Google Scholar]
- Dei Cas, L.; Pugni, F.; Fico, G. Tradition of use on medicinal species in Valfurva (Sondrio, Italy). J. Ethnopharmacol. 2015, 163, 113–134. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Savo, V.; Caneva, G. Traditional uses of plants in the Tolfa–Cerite–Manziate area (Central Italy). Ethnobiol. Lett. 2015, 6, 119–161. [Google Scholar] [CrossRef]
- Menale, B.; Muoio, R. Use of medicinal plants in the South-Eastern area of the Partenio Regional Park (Campania, Southern Italy). J. Ethnopharmacol. 2014, 153, 297–307. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Savo, V.; Bonsangue, G.; Letizia Gargano, M.; Venturella, G.; La Bella, S. Ethnobotanical investigation on wild medicinal plants in the Monti Sicani Regional Park (Sicily, Italy). J. Ethnopharmacol. 2014, 153, 568–586. [Google Scholar] [CrossRef]
- Martini, E. La fitoterapia popolare in val Borbera (Appennino ligure). Webbia 1982, 35, 187–205. [Google Scholar] [CrossRef]
- Di Novella, R.; Di Novella, N.; De Martino, L.; Mancini, E.; De Feo, V. Traditional plant use in the National Park of Cilento and Vallo di Diano, Campania, Southern Italy. J. Ethnopharmacol. 2013, 145, 328–342. [Google Scholar] [CrossRef]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- Maxia, A.; Lancioni, M.C.; Balia, A.N.; Alberghetti, R.; Pieroni, A.; Loi, M.C. Medical ethnobotany of the Tabarkins, a Northern Italian (Ligurian) minority in South-Western Sardinia. Genet. Resour. Crop Evol. 2008, 55, 911–924. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Gargano, M.L.; Venturella, G.; La Bella, S. Plant genetic resources and traditional knowledge on medicinal use of wild shrub and herbaceous plant species in the Etna Regional Park (Eastern Sicily, Italy). J. Ethnopharmacol. 2014, 155, 1362–1381. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Ballero, M.; Bruni, A.; Sacchetti, G.; Mossa, L.; Poli, F. Indagine etnofarmacobotanica del territorio di Arzana (Sardegna orientale). Ann. Bot. 1994, 52, 489–500. [Google Scholar]
- Guarrera, P.M. Il Patrimonio Etnobotanico del Lazio. G. Bot. Ital. 1994, 128, 446. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Terrizzano, L.; Dente, F.; Mariotti, M.G. Ethnobotanical and phytochemical knowledge in the North-Western Ligurian Alps. J. Ethnopharmacol. 2014, 155, 463–484. [Google Scholar] [CrossRef]
- De Natale, A.; Pollio, A. Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy). J. Ethnopharmacol. 2007, 109, 295–303. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Salerno, G.; Caneva, G. Folk phytotherapeutical plants from Maratea area (Basilicata, Italy). J. Ethnopharmacol. 2005, 99, 367–378. [Google Scholar] [CrossRef]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. Blended divergences: Local food and medicinal plant uses among Arbëreshë, Occitans, and autochthonous Calabrians living in Calabria, Southern Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 154, 615–626. [Google Scholar] [CrossRef]
- Motti, R.; Motti, P. An Ethnobotanical Survey of Useful Plants in the Agro Nocerino Sarnese (Campania, Southern Italy). Hum. Ecol. 2017, 45, 865–878. [Google Scholar] [CrossRef]
- Palmese, M.T.; Uncini Manganelli, R.E.; Tomei, P.E. An ethno-pharmacobotanical survey in the Sarrabus district (south-east Sardinia). Fitoterapia 2001, 72, 619–643. [Google Scholar] [CrossRef]
- Pieroni, A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J. Ethnopharmacol. 2000, 70, 235–273. [Google Scholar] [CrossRef]
- Pieroni, A.; Quave, C.L. Traditional pharmacopoeias and medicines among Albanians and Italians in southern Italy: A comparison. J. Ethnopharmacol. 2005, 101, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, A.M.; Motti, R.; Weckerle, C.S. Traditional plant use in the areas of Monte Vesole and Ascea, Cilento National Park (Campania, southern Italy). J. Ethnopharmacol. 2005, 97, 129–143. [Google Scholar] [CrossRef]
- Menale, B.; De Castro, O.; Cascone, C.; Muoio, R. Ethnobotanical investigation on medicinal plants in the Vesuvio National Park (Campania, Southern Italy). J. Ethnopharmacol. 2016, 192, 320–349. [Google Scholar] [CrossRef]
- Camangi, F.; Tomei, P.E. Tradizioni etnofarmacobotaniche nella provincia di Livorno: Il territorio della Valle Benedetta. Inf. Bot. Ital. 2003, 35, 41–54. [Google Scholar]
- Licata, M.; Tuttolomondo, T.; Leto, C.; Virga, G.; Bonsangue, G.; Cammalleri, I.; Gennaro, M.C.; La Bella, S. A survey of wild plant species for food use in Sicily (Italy)–results of a 3-year study in four regional parks. J. Ethnobiol. Ethnomed. 2016, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. Dissymmetry at the Border: Wild Food and Medicinal Ethnobotany of Slovenes and Friulians in NE Italy. Econ. Bot. 2020, 74, 1–14. [Google Scholar] [CrossRef]
- Ballero, M.; Fresu, I. Le piante di uso officinale nella Barbagia di Seui (Sardegna Centrale). Fitoterapia 1993, 64, 141. [Google Scholar]
- Pieroni, A.; Nebel, S.; Quave, C.; Munz, H.; Heinrich, M. Ethnopharmacology of liakra: Traditional weedy vegetables of the Arbereshe of the Vulture area in southern Italy. J. Ethnopharmacol. 2002, 81, 165–185. [Google Scholar] [CrossRef]
- Ballero, M.; Fresu, I. (Piante officinali impiegate in fitoterapia nel territorio del Marganai (Sardegna sud occidentale). Fitoterapia 1991, 62, 524–531. [Google Scholar]
- Lentini, F.; Giani, S.; Amenta, R. L’uso popolare delle piante nelle isole Eolie (Sicilia). Acta Technol. Legis Medicam. 1995, 3, 351–355. [Google Scholar]
- Uncini Manganelli, R.E.; Tomei, P. Indagini farmacobotaniche in Garfagnana (LU): Il versante apuano. Atti Soc. Tosc. Sci. Nat. Mem. 1997, 103, 63–80. [Google Scholar]
- Bandini, A. Le piante della medicina tradizionale nell’alta valle di Vara (Liguria orientale). Webbia 1961, 16, 143–163. [Google Scholar] [CrossRef]
- Zampiva, F. Erbe e piante della Lessinia. Erbor. Domani 1981, 9, 17–25. [Google Scholar]
- Di Sanzo, P.; De Martino, L.; Mancini, E.; Feo, V.D. Medicinal and useful plants in the tradition of Rotonda, Pollino National Park, Southern Italy. J. Ethnobiol. Ethnomed. 2013, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Chimenti Signorini, R.; Fumagalli, M. Indagine etnofarmacobotanica nella Valtournanche (Valle d’Aosta). Webbia 1983, 1, 64–69. [Google Scholar] [CrossRef]
- Bellomaria, B.; Della Mora, L. Novità nell’uso delle piante officinali per la zona di Matelica (Macerata) anche in confronto con altre zone delle Marche. Arch. Bot. Biogeogr. Ital. 1985, 61, 51–81. [Google Scholar]
- Lentini, F.; Aleo, M.; Amenta, R. L’uso popolare delle piante nelle Isole Egadi (Sicilia). Acta Phytoterapeutica 1997, 4, 88–94. [Google Scholar]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Obakiro, S.B.; Kiprop, A.; Kowino, I.; Kigondu, E.; Odero, M.P.; Omara, T.; Bunalema, L. Ethnobotany, ethnopharmacology, and phytochemistry of traditional medicinal plants used in the management of symptoms of tuberculosis in East Africa: A systematic review. Trop. Med. Health 2020, 48, 68. [Google Scholar] [CrossRef]
- Buchbauer, G.; Jäger, W.; Gruber, A.; Dietrich, H. R-(+)-and S-(−)-carvone: Influence of chirality on locomotion activity in mice. Flavour Fragr. J. 2005, 20, 686–689. [Google Scholar] [CrossRef]
- De Sousa, D.P.; de Farias Nobrega, F.F.; de Almeida, R.N. Influence of the chirality of (R)-(−)- and (S)-(+)-carvone in the central nervous system: A comparative study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Urbina, A.V.; Martin, M.L.; Montero, M.J.; Moran, A.; San Roman, L. Sedating and antipyretic activity of the essential oil of Calamintha sylvatica subsp. ascendens. J. Ethnopharmacol. 1989, 25, 165–171. [Google Scholar] [CrossRef]
- Can, O.D.; Ozkay, U.D.; Ozturk, N.; Ozturk, Y. Effects of hawthorn seed and pulp extracts on the central nervous system. Pharm. Biol. 2010, 48, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandanizadeh, K.; Modaresi, M.; Sajjadian, I. Comparing the effect of hawthorn’s extract and chlordiazepoxide on reducing anxiety in laboratory mice. Indo Am. J. Pharm. Sci. 2018, 5, 4435–4440. [Google Scholar]
- Abbasi, M.; Gohari, S.; Ahangar, H.; Bahrami, M.; Kamalinejad, M.; Reshadmanesh, T.; Nazari, S.S. Efficacy of Hawthorn fruit extract on blood pressure and quality of sleep in patients with hypertension along with sleep disorders: A randomized double-blind controlled trial. J. Contemp. Med. Sci. 2021, 7, 196–201. [Google Scholar]
- Abourashed, E.A.; Koetter, U.; Brattstrom, A. In vitro binding experiments with a Valerian, hops and their fixed combination extract (Ze91019) to selected central nervous system receptors. Phytomedicine 2004, 11, 633–638. [Google Scholar] [CrossRef]
- Aoshima, H.; Takeda, K.; Okita, Y.; Hossain, S.J.; Koda, H.; Kiso, Y. Effects of beer and hop on ionotropic gamma-aminobutyric acid receptors. J. Agric. Food Chem. 2006, 54, 2514–2519. [Google Scholar] [CrossRef]
- Meissner, O.; Haberlein, H. Influence of xanthohumol on the binding behavior of GABA(A) receptors and their lateral mobility at hippocampal neurons. Planta Med. 2006, 72, 656–658. [Google Scholar] [CrossRef]
- Wohlfart, R.; Hansel, R.; Schmidt, H. The sedative-hypnotic principle of hops. 4. Pharmacology of the hop substance 2-methyl-3-buten-ol. Planta Med. 1983, 48, 120–123. [Google Scholar] [CrossRef]
- Zanoli, P.; Rivasi, M.; Zavatti, M.; Brusiani, F.; Baraldi, M. New insight in the neuropharmacological activity of Humulus lupulus L. J. Ethnopharmacol. 2005, 102, 102–106. [Google Scholar] [CrossRef]
- Schiller, H.; Forster, A.; Vonhoff, C.; Hegger, M.; Biller, A.; Winterhoff, H. Sedating effects of Humulus lupulus L. extracts. Phytomedicine 2006, 13, 535–541. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Sayyah, M.; Valizadeh, J.; Kamalinejad, M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole- and maximal electroshock-induced seizures. Phytomedicine 2002, 9, 212–216. [Google Scholar] [CrossRef]
- Chioca, L.R.; Ferro, M.M.; Baretta, I.P.; Oliveira, S.M.; Silva, C.R.; Ferreira, J.; Losso, E.M.; Andreatini, R. Anxiolytic-like effect of lavender essential oil inhalation in mice: Participation of serotonergic but not GABAA/benzodiazepine neurotransmission. J. Ethnopharmacol. 2013, 147, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Lopez, V.; Nielsen, B.; Solas, M.; Ramirez, M.J.; Jager, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharm. 2017, 8, 280. [Google Scholar] [CrossRef]
- Schuwald, A.M.; Noldner, M.; Wilmes, T.; Klugbauer, N.; Leuner, K.; Muller, W.E. Lavender oil-potent anxiolytic properties via modulating voltage dependent calcium channels. PLoS ONE 2013, 8, e59998. [Google Scholar] [CrossRef] [Green Version]
- Buchbauer, G.; Jirovetz, L.; Jager, W.; Dietrich, H.; Plank, C. Aromatherapy: Evidence for sedative effects of the essential oil of lavender after inhalation. Z. Nat. C 1991, 46, 1067–1072. [Google Scholar] [CrossRef]
- Bradley, B.F.; Starkey, N.J.; Brown, S.L.; Lea, R.W. Anxiolytic effects of Lavandula angustifolia odour on the Mongolian gerbil elevated plus maze. J. Ethnopharmacol. 2007, 111, 517–525. [Google Scholar] [CrossRef]
- Shaw, D.; Annett, J.M.; Doherty, B.; Leslie, J.C. Anxiolytic effects of lavender oil inhalation on open-field behaviour in rats. Phytomedicine 2007, 14, 613–620. [Google Scholar] [CrossRef]
- Ghadim, M.B.A.; Neisy, A.; Sisakht, M.; Khoshdel, Z. Lavandula angustifolia aqueous extract ameliorates anxiety and depressive-like behaviors in chronic mild stress-treated male rats. J. Med. Plants Res. 2020, 14, 593–603. [Google Scholar]
- Alnamer, R.; Alaoui, K.; Bouidida, E.H.; Benjouad, A.; Cherrah, Y. Sedative and hypnotic activities of the methanolic and aqueous extracts of Lavandula officinalis from Morocco. Adv. Pharmacol. Sci. 2011, 2012. [Google Scholar] [CrossRef]
- Kasper, S.; Gastpar, M.; Muller, W.E.; Volz, H.P.; Moller, H.J.; Dienel, A.; Schlafke, S. Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: A randomized, double-blind, placebo controlled trial. Int. Clin. Psychopharmacol. 2010, 25, 277–287. [Google Scholar] [CrossRef]
- Modaresi, M.; Lohrasbi, S. Effects of Mallow, (Malva sylvestris) Extract on Reducing Anxiety in Animal Model. Avicenna J. Neuro Psycho Physiol. 2017, 4, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, A.; Pashazadeh, M.; Alizadeh, A.; Mirzazadeh, J.; Javanian, S. Study of Sedation, Pre-anesthetic and Anti-anxiety Effects of Malva sylvestris Extract in Comparison with Diazepam in Rats. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 24–28. [Google Scholar]
- Amsterdam, J.D.; Li, Y.; Soeller, I.; Rockwell, K.; Mao, J.J.; Shults, J. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J. Clin. Psychopharmacol. 2009, 29, 378–382. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Keefe, J.R.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Long-term chamomile (Matricaria chamomilla L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine 2016, 23, 1735–1742. [Google Scholar] [CrossRef] [Green Version]
- Medina, J.H.; Viola, H.; Wolfman, C.; Marder, M.; Wasowski, C.; Calvo, D.; Paladini, A.C. Neuroactive flavonoids: New ligands for the Benzodiazepine receptors. Phytomedicine 1998, 5, 235–243. [Google Scholar] [CrossRef]
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 2000, 71 (Suppl. 1), S117–S123. [Google Scholar] [CrossRef]
- Viola, H.; Wasowski, C.; De Stein, M.L.; Wolfman, C.; Silveira, R.; Dajas, F.; Medina, J.H.; Paladini, A.C. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995, 61, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Schreier, P.; Baraldi, M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharm. 2000, 59, 1387–1394. [Google Scholar] [CrossRef]
- Awad, R.; Levac, D.; Cybulska, P.; Merali, Z.; Trudeau, V.L.; Arnason, J.T. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 2007, 85, 933–942. [Google Scholar] [CrossRef]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1075–1081. [Google Scholar]
- Kennedy, D.O.; Little, W.; Scholey, A.B. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (Lemon Balm). Psychosom. Med. 2004, 66, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Ibarra, A.; Feuillere, N.; Roller, M.; Sukkar, S.G. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Med. J. Nutr. Metab. 2011, 4, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Rauniar, G.P.; Das, B.P. Experimental study of various central nervous system effects of eugenol in mice and rats. Health Renaiss. 2012, 10, 208–214. [Google Scholar] [CrossRef]
- Rabbani, M.; Sajjadi, S.E.; Vaezi, A. Evaluation of anxiolytic and sedative effect of essential oil and hydroalcoholic extract of Ocimum basilicum L. and chemical composition of its essential oil. Res. Pharm. Sci. 2015, 10, 535–543. [Google Scholar]
- Hirai, M.; Ito, M. Sedative effects of the essential oil and headspace air of Ocimum basilicum by inhalation in mice. J. Nat. Med. 2019, 73, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Hillenbrand, M.; Zapp, J.; Becker, H. Depsides from the petals of Papaver rhoeas. Planta Med. 2004, 70, 380–382. [Google Scholar]
- Grauso, L.; de Falco, B.; Motti, R.; Lanzotti, V. Corn poppy, Papaver rhoeas L.: A critical review of its botany, phytochemistry and pharmacology. Phytochem. Rev. 2020, 20, 227–248. [Google Scholar] [CrossRef]
- Labanca, F.; Ovesna, J.; Milella, L. Papaver somniferum L. taxonomy, uses and new insight in poppy alkaloid pathways. Phytochem. Rev. 2018, 17, 853–871. [Google Scholar] [CrossRef]
- Listos, J.; Lupina, M.; Talarek, S.; Mazur, A.; Orzelska-Gorka, J.; Kotlinska, J. The Mechanisms Involved in Morphine Addiction: An Overview. Int. J. Mol. Sci. 2019, 20, 4302. [Google Scholar] [CrossRef] [Green Version]
- El Alaoui, C.; Chemin, J.; Fechtali, T.; Lory, P. Modulation of T-type Ca2+ channels by Lavender and Rosemary extracts. PLoS ONE 2017, 12, e0186864. [Google Scholar]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.; Hanrahan, J. Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus Officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Hernández, E.; González-Trujano, M.; Terrazas, T.; Herrera Santoyo, J.; Guevara-Fefer, P. Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation. Salud Ment. 2016, 39, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Allio, A.; Calorio, C.; Franchino, C.; Gavello, D.; Carbone, E.; Marcantoni, A. Bud extracts from Tilia tomentosa Moench inhibit hippocampal neuronal firing through GABAA and benzodiazepine receptors activation. J. Ethnopharmacol. 2015, 172, 288–296. [Google Scholar] [CrossRef]
- Cavadas, C.; Fontes Ribeiro, C.A.; Santos, M.S.; Cunha, A.P.; Macedo, T.; Caramona, M.M.; Cotrim, M.D. In vitro study of the interaction of Tilia europeae L. aqueous extract with GABAA receptors in rat brain. Phytother. Res. 1997, 11, 17–21. [Google Scholar] [CrossRef]
- Dietz, B.M.; Mahady, G.B.; Pauli, G.F.; Farnsworth, N.R. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res. Mol. Brain Res. 2005, 138, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Khom, S.; Baburin, I.; Timin, E.; Hohaus, A.; Trauner, G.; Kopp, B.; Hering, S. Valerenic acid potentiates and inhibits GABAA receptors: Molecular mechanism and subunits pecificity. Neuropharmacology 2007, 53, 178–187. [Google Scholar] [CrossRef]
- Benke, D.; Barberis, A.; Kopp, S.; Altmann, K.H.; Schubiger, M.; Vogt, K.E.; Rudolph, U.; Mohler, H. GABA A receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology 2009, 56, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Aliakbari, F.; Rafieian, M. The effectiveness of Valeriana officinalis on sleep disturbance in patients with chronic heart failure. Int. J. Pharm. Investig. 2018, 8, 145–150. [Google Scholar]
- Foddis, C.; Maxia, A. Le piante utilizzate nella medicina popolare dell’Ogliastra (Sardegna centro-orientale) per la cura delle patologie del sistema muscolo-scheletrico. Rend. Semin. Fac. Sci. Univ. Cagliari 2006, 76, 17–28. [Google Scholar]
- Sanna, C.; Ballero, M.; Maxia, A. Le piante medicinali utilizzate contro le patologie epidermiche in Ogliastra (Sardegna centro-orientale). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2006, 113, 73–82. [Google Scholar]
- Boukhary, R.; Aboul-ElA, M.; El-Lakany, A. Review on chemical constituents and biological activities of genus Anthemis. Pharmacogn. J. 2019, 11, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Vučković, I.; Vujisić, L.; Vajs, V.; Tešević, V.; Macura, S.; Janaćković, P.; Milosavljević, S. Sesquiterpene lactones from the aerial parts of Anthemis arvensis L. Biochem. Syst. Ecol. 2006, 34, 303–309. [Google Scholar] [CrossRef]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motti, R. Wild Plants Used as Herbs and Spices in Italy: An Ethnobotanical Review. Plants 2021, 10, 563. [Google Scholar] [CrossRef]
- Motti, R.; Ippolito, F.; Bonanomi, G. Folk phytotherapy used in paediatric health care in central and southern Italy regions: A review. Hum. Ecol. 2018, 46, 573–585. [Google Scholar] [CrossRef]
- Bozovic, M.; Ragno, R. Calamintha nepeta (L.) Savi and its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry. Molecules 2017, 22, 290. [Google Scholar] [CrossRef]
- Debbabi, H.; Mokni, R.E.; Chaieb, I.; Nardoni, S.; Maggi, F.; Caprioli, G.; Hammami, S. Chemical Composition, Antifungal and Insecticidal Activities of the Essential Oils from Tunisian Clinopodium Nepeta Subsp. nepeta and Clinopodium nepeta Subsp. Glandulosum. Molecules 2020, 25, 2137. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Goncalves, M.J.; Cavaleiro, C.; Salgueiro, L. Chemical composition and biological assays of essential oils of Calamintha nepeta (L.) Savi subsp. nepeta (Lamiaceae). Nat. Prod. Res. 2010, 24, 1734–1742. [Google Scholar] [CrossRef]
- Yang, B.; Liu, P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. Comparing the composition and bioactivity of Crataegus Monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.M. Polyphenolic Composition of Crataegus monogyna Jacq.: From Chemistry to Medical Applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef]
- Rodrigues, S.; Calhelha, R.C.; Barreira, J.C.; Dueñas, M.; Carvalho, A.M.; Abreu, R.M.; Santos-Buelga, C.; Ferreira, I.C. Crataegus monogyna buds and fruits phenolic extracts: Growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC–DAD–ESI/MS. Food Res. Int. 2012, 49, 516–523. [Google Scholar] [CrossRef]
- Dahmer, S.; Scott, E. Health effects of hawthorn. Am. Fam. Physician 2010, 81, 465–468. [Google Scholar]
- Pittler, M.H.; Guo, R.; Ernst, E. Hawthorn extract for treating chronic heart failure. Cochrane Database Syst. Rev. 2008, 1, CD005312. [Google Scholar]
- Rigelsky, J.M.; Sweet, B.V. Hawthorn: Pharmacology and therapeutic uses. Am. J. Health Syst. Pharm. 2002, 59, 417–422. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Emrick, S.; Lanzotti, V. Traditional Herbal Remedies Used in women’s Health Care in Italy: A Review. Hum. Ecol. 2019, 47, 941–972. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L. a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Akazawa, H.; Kohno, H.; Tokuda, H.; Suzuki, N.; Yasukawa, K.; Kimura, Y.; Manosroi, A.; Manosroi, J.; Akihisa, T. Anti-inflammatory and anti-tumor-promoting effects of 5-deprenyllupulonol C and other compounds from Hop (Humulus lupulus L.). Chem. Biodivers. 2012, 9, 1045–1054. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, L.; Sanchez, C.; Bravo, R.; Rodriguez, A.; Barriga, C.; Juanez, J.C. The sedative effects of hops (Humulus lupulus), a component of beer, on the activity/rest rhythm. Acta Physiol. Hung. 2012, 99, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Koetter, U.; Schrader, E.; Kaufeler, R.; Brattstrom, A. A randomized, double blind, placebo-controlled, prospective clinical study to demonstrate clinical efficacy of a fixed valerian hops extract combination (Ze 91019) in patients suffering from non-organic sleep disorder. Phytother. Res. 2007, 21, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Bland, J.S.; Katke, J.; Darland, G.; Hall, A.; Lerman, R.H.; Lamb, J.; Carroll, B.; Tripp, M. Clinical safety and efficacy of NG440: A novel combination of rho iso-alpha acids from hops, rosemary, and oleanolic acid for inflammatory conditions. Can. J. Physiol. Pharm. 2007, 85, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Dimpfel, W.; Suter, A. Sleep improving effects of a single dose administration of a valerian/hops fluid extract—A double blind, randomized, placebo-controlled sleep-EEG study in a parallel design using electrohypnograms. Eur. J. Med. Res. 2008, 13, 200–204. [Google Scholar] [CrossRef] [Green Version]
- EMA. Hop Strobile, Humulus lupulus L. Flos; EMA/HMPC/304459/2016; EMA: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Motti, R.; Bonanomi, G.; Lanzotti, V.; Sacchi, R. The Contribution of Wild Edible Plants to the Mediterranean Diet: An Ethnobotanical Case Study Along the Coast of Campania (Southern Italy). Econ. Bot. 2020, 74, 249–272. [Google Scholar] [CrossRef]
- Musarella, C.M.; Paglianiti, I.; Cano-Ortiz, A.; Spampinato, G. Indagine etnobotanica nel territorio del Poro e delle Preserre calabresi (Vibo Valentia, S-Italia). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2019, 126, 13–28. [Google Scholar]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical Composition and Antimicrobial Activity of Laurus nobilis L. Essential Oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef] [Green Version]
- Said, C.M.; Hussein, K. Determination of the chemical and genetic differences of Laurus collected from three different geographic and climatic areas in Lebanon. Eur. Sci. J. 2014, 2, 412–419. [Google Scholar]
- Chahal, K.K.; Kaur, M.; Bhardwaj, U.; Singla, N.; Kaur, A. A review on chemistry and biological activities of Laurus nobilis L. essential oil. J. Pharmacogn. Phytochem. 2017, 6, 1153–1161. [Google Scholar]
- Sayyah, M.; Saroukhani, G.; Peirovi, A.; Kamalinejad, M. Analgesic and anti-inflammatory activity of the leaf essential oil of Laurus nobilis Linn. Phytother. Res. 2003, 17, 733–736. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A natural compound with versatile pharmacological actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, D.P.; Militao, G.C.G.; de Morais, M.C.; de Sousa, D.P. The Dual Antioxidant/Prooxidant Effect of Eugenol and Its Action in Cancer Development and Treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Chauhan, A.; Yadav, A.; Yadav, A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef]
- Lim, W.C.; Seo, J.M.; Lee, C.I.; Pyo, H.B.; Lee, B.C. Stimulative and sedative effects of essential oils upon inhalation in mice. Arch. Pharm. Res. 2005, 28, 770–774. [Google Scholar] [CrossRef]
- Woelk, H.; Schlafke, S. A multi-center, double-blind, randomised study of the Lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine 2010, 17, 94–99. [Google Scholar] [CrossRef]
- Bradley, B.F.; Brown, S.L.; Chu, S.; Lea, R.W. Effects of orally administered lavender essential oil on responses to anxiety-provoking film clips. Hum. Psychopharmacol. Clin. Exp. 2009, 24, 319–330. [Google Scholar] [CrossRef]
- Moeini, M.; Khadibi, M.; Bekhradi, R.; Mahmoudian, S.A.; Nazari, F. Effect of aromatherapy on the quality of sleep in ischemic heart disease patients hospitalized in intensive care units of heart hospitals of the Isfahan University of Medical Sciences. Iran. J. Nurs. Midwifery Res. 2010, 15, 234–239. [Google Scholar]
- Chien, L.W.; Cheng, S.L.; Liu, C.F. The effect of lavender aromatherapy on autonomic nervous system in midlife women with insomnia. Evid.-Based Complement. Altern. Med. 2012, 2012, 740813. [Google Scholar] [CrossRef] [PubMed]
- Lewith, G.T.; Godfrey, A.D.; Prescott, P. A single-blinded, randomized pilot study evaluating the aroma of Lavandula augustifolia as a treatment for mild insomnia. J. Altern. Complement. Med. 2005, 11, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.C.; Martins, C.A.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine. J. Pharm. Pharmacol. 2012, 64, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef]
- Billeter, M.; Meier, B.; Sticher, O. 8-Hydroxyflavonoid glucuronides from Malva sylvestris. Phytochemistry 1991, 30, 987–990. [Google Scholar] [CrossRef]
- Farina, A.; Doldo, A.; Cotichini, V.; Rajevic, M.; Quaglia, M.G.; Mulinacci, N.; Vincieri, F.F. HPTLC and reflectance mode densitometry of anthocyanins in Malva silvestris L.: A comparison with gradient-elution reversed-phase HPLC. J. Pharm. Biomed. Anal. 1995, 14, 203–211. [Google Scholar] [CrossRef]
- Takeda, K.; Enoki, S.; Harborne, J.B.; Eagles, J. Malonated anthocyanins in Malvaceae: Malonylmalvin from Malva sylvestris. Phytochemistry 1989, 28, 499–500. [Google Scholar] [CrossRef]
- Daniela, A.; Pichichero, E.; Canuti, L.; Cicconi, R.; Karou, D.; D’Arcangelo, G.; Canini, A. Identification of phenolic compounds from medicinal and melliferous plants and their cytotoxic activity in cancer cells. Caryologia 2007, 60, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Cutillo, F.; D’Abrosca, B.; Dellagreca, M.; Fiorentino, A.; Zarrelli, A. Terpenoids and phenol derivatives from Malva silvestris. Phytochemistry 2006, 67, 481–485. [Google Scholar] [CrossRef]
- Mann, C.; Staba, E.J. The chemistry, pharmacology, and commercial formulations of Chamomile. J. Herbs Spices Med. Plants 1986, 1, 235–280. [Google Scholar]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Tavares, M.F.; Horvath, C. Capillary electrochromatography of selected phenolic compounds of Chamomilla recutita. J. Chromatogr. A 2007, 1154, 390–399. [Google Scholar] [CrossRef]
- Jenabi, E.; Ebrahimzadeh, S. The effect of Matricaria chamomilla tea on primary dysmenorrheal. J. Med. Plants 2009, 8, 39–42. [Google Scholar]
- Niazi, A.; Moradi, M. The Effect of Chamomile on Pain and Menstrual Bleeding in Primary Dysmenorrhea: A Systematic Review. Int. J. Community Based Nurs. Midwifery 2021, 9, 174–186. [Google Scholar]
- Sharafzadeh, S.; Alizadeh, O. German and Roman chamomile. J. Appl. Pharm. Sci. 2011, 1, 1–5. [Google Scholar]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharm. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Cemek, M.; Kaga, S.; Simsek, N.; Buyukokuroglu, M.E.; Konuk, M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J. Nat. Med. 2008, 62, 284–293. [Google Scholar] [CrossRef]
- Ranjbar, A.; Mohsenzadeh, F.; Chehregani, A.; Khajavi, F.; Zijoud, S.M.; Ghasemi, H. Ameliorative effect of Matricaria chamomilla.L on paraquat: Induced oxidative damage in lung rats. Pharmacogn. Res 2014, 6, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, J.K.; Gupta, S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J. Agric. Food Chem. 2007, 55, 9470–9478. [Google Scholar] [CrossRef]
- Avallone, R.; Zanoli, P.; Corsi, L.; Cannazza, G.; Baraldi, M. Benzodiazepine-like compounds and GABA in flower head of Matricaria chamomilla. Phytother. Res. 1996, 10, 177–179. [Google Scholar]
- WCSP World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens. Available online: http://wcsp.science.kew.org/ (accessed on 21 April 2021).
- Sansanelli, S.; Tassoni, A. Wild food plants traditionally consumed in the area of Bologna (Emilia Romagna region, Italy). J. Ethnobiol. Ethnomed. 2014, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, P.M.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef]
- Vitalini, S.; Tome, F.; Fico, G. Traditional uses of medicinal plants in Valvestino (Italy). J. Ethnopharmacol. 2009, 121, 106–116. [Google Scholar] [CrossRef]
- Miraj, S.; Azizi, N.; Kiani, S. A review of chemical components and pharmacological effects of Melissa officinalis L. Der Pharm. Lett. 2016, 8, 229–237. [Google Scholar]
- Abdellatif, F.; Boudjella, H.; Zitouni, A.; Hassani, A. Chemical composition and antimicrobial activity of the essential oil from leaves of Algerian Melissa officinalis L. EXCLI J. 2014, 13, 772–781. [Google Scholar]
- Hancianu, M.; Aprotosoaie, A.C.; Gille, E.; Poiata, A.; Tuchilus, C.; Spac, A.; Stanescu, U. Chemical composition and in vitro antimicrobial activity of essential oil of Melissa officinalis L. from Romania. Rev. Med. Chir. Soc. Med. Nat. Iasi 2008, 112, 843–847. [Google Scholar] [PubMed]
- Taherpour, A.A.; Maroofi, H.; Rafie, Z.; Larijani, K. Chemical composition analysis of the essential oil of Melissa officinalis L. from Kurdistan, Iran by HS/SPME method and calculation of the biophysicochemical coefficients of the components. Nat. Prod. Res. 2012, 26, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Tittel, G.; Wagner, H.; Bos, R. Chemical composition of the essential oil from melissa. Planta Med. 1982, 46, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Adinee, J.; Piri, K.; Karami, O. Essential oil component in flower of lemon balm (Melissa officinalis L.). Am. J. Biochem. Biotechnol. 2008, 4, 277–278. [Google Scholar] [CrossRef]
- Ibarra, A.; Feuillere, N.; Roller, M.; Lesburgere, E.; Beracochea, D. Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine 2010, 17, 397–403. [Google Scholar] [CrossRef]
- Al Abbasy, D.W.; Pathare, N.; Al-Sabahi, J.N.; Khan, S.A. Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum Linn.). Asian Pac. J. Trop. Dis. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Li, H.; Ge, Y.; Luo, Z.; Zhou, Y.; Zhang, X.; Zhang, J.; Fu, Q. Evaluation of the chemical composition, antioxidant and anti-inflammatory activities of distillate and residue fractions of sweet basil essential oil. J. Food Sci. Technol. 2017, 54, 1882–1890. [Google Scholar] [CrossRef]
- McCance, K.R.; Flanigan, P.M.; Quick, M.M.; Niemeyer, E.D. Influence of plant maturity on anthocyanin concentrations, phenolic composition, and antioxidant properties of 3 purple basil (Ocimum basilicum L.) cultivars. J. Food Compos. Anal. 2016, 53, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I.; et al. A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum basilicum. Food Rev. Int. 2021, 1–29. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.H.H.; Nguyen, D.C.; Nguyen, T.Q.; Tan, H.; Nhan, L.T.H.; Tran, L.D.; Do, S.T.; Nguyen, T.D. Optimization of microwave-assisted extraction of essential oil from vietnamese basil (Ocimum basilicum L.) using response surface methodology. Processes 2018, 6, 206. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, R.Y.M.; Mansour, S.M.; Elkady, W.M. Phytochemical profile and protective effect of Ocimum basilicum aqueous extract in doxorubicin/irradiation-induced testicular injury. J. Pharm. Pharmcol. 2020, 72, 101–110. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Ghorbani, A.; Rakhshandeh, H. Hypnotic Effect of Ocimum basilicum on Pentobarbital-Induced Sleep in Mice. Iran. Red Crescent Med. J. 2016, 18, e24261. [Google Scholar] [CrossRef] [Green Version]
- Venancio, A.M.; Onofre, A.S.; Lira, A.F.; Alves, P.B.; Blank, A.F.; Antoniolli, A.R.; Marchioro, M.; Estevam Cdos, S.; de Araujo, B.S. Chemical composition, acute toxicity, and antinociceptive activity of the essential oil of a plant breeding cultivar of basil (Ocimum basilicum L.). Planta Med. 2011, 77, 825–829. [Google Scholar] [CrossRef]
- Lapczynski, A.; Letizia, C.S.; Api, A.M. Addendum to Fragrance material review on linalool. Food Chem. Toxicol. 2008, 46 (Suppl. 11), S190–S192. [Google Scholar] [CrossRef]
- Pignatti, S. Flora D’italia; Edagricole: Bologna, Italy, 1982; Volume II. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea 1, 2nd ed; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Coban, I.; Toplan, G.G.; Ozbek, B.; Gurer, C.U.; Sariyar, G. Variation of alkaloid contents and antimicrobial activities of Papaver rhoeas L. growing in Turkey and northern Cyprus. Pharm. Biol. 2017, 55, 1894–1898. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.H.; Ha, I.J.; Lee, M.Y.; Kim, E.O.; Park, D.; Lee, J.H.; Lee, S.G.; Kim, D.W.; Lee, T.H.; Lee, E.J.; et al. Identification and metabolite profiling of alkaloids in aerial parts of Papaver rhoeas by liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2018, 41, 2517–2527. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Ferreira, I.C.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT-Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Dogan, G.; Bagci, E. Essential Oil Composition of Papaver rhoeas L. Corn poppy Papaveraceae from Turkey. Hacet. J. Biol. Chem. 2014, 42, 545–549. [Google Scholar]
- Velickovic, J.M.; Mitic, M.N.; Arsic, B.B.; Paunovic, D.Đ.; Stojanovic, B.T.; Veljkovic, J.N.; Dimitrijecic, D.S.; Stevanovic, S.D.; Kostic, D.A. HPLC analysis of estracts of fresh petals of Papaver rhoeas L. Studia UBB Chem. 2019, 64, 239–247. [Google Scholar] [CrossRef]
- Soulimani, R.; Younos, C.; Jarmouni-Idrissi, S.; Bousta, D.; Khallouki, F.; Laila, A. Behavioral and pharmaco-toxicological study of Papaver rhoeas L. in mice. J. Ethnopharmacol. 2001, 74, 265–274. [Google Scholar] [CrossRef]
- Ranjbaran, M.; Mirzaei, P.; Lotfi, F.; Behzadi, S.; Sahraei, H. Reduction of metabolic signs of acute stress in male mice by Papaver rhoaes hydro-alcoholic extract. Pak. J. Biol. Sci. 2013, 16, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Hamzavi, S.; Aghababa, H. The effects of alcoholic extract of red poppy (Papaver rhoeas) on anxiety induced by elevated plus maze and the plasma corticosterone levels in adult male wistar rats. J. Sabzevar Univ. Med. Sci. 2015, 22, 557–564. [Google Scholar]
- Masihuddin, M.; Jafri, M.A.; Siddiqui, A.; Chaudhary, S. Traditional uses, phytochemistry and pharmacological activities of papaver somniferum with special reference of unani medicine an updated review. J. Drug Deliv. Ther. 2018, 8, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Özcan, M.M.; Atalay, Ç. Determination of seed and oil properties of some poppy (Papaver somniferum L.) varieties. Grasas Aceites 2006, 57, 169–174. [Google Scholar]
- Heinrich, M.; Kufer, J.; Leonti, M.; Pardo-de-Santayana, M. Ethnobotany and ethnopharmacology—Interdisciplinary links with the historical sciences. J. Ethnopharmacol. 2006, 107, 157–160. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, C.; Abrams, T.R.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; Kirkwood, C.D.; Giese, N.; Hoehn, K.; Iovin, R.; et al. An evidence-based systematic review of rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2010, 7, 351–413. [Google Scholar] [CrossRef]
- Diego, M.A.; Jones, N.A.; Field, T.; Hernandez-Reif, M.; Schanberg, S.; Kuhn, C.; McAdam, V.; Galamaga, R.; Galamaga, M. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int. J. Neurosci. 1998, 96, 217–224. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, E.S. The effect of aroma inhalation method on stress responses of nursing students. J. Korean Acad. Nurs. 2004, 34, 344–351. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Katsikoudi, A.; Kontogianni, V.G.; Kellici, T.F.; Iatrou, G.; Lamari, F.N.; Tzakos, A.G.; Margarity, M. Rosemary tea consumption results to anxiolytic- and anti-depressant-like behavior of adult male mice and inhibits all cerebral area and liver cholinesterase activity; phytochemical investigation and in silico studies. Chem. Biol. Interact. 2015, 237, 47–57. [Google Scholar] [CrossRef]
- Noori Ahmad Abadi, M.; Mortazavi, M.; Kalani, N.; Marzouni, H.Z.; Kooti, W.; Ali-Akbari, S. Effect of Hydroalcoholic Extract of Rosmarinus officinalis L. Leaf on Anxiety in Mice. J. Evid. Based Complement. Altern. Med. 2016, 21, NP85–NP90. [Google Scholar] [CrossRef]
- Daher, C.F.; Kashour, B.M. Rosmarinus officinalis leaves water extract: A possible anti-inflammatory and anti-ulcerogenic remedy. Planta Med. 2008, 74, PA203. [Google Scholar] [CrossRef]
- EMA. Assessment Report on Rosmarinus officinalis L. Aetheroleum and Rosmarinus officinalis L. Folium; EMA/HMPC/13631/2009; EMA: Amsterdam, The Netherlands, 2009. [Google Scholar]
- McCaffrey, R.; Thomas, D.J.; Kinzelman, A.O. The effects of lavender and rosemary essential oils on test-taking anxiety among graduate nursing students. Holist. Nurs. Pract. 2009, 23, 88–93. [Google Scholar] [CrossRef]
- Solhi, H.; Salehi, B.; Alimoradian, A.; Pazouki, S.; Taghizadeh, M.; Saleh, A.M.; Kazemifar, A.M. Beneficial Effects of Rosmarinus Officinalis for Treatment of Opium Withdrawal Syndrome during Addiction Treatment Programs: A Clinical Trial. Addict. Health 2013, 5, 90–94. [Google Scholar]
- Nematolahi, P.; Mehrabani, M.; Karami-Mohajeri, S.; Dabaghzadeh, F. Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 30, 24–28. [Google Scholar] [CrossRef]
- Achour, M.; Ben Salem, I.; Ferdousi, F.; Nouira, M.; Ben Fredj, M.; Mtiraoui, A.; Isoda, H.; Saguem, S. Rosemary Tea Consumption Alters Peripheral Anxiety and Depression Biomarkers: A Pilot Study in Limited Healthy Volunteers. J. Am. Coll. Nutr. 2021, 1–10. [Google Scholar] [CrossRef]
- Karioti, A.; Chiarabini, L.; Alachkar, A.; Chehna, M.F.; Vincieri, F.F.; Bilia, A.R. HPLC–DAD and HPLC–ESI-MS analyses of Tiliae flos and its preparations. J. Pharm. Biomed. Anal. 2014, 100, 205–214. [Google Scholar] [CrossRef]
- Symma, N.; Sendker, J.; Petereit, F.; Hensel, A. Multistep Analysis of Diol-LC-ESI-HRMS Data Reveals Proanthocyanidin Composition of Complex Plant Extracts (PAComics). J. Agric. Food Chem. 2020, 68, 8040–8049. [Google Scholar] [CrossRef]
- Toker, G.; Baser, K.H.C.; Kürkçüoglu, M.; Özek, T. The composition of essential oils from Tilia L. species growing in Turkey. J. Essent. Oil Res. 1999, 11, 369–374. [Google Scholar] [CrossRef]
- Symma, N.; Butergerds, M.; Sendker, J.; Petereit, F.; Hake, A.; Dufer, M.; Hensel, A. Novel Piperidine and 3,4-dihydro-2H-pyrrole Alkaloids from Tilia platyphyllos and Tilia cordata Flowers. Planta Med. 2021, 87, 686–700. [Google Scholar] [CrossRef]
- EMA. Community Herbal Monograph on Tilia Cordata Miller, Tilia platyphyllos Scop. Tilia x Vulgaris Heyne or Their Mixtures, Flos; EMA/HMPC/337066/2011; EMA: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Aguirre-Hernandez, E.; Martinez, A.L.; Gonzalez-Trujano, M.E.; Moreno, J.; Vibrans, H.; Soto-Hernandez, M. Pharmacological evaluation of the anxiolytic and sedative effects of Tilia americana L. var. mexicana in mice. J. Ethnopharmacol. 2007, 109, 140–145. [Google Scholar] [CrossRef]
- Cotrim, M.D.; Figueiredo, I.V.; Cavadas, C.; Cunha, A.; Caramona, M.M.; Macedo, T.R.A. Pharmacological properties of Tilia europeæ aqueous: Screening anxiolytic/sedative activity in mice. Arq. Patol. Geral Anat. Patol. Univ. Coimbra 2005, 31, 23–25. [Google Scholar]
- Herrera-Ruiz, M.; Roman-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Jimenez-Ferrer, J.E. Flavonoids from Tilia americana with anxiolytic activity in plus-maze test. J. Ethnopharmacol. 2008, 118, 312–317. [Google Scholar] [CrossRef]
- Negri, G.; Santi, D.D.; Tabach, R. Flavonol glycosides found in hydroethanolic extracts from Tilia cordata, a species utilized as anxiolytics. Rev. Bras. Plantas Med. 2013, 15, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ortega, G.; Guevara-Fefer, P.; Chávez, M.; Herrera, J.; Martínez, A.; Martínez, A.L.; González-Trujano, M.E. Sedative and anxiolytic efficacy of Tilia americana var. mexicana inflorescences used traditionally by communities of State of Michoacan, Mexico. J. Ethnopharmacol. 2008, 116, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, R.; De Marco, E.V.; Gallo, O.; Tagarelli, G. Italian folk plant-based remedies to heal headache (XIX-XX century). J. Ethnopharmacol. 2018, 210, 417–433. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.C.; Liu, Y.W.; Fang, Y. Studies on chemical constituents of Valeriana officinalis. J. Chin. Med. Mater. 2007, 30, 1391–1393. [Google Scholar]
- Janot, M.M.; Guilhem, J.; Contz, O.; Venera, G.; Cionga, E. Contribution to the study of valerian alcaloids (Valeriana officinalis, L.): Actinidine and naphthyridylmethylketone, a new alkaloid (author’s transl). Ann. Pharm. Fr. 1979, 37, 413–420. [Google Scholar]
- Torssell, K.; Wahlberg, K. Isolation, structure and synthesis of alkaloids from Valeriana officinalis L. Acta Chem. Scand. 1967, 21, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Patočka, J.; Jakl, J. Biomedically relevant chemical constituents of Valeriana officinalis. J. Appl. Biomed. 2010, 8, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Michael, T.; Murray, N.D. Valeriana officinalis (Valerian). In Textbook of Natural Medicine, 5th ed.; ScienceDirect: Amsterdam, The Netherlands, 2020; Volume 1, pp. 902–905. [Google Scholar]
- Bos, R.; Woerdenbag, H.J.; Pras, N. Determination of valepotriates. J. Chromatogr. A 2002, 967, 131–146. [Google Scholar] [CrossRef]
- Nandhini, S.; Narayanan, K.B.; Ilango, K. Valeriana Officinalis: A review of its traditional uses, phytochemistry and pharmacology. Asian J. Pharm. Clin. Res. 2018, 11, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Trauner, G.; Khom, S.; Baburin, I.; Benedek, B.; Hering, S.; Kopp, B. Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid. Planta Med. 2008, 74, 19–24. [Google Scholar] [CrossRef]
- Murphy, K.; Kubin, Z.J.; Shepherd, J.N.; Ettinger, R.H. Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine 2010, 17, 674–678. [Google Scholar] [CrossRef]
- Bent, S.; Padula, A.; Moore, D.; Patterson, M.; Mehling, W. Valerian for sleep: A systematic review and meta-analysis. Am. J. Med. 2006, 119, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-San-Martin, M.I.; Masa-Font, R.; Palacios-Soler, L.; Sancho-Gomez, P.; Calbo-Caldentey, C.; Flores-Mateo, G. Effectiveness of Valerian on insomnia: A meta-analysis of randomized placebo-controlled trials. Sleep Med. 2010, 11, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid. Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef] [PubMed]
- Stevinson, C.; Ernst, E. Valerian for insomnia: A systematic review of randomized clinical trials. Sleep Med. 2000, 1, 91–99. [Google Scholar] [CrossRef]
- Taibi, D.M.; Landis, C.A.; Petry, H.; Vitiello, M.V. A systematic review of valerian as a sleep aid: Safe but not effective. Sleep Med. Rev. 2007, 11, 209–230. [Google Scholar] [CrossRef]


| Botanical Name | Family | Parts Used | Preparation | References |
|---|---|---|---|---|
| Achillea moschata L. | Asteraceae | Aerial parts | Infusion | [42,43] |
| Adiantum capillus-veneris L. | Adiantaceae | Aerial parts | Decoction | [44,45,46] |
| Agrimonia eupatoria L. | Rosaceae | Aerial parts | Infusion | [43] |
| Alchemilla group alpina L. | Asteraceae | Leaves | Infusion | [33] |
| Alchemilla group vulgaris L. | Asteraceae | Leaves | Infusion | [33] |
| Aloysia citrodora Paláu (=Lippia triphylla (L’Hér.) Kuntze) | Verbenaceae | Leaves | Infusion | [31,45] |
| Angelica archangelica L. | Apiaceae | Roots | Extract | [47] |
| Angelica sylvestris L. | Apiaceae | Leaves | Infusion | [43] |
| Anthemis arvensis L. | Asteraceae | Flowers | Infusion | [44,45,46,48] |
| Artemisia absinthium L. | Asteraceae | Leaves | Infusion | [49] |
| Artemisia arborescens L. | Asteraceae | Flowers | Infusion | [50] |
| Artemisia vulgaris L. | Asteraceae | Leaves, roots | [47,51,52] | |
| Arum italicum Miller | Araceae | Leaves | Infusion | [53] |
| Arum pictum L. f. | Araceae | Leaves | Infusion | [53] |
| Avena fatua L. | Poaceae | Fruits | Decoction | [54] |
| Avena sativa L. | Poaceae | Fruits | Decoction | [54] |
| Ballota nigra L. | Solanaceae | Whole plant | [47] | |
| Borago officinalis L. | Boraginaceae | Leaves | Infusion | [43] |
| Calendula officinalis L. | Asteraceae | Whole plant | [47] | |
| Centranthus ruber (L.) DC. | Valerianaceae | Whole plant, rhizome | Infusion | [31,55] |
| Chamaemelum nobilis All. | Asteraceae | Aerial parts | Infusion | [56] |
| Citrus aurantium L. | Rutaceae | Leaves | Infusion | [55] |
| Citrus limon L. | Rutaceae | Fruits | Juice | [57,58] |
| Citrus x sinensis (L.) Osbeck | Rutaceae | Flowers | Infusion | [59] |
| Clinopodium nepeta (L.) Kuntze (=Calamintha nepeta (L.) Savi) | Lamiaceae | Aerial parts | Decoction | [47,49,54,59,60,61] |
| Conium maculatum L. | Apiaceae | Leaves | Infusion | [62] |
| Corydalis cava (L.) Schweigg. and Körte | Papaveraceae | Tubers | Infusion | [47] |
| Crataegus laevigata (Poir.) DC. | Rosaceae | Flowers | Infusion | [43,63,64] |
| Crataegus monogyna Jacq. | Rosaceae | Flowers, leaves | Decoction | [31,43,44,45,49,54,65,66,67,68] |
| Crocus sativus L. | Iridaceae | Pistil | Infusion | [51] |
| Cydonia oblonga Mill. | Rosaceae | Fruits | Decoction | [44] |
| Cynodon dactylon (L.) Pers | Poaceae | Roots | Decoction | [60] |
| Dianthus seguieri Vill. | Caryophyllaceae | [69] | ||
| Ecballium elaterium (L.) A. Rich | Cucurbitaceae | Roots, fruits | Decoction | [54] |
| Foeniculum vulgare Miller | Apiaceae | Fruits | Decoction | [32,60] |
| Humulus lupulus L. | Cannabaceae | Flowers | Infusion | [32,43,53,55,60,70,71] |
| Hyoscyamus niger L. | Solanaceae | Seeds | [47] | |
| Hypericum perforatum L. | Flowers | Infusion | [65] | |
| Ilex aquifolium L. | Aquifoliaceae | Leaves | Infusion | [53,62] |
| Jacobaea delphiniifolia (Vahl) Pelser and Veldkamp | Asteraceae | Flowers | Infusion | [62] |
| Lactuca sativa L. | Asteraceae | Leaves | Decoction | [32,43,55] |
| Lactuca virosa L. | Asteraceae | Latex | Raw | [47] |
| Laurus nobilis L. | Lauraceae | Leaves | Infusion | [52,55,57,61,72] |
| Lavandula angustifolia Miller | Lamiaceae | Flowers | Infusion | [31,62,65,73] |
| Lavandula officinalis Chaix | Lamiaceae | Flowers | Infusion | [65] |
| Lavandula stoechas L. | Lamiaceae | Flowers | Infusion | [57,72] |
| Leucanthemum alpinum Lam. | Asteraceae | Leaves | Infusion | [43] |
| Lolium multiflorum Lam. | Poaceae | Fruits | Decoction | [51] |
| Lolium perenne L. | Poaceae | Fruits | Decoction | [51,74] |
| Lotus corniculatus L. | Fabaceae | Flowers | Infusion | [47,75,76] |
| Malva cretica Cav. | Malvaceae | Aerial parts | Decoction | [59] |
| Malva neglecta Wallr. | Malvaceae | Aerial parts | Decoction | [59] |
| Malva sylvestris L. | Malvaceae | Aerial parts | Decoction | [58,59,60,77] |
| Matricaria camomilla L. (=Chamomilla recutita (L.) Rauschert) | Asteraceae | Flowers | Infusion | [31,33,42,43,45,47,49,50,51,53,55,56,57,58,59,60,63,64,65,68,72,73,74,78,79,80,81,82,83,84,85,86] |
| Matricaria discoidea DC. | Asteraceae | Flowers | Infusion | [33] |
| Melilotus officinalis Pall. | Fabaceae | Flowers | Infusion | [43] |
| Melissa officinalis L. | Lamiaceae | Whole plant | Infusion | [33,43,45,49,57,59,61,63,73,87,88] |
| Melittis melissophyllum L. | Lamiaceae | Flowers | Infusion | [77] |
| Mentha aquatica L. | Lamiaceae | Aerial parts | Infusion | [59,62] |
| Mentha piperita L. | Lamiaceae | Whole plant | Infusion | [59,61] |
| Mentha spicata L. (=M. spicata L. subsp. glabrata (Lej. and Courtois) Lebeau) | Lamiaceae | Aerial parts | Infusion | [59,62] |
| Mentha suaveolens Ehrh. subsp. suaveolens | Lamiaceae | Aerial parts | Infusion | [62] |
| Mentha viridis L. | Lamiaceae | Whole plant | Infusion | [57] |
| Myrtus communis L. | Myrtaceae | Leaves | Infusion | [44] |
| Nepeta cataria L. | Lamiaceae | Aerial parts | [43,73,89] | |
| Ocimum basilicum L. | Lamiaceae | Leaves, flowers | Infusion | [43,49,54,63,72,82,90] |
| Olea europaea L. | Oleaceae | Leaves | Infusion | [90] |
| Opuntia ficus-indica (L.) Mill. | Cactaceae | Flowers | Infusion | [59] |
| Origanum majorana L. | Lamiaceae | Leaves | Infusion | [44] |
| Origanum vulgare L. subsp. viridulum (Martrin-Donos) Nyman | Lamiaceae | Leaves | Infusion | [43,86] |
| Paeonia mascula (L.) Mill. | Paeoniaceae | [91] | ||
| Papaver rhoeas L. | Papaveraceae | Petals, fruits | Infusion | [31,32,43,44,45,46,47,48,50,53,59,60,61,62,63,64,66,67,72,73,77,79,82,85,86,88,92] |
| Papaver setigerum DC. | Papaveraceae | Fruits | Decoction | [45,46] |
| Papaver somniferum L. | Papaveraceae | Fruits | Infusion | [46,47,48,53,68] |
| Passiflora caerulea L. | Passifloraceae | Fruits, seeds | Decoction | [32,45,46] |
| Pimpinella anisum L. | Apiaceae | Fruits | Infusion | [43,57] |
| Polypodium vulgare L. | Polypodiaceae | Leaves | Infusion | [43] |
| Primula veris L. | Primulaceae | Flowers, leaves | Infusion | [43,65] |
| Primula vulgaris Huds. | Primulaceae | Flowers | Decoction | [45,71] |
| Prunus persica (L.) Batsch | Rosaceae | Flowers | Decoction | [55] |
| Robinia pseudacacia L. | Fabaceae | Flowers | Decoction | [63] |
| Rosmarinus officinalis L. | Lamiaceae | Aerial parts | Infusion | [44,45,60,61,77] |
| Ruta chalepensis L. | Rutaceae | Leaves | Infusion | [82] |
| Salix alba L. | Salicaceae | [76] | ||
| Salvia officinalis L. | Lamiaceae | Leaves | Infusion | [49,54] |
| Santolina insularis (Gennari ex Fiori) Arrigoni | Asteraceae | Leaves | Infusion | [44] |
| Senecio delphinifolius Vahl. | Asteraceae | Aerial parts | Decoction | [68] |
| Solanum nigrum L. | Solanaceae | [91,93] | ||
| Sonchus oleraceus L. | Asteraceae | Leaves | Infusion | [94] |
| Stachys recta L. | Lamiaceae | Aerial parts | Infusion | [45,95] |
| Tanacetum balsamita L. (=Balsamita major Desf.) | Asteraceae | Leaves | Infusion | [43,47,76,96,97] |
| Tanacetum parthenium (L.) Sch.-Bip. | Asteraceae | Flowers | Infusion | [45,46] |
| Thalictrum aquilegiifolium L. | Ranunculaceae | Leaves | [47] | |
| Thymbra capitata (L.) Cav. | Lamiaceae | Aerial parts | [89] | |
| Thymus serpyllum L. | Lamiaceae | Aerial parts | Infusion | [65] |
| Tilia cordata Mill. | Malvaceae | Leaves | Infusion | [45,81,88] |
| Tilia platyphyllus Scop. | Malvaceae | Flowers | Infusion | [31,43,59,60,84] |
| Tilia sp. | Malvaceae | Flowers | Infusion | [42,66,82] |
| Tussilago farfara L. | Asteraceae | Flowers | Infusion | [98] |
| Urtica dioica L. | Urticaceae | Leaves | Infusion | [49] |
| Valeriana montana L. | Valerianaceae | Rhizome | Decoction | [51] |
| Valeriana officinalis L. | Valerianaceae | Rhizome, fruits | Decoction | [43,45,47,51,60,63,64,68,74] |
| Valeriana tripteris L. | Valerianaceae | [99] | ||
| Verbena officinalis L. | Verbenaceae | Flowers, leaves | Infusion | [44,45,46,100,101] |
| Zea mays L. | Poaceae | Stigmas and styles | Infusion | [53] |
| Ziziphus jujuba Mill. (=Ziziphus sativa Gaertn.) | Rhamnaceae | Fruits | Decoction | [44] |
| Plants | Major Bioactive Compounds | Mechanism of Action | Actions | Clinical Evidence | References |
| Anthemis arvensis L. | n. a. | n. a. | S | N | [48] |
| Clinopodium nepeta L. | Monoterpenoids: pulegone, menthone, 1,8-cineole and carvone | n. a. | S | N | [104,105,106] |
| Crataegus monogyna Jacq. | Polyphenol: epicatechin Flavonol: hyperoside | n. a. | A, S | Y | [107,108,109] |
| Humulus lupulus L. | Terpenes bitter acids: humulone and lupulone Chalcone: Xanthohumol Alcohol: 2-methyl-3-buten-2-ol |
| S | Only in combination with other herbs | [110,111,112,113,114,115] |
| Laurus nobilis L. | Phenylpropanoids: eugenol and methyl eugenol Monoterpenoid: 1,8-cineole | n. a. | S | N | [116,117] |
| Lavandula angustifolia Mill. | Monoterpenoids: linalool and linalyl acetate |
| A, S | Y | [118,119,120,121,122,123,124,125,126] |
| Malva sylvestris L. | Flavonoids: malvin, malonylmalvin, alvidin, apigenin Monoterpenoid: linalool | n. a. | A, S | N | [127,128] |
| Matricaria chamomilla L. | Flavonoid: apigenin |
| A, S | Y | [129,130,131,132,133,134,135] |
| Melissa officinalis L. | Phenols: rosmarinic acid, oleanolic acid, and ursolic acid |
| A | Y | [135,136,137,138] |
| Ocimum basilicum L. | Monoterpenoid: linalool Phenylpropanoid: eugenol Phenylpropene: methyl chavicol | n. a. | A, S | N | [139,140,141] |
| Papaver rhoeas L. | Flavonol: hyperoside | n. a. | A, S | N | [142,143] |
| Papaver somniferum L. | Alkaloids: morphine, codeine, noscapine, and tubocurarine |
| S | N | [144,145] |
| Rosmarisus officinalis L. | Phenols: rosmarinic acid, caffeic acid Flavones: cirsimaritin, salvigenin Monoterpenoid: 1,8 cineole Diterpenoids: carnosic acid, carnosol, and rosmanol, Triterpenoid: ursolic acid |
| A, S | Y | [146,147] |
| Tilia platyphyllus Scop. | Flavonoids: quercetin, isoquercitrin, and rutin |
| A, S | N | [148,149,150] |
| Valeriana officinalis L. | Sesquiterpenes: valerenic acid and valerenol Valepotriates |
| A, S | Y | [135,151,152,153,154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motti, R.; de Falco, B. Traditional Herbal Remedies Used for Managing Anxiety and Insomnia in Italy: An Ethnopharmacological Overview. Horticulturae 2021, 7, 523. https://doi.org/10.3390/horticulturae7120523
Motti R, de Falco B. Traditional Herbal Remedies Used for Managing Anxiety and Insomnia in Italy: An Ethnopharmacological Overview. Horticulturae. 2021; 7(12):523. https://doi.org/10.3390/horticulturae7120523
Chicago/Turabian StyleMotti, Riccardo, and Bruna de Falco. 2021. "Traditional Herbal Remedies Used for Managing Anxiety and Insomnia in Italy: An Ethnopharmacological Overview" Horticulturae 7, no. 12: 523. https://doi.org/10.3390/horticulturae7120523
APA StyleMotti, R., & de Falco, B. (2021). Traditional Herbal Remedies Used for Managing Anxiety and Insomnia in Italy: An Ethnopharmacological Overview. Horticulturae, 7(12), 523. https://doi.org/10.3390/horticulturae7120523







