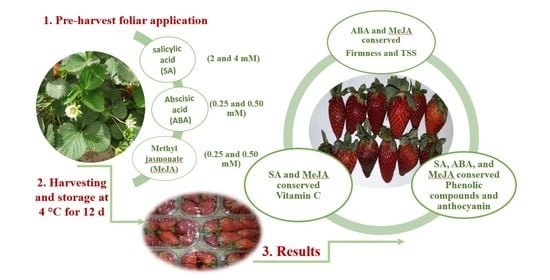
Pre-Harvest Application of Salicylic Acid, Abscisic Acid, and Methyl Jasmonate Conserve Bioactive Compounds of Strawberry Fruits during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Treatments
2.2. Storage Experiment Design
2.3. Chemicals
2.4. Physico-Chemical Quality
2.5. Phytochemicals Profile
2.6. Statistical Analyses
3. Results
3.1. Influence of Treatments on Weight Loss, Firmness, and Color
3.2. Influence of Treatments on TSS, pH, and TA
3.3. Influence of Treatments on Ascorbic Acid, Total Phenolic, and Anthocyanin Content
3.4. Correlation Study among Weight Loss (WL), Firmness, TSS, pH, Titratable Acidity (TA), Ascorbic Acid Content (ASA), Phenolic Compounds, and Anthocyanin Content
4. Discussion
4.1. Influence of Treatments on Weight Loss, Firmness, and Color
4.2. Influence of Treatments on TSS, pH and TA
4.3. Influence of Treatments on Ascorbic Acid, Total Phenolic, and Anthocyanin Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Mogy, M.M.; Ludlow, R.A.; Roberts, C.; Müller, C.T.; Rogers, H.J. Postharvest exogenous melatonin treatment of strawberry reduces postharvest spoilage but affects components of the aroma profile. J. Berry Res. 2019, 9, 297–307. [Google Scholar] [CrossRef]
- Hadi, A.; Askarpour, M.; Miraghajani, M.; Symonds, M.E.; Sheikhi, A.; Ghaedi, E. Effects of strawberry supplementation on cardiovascular risk factors: A comprehensive systematic review and meta-analysis of randomized controlled trials. Food Funct. 2019, 10, 6987–6998. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Alsanius, B.W. Cassia oil for controlling plant and human pathogens on fresh strawberries. Food Control 2012, 28, 157–162. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The combined effects of tea tree oil and hot air treatment on the quality and sensory characteristics and decay of strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowicz, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the ethylene-action inhibitor 1-methylcyclopropene on postharvest quality of non-climacteric fruit crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyen, H.V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria × ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. UV-C treatment maintains quality and enhances antioxidant capacity of fresh-cut strawberries. Postharvest Biol. Technol. 2019, 156, 110945. [Google Scholar] [CrossRef]
- Shehata, S.A.; El-Mogy, M.M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Mahmoud, A.W.M.; El-Sawy, M.B.I.; Parmar, A. Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli. Agronomy 2019, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- Shehata, S.A.; Abdelgawad, K.F.; El-Mogy, M.M. Quality and Shelf-Life of Onion Bulbs Influenced by Biostimulants. Int. J. Veg. Sci. 2017, 23, 362–371. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; García-Pastor, M.E.; Zapata, P.J.; Guillén, F.; Serrano, M.; Valero, D.; Gironés-Vilaplana, A. Preharvest application of oxalic acid improves quality and phytochemical content of artichoke (Cynara scolymus L.) at harvest and during storage. Food Chem. 2017, 230, 343–349. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-Harvest Treatment of Chitosan Oligosaccharides Improved Strawberry Fruit Quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Bi, Y. The role of signal production and transduction in induced resistance of harvested fruits and vegetables. Food Qual. Saf. 2021, 5, fyab011. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M. Exogenous applications of salicylic acid affect quality and yield of strawberry grown under antifrost heated greenhouse conditions. J. Plant Nutr. Soil Sci. 2009, 172, 270–276. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Blanch, G.P.; del Castillo, M.L.R. Changes in strawberry volatile constituents after pre-harvest treatment with natural hormonal compounds. Flavour Fragr. J. 2012, 27, 180–187. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef]
- Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef]
- Ali, M.R.; Mohamed, R.M.; Abedelmaksoud, T.G. Functional strawberry and red beetroot jelly candies rich in fibers and phenolic compounds. Food Syst. 2021, 4, 12–18. [Google Scholar] [CrossRef]
- Abdelgawad, K.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, I.S.; Abdelgawad, K.F.; El-Mogy, M.M.; El-Sawy, M.B.I.; Mahmoud, H.A.; Fahmy, M.A.M. Weed Control, Growth, Nodulation, Quality and Storability of Peas as Affected by Pre- and Postemergence Herbicides. Horticulturae 2021, 7, 307. [Google Scholar] [CrossRef]
- Tonu, T.; Ulvi, M.; Lech, S. Strawberry anthocyanin determination by pH differential spectroscopic method- how to get true results? Acta Sci. Pol. Hortorum Cultus 2014, 13, 35–47. Available online: https://czasopisma.up.lublin.pl/index.php/asphc/article/view/2729 (accessed on 1 December 2021).
- Baninaiem, E.; Mirzaaliandastjerdi, A.M.; Rastegar, S.; Kh, A. Effect of pre- and postharvest salicylic acid treatment on quality characteristics of tomato during cold storage. Adv. Hortic. Sci. 2017, 30, 183–192. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Lobos, T.; Cardemil, L.; Nunes-Nesi, A.; Retamales, J.; Jaakola, L.; Alberdi, M.; Ribera-Fonseca, A. Methyl Jasmonate: An Alternative for Improving the Quality and Health Properties of Fresh Fruits. Molecules 2016, 21, 567. [Google Scholar] [CrossRef] [Green Version]
- Zuñiga, P.E.; Castañeda, Y.; Arrey-Salas, O.; Fuentes, L.; Aburto, F.; Figueroa, C.R. Methyl Jasmonate Applications From Flowering to Ripe Fruit Stages of Strawberry (Fragaria × ananassa ‘Camarosa’) Reinforce the Fruit Antioxidant Response at Post-harvest. Front. Plant Sci. 2020, 11, 538. [Google Scholar] [CrossRef]
- El Kayal, W.; El-Sharkawy, I.; Dowling, C.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Effect of preharvest application of hexanal and growth regulators in enhancing shelf life and regulation of membrane-associated genes in strawberry. Can. J. Plant Sci. 2017, 97, 1109–1120. [Google Scholar] [CrossRef]
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef] [Green Version]
- Teribia, N.; Tijero, V.; Munné-Bosch, S. Linking hormonal profiles with variations in sugar and anthocyanin contents during the natural development and ripening of sweet cherries. N. Biotechnol. 2016, 33, 824–833. [Google Scholar] [CrossRef]
- Li, Y.; Lua, Y.; Lia, L.; Chu, Z.; Zhang, H.; Li, H. Impairment of hormone pathways results in a general disturbance of fruit primary metabolism in tomato. Food Chem. 2019, 274, 170–179. [Google Scholar] [CrossRef]
- Leng, P.; Yuan, B.; Guo, Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lara, R.; Gordillo, B.; Rodríguez-Pulido, F.J.; Lourdes González-Miret, M.; del Villar-Martínez, A.A.; Dávila-Ortiz, G.; Heredia, F.J. Assessment of the differences in the phenolic composition and color characteristics of new strawberry (Fragaria × ananassa Duch.) cultivars by HPLC–MS and Imaging Tristimulus Colorimetry. Food Res. Int. 2015, 76, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of humic acid and salicylic acid on yield, fruit quality, and leaf mineral elements of strawberry (Fragaria × Ananassa duch.) cv. Camarosa. J. Plant Nutr. 2016, 39, 1821–1829. [Google Scholar] [CrossRef]
- Ayub, R.A.; Bosetto, L.; Galvão, C.W.; Etto, R.M.; Inaba, J.; Lopes, P.Z. Abscisic acid involvement on expression of related gene and phytochemicals during ripening in strawberry fruit Fragaria × ananassa cv. Camino Real. Sci. Hortic. 2016, 203, 178–184. [Google Scholar] [CrossRef]
- Ren, J.; Sun, L.; Wang, C.; Zhao, S.; Leng, P. Expression analysis of the cDNA for magnesium chelatase H subunit (CHLH) during sweet cherry fruit ripening and under stress conditions. Plant Growth Regul. 2011, 63, 301–307. [Google Scholar] [CrossRef]
- Lopes, P.Z.; Fornazzari, I.M.; Almeida, A.T.; Galvão, C.W.; Etto, R.M.; Inaba, J.; Ayub, R.A. Effect of ethylene treatment on phytochemical and ethylene-related gene expression during ripening in strawberry fruit Fragaria × ananassa cv. Camino Real. Genet. Mol. Res. 2015, 14, 16113–16125. [Google Scholar] [CrossRef]
- Fan, M.; Cong, G.; Yanling, L.; Hao-Ru, T.; Qing, C.; Bo, S.; Yong, Z.; Ya, L. Abscisic Acid Affects Strawberry Fruit Quality. In Proceedings of the 2018 International Conference on Management, Economics, Education, Arts and Humanities (MEEAH 2018), Shenzhen, China, 14–15 July 2018; December 2018; pp. 24–29. [Google Scholar]
- Lolaei, A.; Zamani, S.; Ahmadian, E.; Mobasheri, S. Effect of methyl jasmonate on the composition of yield and growth of strawberry (Selva and Queen Elisa). Int. J. Agric. Crop. Sci. 2013, 5, 200–206. [Google Scholar]
- Lolaei, A.; Kaviani, B.; Rezaei, M.A.; Raad, M.K.; Mohammadipour, R. Effect of pre- and postharvest treatment of salicylic acid on ripening of fruit and overall quality of strawberry (Fragaria ananasa Duch cv.Camarosa) fruit. Ann. Biol. Res. 2012, 3, 4680–4684. [Google Scholar]
- Lu, X.; Sun, D.; Li, Y.; Shi, W.; Sun, G. Pre- and post-harvest salicylic acid treatments alleviate internal browning and maintain quality of winter pineapple fruit. Sci. Hortic. 2011, 130, 97–101. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Müller, M.; Munné-Bosch, S. Biosynthesis, Metabolism and Function of Auxin, Salicylic Acid and Melatonin in Climacteric and Non-climacteric Fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2017, 98, 2742–2750. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Kong, W.; Li, S.; Archbold, D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Hadian-Deljou, M.; Esna-Ashari, M.; Sarikhani, H. Effect of pre- and post-harvest salicylic acid treatments on quality and antioxidant properties of ‘Red Delicious’ apples during cold storage. Adv. Hortic. Sci. 2017, 31, 31–38. [Google Scholar]
- Geransayeh, M.; Sepahvand, S.; Abdossi, V. Extending Postharvest Longevity and Improving Quality of Strawberry (Fragaria Ananasa Duch Cv. ‘Gaviota’) Fruit by Postharvest Salicylic Acid Treatment. J. Agric. Stud. 2015, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Loyola-Vargas, V.M. Effect of acetylsalicylic acid on secondary metabolism ofCatharanthus roseus tumor suspension cultures. Plant Cell Rep. 1997, 16, 287–290. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Delgado, L.D.; Zúñiga, P.E.; Figueroa, N.E.; Pastene, E.; Escobar-Sepúlveda, H.F.; Figueroa, P.M.; Garrido-Bigotes, A.; Figueroa, C.R. Application of a JA-Ile Biosynthesis Inhibitor to Methyl Jasmonate-Treated Strawberry Fruit Induces Upregulation of Specific MBW Complex-Related Genes and Accumulation of Proanthocyanidins. Molecules 2018, 23, 1433. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Bigotes, A.; Figueroa, P.M.; Figueroa, C.R. Jasmonate Metabolism and Its Relationship with Abscisic Acid During Strawberry Fruit Development and Ripening. J. Plant Growth Regul. 2018, 37, 101–113. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Kumar, S.P.; Maurer, D.; Feygenberg, O.; Love, C.; Alkan, N. Improving the Red Color and Fruit Quality of ‘Kent’ Mango Fruit by Pruning and Preharvest Spraying of Prohydrojasmon or Abscisic Acid. Agronomy 2020, 10, 944. [Google Scholar] [CrossRef]






| Time | Treatments | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|---|
| 0 days | 2 SA | 37.65 ± 1.90 a | 34.06 ± 1.04 a | 24.09 ± 3.36 a | 41.88 ± 2.41 a | 34.95 ± 3.43 a |
| 4 SA | 35.52 ± 1.14 a | 32.21 ± 1.64 a | 22.98 ± 2.43 a | 39.58 ± 2.72 a | 35.26 ± 1.58 a | |
| 0.25 ABA | 32.59 ± 1.85 a | 28.98 ± 2.69 a | 16.36 ± 3.16 a | 33.57 ± 3.75 a | 28.68 ± 2.45 a | |
| 0.50 ABA | 30.38 ± 1.94 a | 23.72 ± 4.11 a | 13.13 ± 3.40 a | 27.16 ± 5.20 a | 27.89 ± 2.86 a | |
| 0.25 MeJA | 36.89 ± 2.42 a | 30.34 ± 1.49 a | 22.4 ± 4.67 a | 37.94 ± 3.52 a | 35.77 ± 4.81 a | |
| 0.50 MeJA | 30.79 ± 1.12 a | 24.49 ± 0.92 a | 13.44 ± 1.55 a | 27.98 ± 1.39 a | 28.47 ± 2.18 a | |
| Control | 31.94 ± 0.24 a | 31.57 ± 1.15 a | 19.39 ± 1.67 a | 37.15 ± 1.89 a | 31.62 ± 1.43 a | |
| 4 days | 2 SA | 30.03 ± 0.32 a | 27.13 ± 0.72 a | 15.51 ± 0.70 ab | 31.07 ± 0.40 a | 29.80 ± 1.77 ab |
| 4 SA | 31.14 ± 0.19 a | 27.54 ± 1.98 a | 18.29 ± 0.79 ab | 33.07 ± 2.08 a | 33.74 ± 0.78 a | |
| 0.25 ABA | 29.84 ± 0.65 a | 26.00 ± 0.05 a | 13.43 ± 0.16 b | 30.15 ± 0.12 a | 26.44 ± 0.23 b | |
| 0.50 ABA | 30.44 ± 1.33 a | 26.11 ± 1.86 a | 18.65 ± 2.32 a | 32.12 ± 2.86 a | 35.15 ± 1.47 a | |
| 0.25 MeJA | 31.19 ± 0.04 a | 27.92 ± 0.54 a | 16.98 ± 0.80 ab | 32.68 ± 0.88 a | 31.24 ± 0.71 ab | |
| 0.50 MeJA | 30.23 ± 0.53 a | 26.77 ± 0.93 a | 15.75 ± 0.21 ab | 31.08 ± 0.70 a | 30.56 ± 1.20 ab | |
| Control | 29.16 ± 0.31 a | 26.97 ± 0.27 a | 15.78 ± 0.57 ab | 31.27 ± 0.05 a | 30.33 ± 1.16 ab | |
| 8 days | 2 SA | 32.79 ± 2.37 a | 28.81 ± 2.37 a | 18.20 ± 3.58 a | 34.18 ± 3.89 a | 31.6 ± 3.05 a |
| 4 SA | 34.80 ± 0.47 a | 31.38 ± 0.41 a | 24.00 ± 0.51 a | 39.52 ± 0.52 a | 37.44 ± 0.53 a | |
| 0.25 ABA | 31.42 ± 1.44 a | 28.51 ± 1.10 a | 18.71 ± 2.12 a | 34.28 ± 1.26 a | 32.99 ± 2.83 a | |
| 0.50 ABA | 39.27 ± 0.47 a | 34.62 ± 0.51 a | 29.74 ± 2.12 a | 45.68 ± 1.70 a | 40.61 ± 1.58 a | |
| 0.25 MeJA | 36.33 ± 3.64 a | 31.22 ± 2.41 a | 27.07 ± 5.50 a | 41.59 ± 5.02 a | 39.88 ± 4.92 a | |
| 0.50 MeJA | 33.30 ± 0.77 a | 32.64 ± 0.97 a | 21.05 ± 3.32 a | 39.30 ± 2.28 a | 33.34 ± 2.87 a | |
| Control | 37.87 ± 2.06 a | 32.59 ± 1.72 a | 27.73 ± 2.64 a | 42.90 ± 2.23 a | 40.28 ± 3.02 a | |
| 12 days | 2 SA | 30.75 ± 0.02 ab | 30.45 ± 1.40 ab | 17.96 ± 1.40 ab | 35.42 ± 1.96 a | 30.71 ± 0.99 ab |
| 4 SA | 27.39 ± 0.79 b | 28.24 ± 0.94 ab | 13.61 ± 1.43 b | 31.38 ± 1.46 a | 25.50 ± 1.62 b | |
| 0.25 ABA | 29.37 ± 0.24 b | 27.50 ± 0.76 b | 17.15 ± 0.22 ab | 32.43 ± 0.53 a | 32.0 ± 1.04 ab | |
| 0.50 ABA | 34.54 ± 2.07 a | 31.85 ± 1.32 ab | 24.38 ± 4.57 a | 40.41 ± 3.80 a | 36.26 ± 4.13 a | |
| 0.25 MeJA | 30.14 ± 1.06 ab | 29.81 ± 0.46 ab | 19.13 ± 2.62 ab | 35.67 ± 1.84 a | 32.11 ± 3.13 ab | |
| 0.50 MeJA | 33.34 ± 0.32 ab | 32.57 ± 0.76 ab | 23.51 ± 0.42 ab | 40.17 ± 0.86 a | 35.83 ± 0.15 ab | |
| Control | 33.29 ± 1.05 ab | 31.35 ± 0.67 ab | 24.21 ± 1.52 ab | 39.38 ± 1.31 a | 37.13 ± 0.91 a |
| WL | Firmness | TSS | pH | TA | ASA | TPC | |
|---|---|---|---|---|---|---|---|
| Firmness | −0.889 * | ||||||
| TSS | 0.11 | −0.13 | |||||
| pH | 0.305 * | −0.383 * | −0.007 | ||||
| TA | −0.706 * | 0.766 * | −0.173 | −0.712 * | |||
| ASA | −0.544 * | 0.571 * | 0.051 | 0.042 | 0.308 * | ||
| TPC | 0.871 * | −0.813 * | 0.213 | 0.327 * | −0.657 * | −0.413 * | |
| Anthocyanin | 0.722 * | −0.663 * | −0.08 | 0.322 * | −0.559 * | −0.375 * | 0.643 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, O.S.; Ali, M.R.; Khojah, E.; Samra, B.N.; Ramadan, K.M.A.; El-Mogy, M.M. Pre-Harvest Application of Salicylic Acid, Abscisic Acid, and Methyl Jasmonate Conserve Bioactive Compounds of Strawberry Fruits during Refrigerated Storage. Horticulturae 2021, 7, 568. https://doi.org/10.3390/horticulturae7120568
Darwish OS, Ali MR, Khojah E, Samra BN, Ramadan KMA, El-Mogy MM. Pre-Harvest Application of Salicylic Acid, Abscisic Acid, and Methyl Jasmonate Conserve Bioactive Compounds of Strawberry Fruits during Refrigerated Storage. Horticulturae. 2021; 7(12):568. https://doi.org/10.3390/horticulturae7120568
Chicago/Turabian StyleDarwish, Omaima S., Marwa R. Ali, Ebtihal Khojah, Bassem N. Samra, Khaled M. A. Ramadan, and Mohamed M. El-Mogy. 2021. "Pre-Harvest Application of Salicylic Acid, Abscisic Acid, and Methyl Jasmonate Conserve Bioactive Compounds of Strawberry Fruits during Refrigerated Storage" Horticulturae 7, no. 12: 568. https://doi.org/10.3390/horticulturae7120568
APA StyleDarwish, O. S., Ali, M. R., Khojah, E., Samra, B. N., Ramadan, K. M. A., & El-Mogy, M. M. (2021). Pre-Harvest Application of Salicylic Acid, Abscisic Acid, and Methyl Jasmonate Conserve Bioactive Compounds of Strawberry Fruits during Refrigerated Storage. Horticulturae, 7(12), 568. https://doi.org/10.3390/horticulturae7120568