Response Mechanism of Plants to Drought Stress
Abstract
:1. Introduction
2. Effects of Drought Stress on Plant Morphological Characteristics
2.1. Drought Stress and the External form of Plants
2.2. Drought Stress, the Internal Structure, and Physical Property of Plants
3. Effects of Drought Stress on Plant Physiological and Biochemical Characteristics
3.1. Photosynthetic Capacity
3.2. Osmotic Regulation Metabolism
3.3. Drought-Induced Proteins
3.3.1. Late Embryogenesis Abundant Protein
3.3.2. Dehydrin
3.3.3. Aquaporin
3.4. Reactive Oxygen Metabolism
3.4.1. Production and Basic Function of Reactive Oxygen Species
3.4.2. Reactive Oxygen Scavenging System
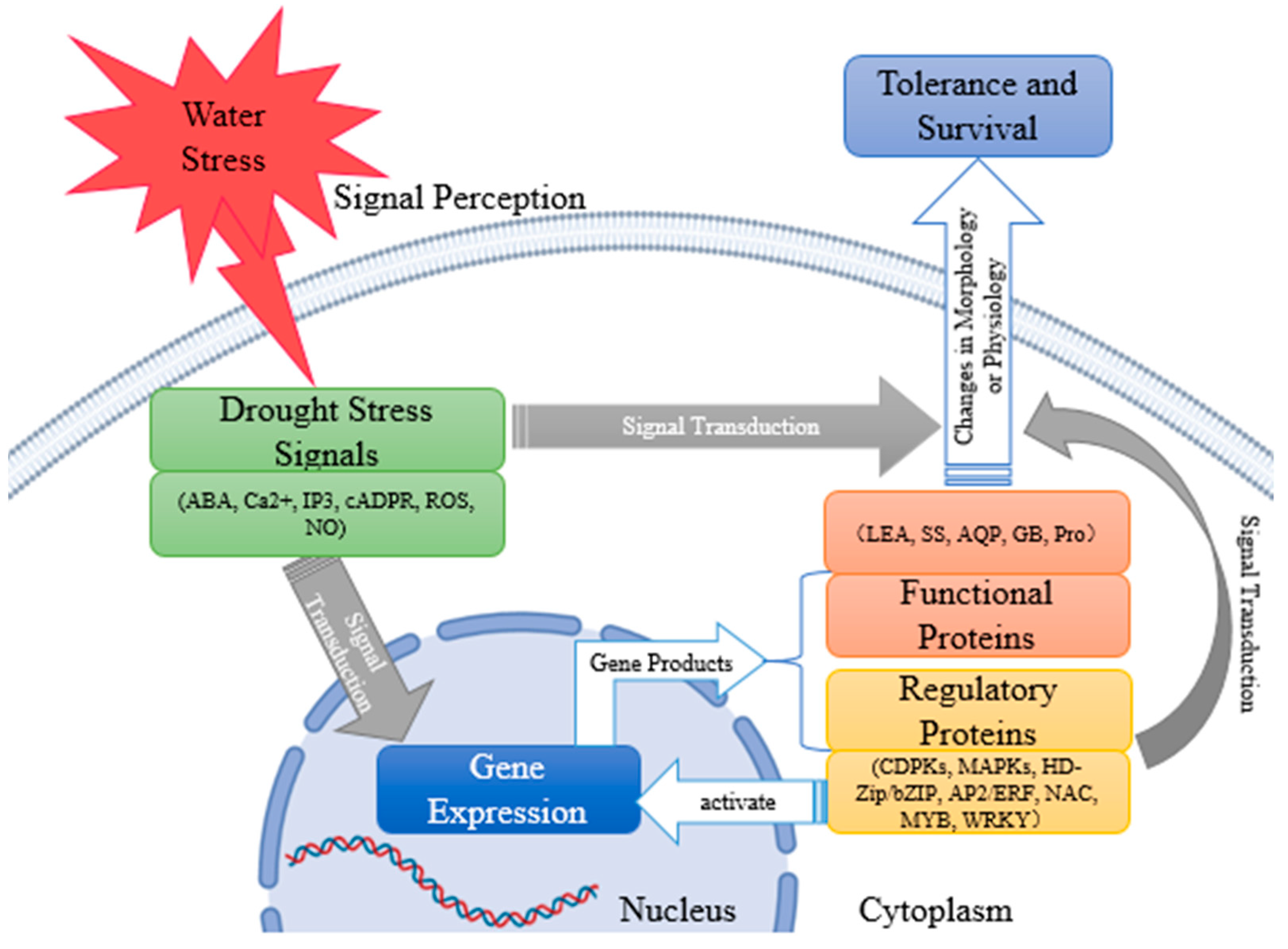
4. Drought Stress Signal Transduction in Plants
4.1. Plant Drought Stress Signal
4.2. Intracellular Transduction Pathways and Regulation Mechanisms of Plant Drought Stress Signals
5. Drought Stress Signal Transduction in Plants
5.1. Functional Genes
5.1.1. Osmotic Adjustment Related Genes
5.1.2. Drought-Induced Protein Genes
5.2. Regulatory Genes
5.2.1. Signal Transduction Related Genes
5.2.2. Transcription Factor Genes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khan, M.A.; Iqbal, M.; Akram, M.; Ahmad, M.; Hassan, M.W.; Jamil, M. Recent advances in molecular tool development for drought tolerance breeding in cereal crops: A review. Zemdirb. Agric. 2013, 100, 325–334. [Google Scholar] [CrossRef]
- Escalona, J.M.; Flexas, J.; Medrano, H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Aust. J. Plant Physiol. 1999, 27, 421–433. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhang, Y.; Wang, R.; Xie, Z. Impacts of drought stress on the morphology, physiology, and sugar content of Lanzhou lily (Lilium davidii var. unicolor). Acta Physiol. Plant. 2020, 42, 127. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.; Solomon, S.; Mall, A.K.; Prajapati, C.P.; Hashem, A.; Abd Allah, E.F.; Ansari, M.I. Morphological assessment of water stressed sugarcane: A comparison of waterlogged and drought affected crop. Saudi J. Biol. Sci. 2020, 27, 1228–1236. [Google Scholar] [CrossRef]
- Patmi, Y.S.; Pitoyo, A.; Solichatun; Sutarno. Effect of drought stress on morphological, anatomical, and physiological characteristics of Cempo Ireng Cultivar Mutant Rice (Oryza sativa l.) strain 51 irradiated by gamma-ray. J. Phys. Conf. Ser. 2020, 1436, 012015. [Google Scholar] [CrossRef]
- Werner, C.; Correia, O.; Beyschlag, W. Two different strategies of Mediterranean macchia plants to avoid photoinhibitory damage by excessive radiation levels during summer drought. Acta Oecologica 1999, 20, 15–23. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology and Development; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
- Rucker, K.S.; Kvien, C.K.; Holbrook, C.C.; Hook, J.E. Identification of Peanut Genotypes with Improved Drought Avoidance Traits 1. Peanut Sci. 1995, 22, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Bhusal, N.; Lee, M.; Han, R.; Han, A.; Kim, H. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Entific Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol. Biochem. 2017, 118, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.K.; Srivastava, J.P.; Lal, J.P. Drought Resistance in Lentil (Lens culinaris Medik.) in Relation to Morphological, Physiological Parameters and Phenological Developments. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2288–2304. [Google Scholar] [CrossRef]
- Asl, K.K.; Ghorbanpour, M.; Khameneh, M.M.; Hatami, M. Influence of Drought Stress, Biofertilizers and Zeolite on Morphological Traits and Essential Oil Constituents in Dracocephalum moldavica L. J. Med. Plants 2018, 17, 91–112. [Google Scholar]
- Willick, I.R.; Lahlali, R.; Vijayan, P.; Muir, D.; Karunakaran, C.; Tanino, K.K. Wheat flag leaf epicuticular wax morphology and composition in response to moderate drought stress are revealed by SEM, FTIR-ATR and synchrotron X-ray spectroscopy. Physiol. Plant. 2018, 162, 316–332. [Google Scholar] [CrossRef] [Green Version]
- Rueda, M.; Godoy, O.; Hawkins, B.A. Spatial and evolutionary parallelism between shade and drought tolerance explains the distributions of conifers in the conterminous United States. Glob. Ecol. Biogeogr. 2017, 26, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Picotte, J.J.; Rhode, J.M.; Cruzan, M.B. Leaf morphological responses to variation in water availability for plants in the Piriqueta caroliniana complex. Plant Ecol. 2008, 200, 267–275. [Google Scholar] [CrossRef]
- Lobet, G.; Draye, X. Novel scanning procedure enabling the vectorization of entire rhizotron-grown root systems. Plant Methods 2013, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Gowda, V.R.P.; Henry, A.; Yamauchi, A.; Shashidhar, H.E.; Serraj, R. Root biology and genetic improvement for drought avoidance in rice. Field Crop. Res. 2011, 122, 1–13. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.; Zhang, D.; Shu, B.; Xiao, J.; Xia, R. Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under sand culture. Sci. Hortic. 2013, 162, 100–105. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Zhang, Y.; Sun, H.; Zhang, K.; Bai, Z.; Dong, H.; Li, C. Fine root and root hair morphology of cotton under drought stress revealed with RhizoPot. J. Agron. Crop Sci. 2020, 206, 679–693. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, B.; Chen, Q.; Ge, X.; Shi, Y. Effects of drought on root architecture and non-structural carbohydrate of Cunninghamia lanceolata. Acta Ecol. Sin. 2018, 38, 6729–6740. [Google Scholar]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced Lateral Root Branching Density Improves Drought Tolerance in Maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Benjamin, J.G.; Nielsen, D.C. Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crop. Res. 2006, 97, 248–253. [Google Scholar] [CrossRef]
- Bismillah Khan, M.; Hussain, M.; Raza, A.; Farooq, S.; Jabran, K. Seed priming with CaCl2 and ridge planting for improved drought resistance in maize. Turk. J. Agric. For. 2015, 39, 937–952. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lu, S.; Joubes, J.; Jenks, M.A. The Impact of Water Deficiency on Leaf Cuticle Lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zhu, X.; Zhang, Y.; Du, Z.; Chen, X.; Kong, X.; Sun, W.; Chen, C. Drought stress modify cuticle of tender tea leaf and mature leaf for transpiration barrier enhancement through common and distinct modes. Sci. Rep. 2020, 10, 6696. [Google Scholar] [CrossRef] [Green Version]
- Chartzoulakis, K.; Patakas, A.; Kofidis, G.; Bosabalidis, A.; Nastou, A. Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Sci. Hortic. 2002, 95, 39–50. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Li, Z.; Song, C.; Song, S.; Zheng, X. Contrasting Drought Tolerance in Two Apple Cultivars Associated with Difference in Leaf Morphology and Anatomy. Am. J. Plant Sci. 2019, 10, 709–722. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yang, H.; Wu, W.; Li, W. Effect of drought stress on physiological changes and leaf surface morphology in the blackberry. Braz. J. Bot. 2017, 40, 625–634. [Google Scholar] [CrossRef]
- Vincent, D.; Lapierre, C.; Pollet, B.; Cornic, G.; Negroni, L.; Zivy, M. Water deficits affect caffeate O-methyltransferase, lignification, and related enzymes in maize leaves. A proteomic investigation. Plant Physiol. 2005, 137, 949–960. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.-W.; Li, J.-N.; Liu, X.; Lian, J.-P.; Fu, C.; Li, W.; Jiang, J.-Y.; Xue, Y.-F.; Wang, J.; Chai, Y.-R. Lignification Response and the Difference between Stem and Root of Brassica napus under Heat and Drought Compound Stress. Acta Agron. Sin. 2017, 43, 1689. [Google Scholar] [CrossRef]
- Zhao, T.-J.; Sun, S.; Liu, Y.; Liu, J.-M.; Liu, Q.; Yan, Y.-B.; Zhou, H.-M. Regulating the Drought-responsive Element (DRE)-mediated Signaling Pathway by Synergic Functions of Trans-active and Trans-inactive DRE Binding Factors in Brassica napus. J. Biol. Chem. 2006, 281, 10752–10759. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Hashemi Garmdareh, S.E.; Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 253, 128–133. [Google Scholar] [CrossRef]
- Bidgoli, R.D. Effect of drought stress on some morphological characteristics, quantity and quality of essential oil in Rosemary (Rosmarinus officinalis L.). Adv. Med. Plant Res. 2018, 6, 40–45. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.; Ferreira, M.J.; Rodrigues, J.; Garcia, M.N.; Ceron, J.V.B.; Nelson, B.W.; Saleska, S.R. Causes of reduced leaf-level photosynthesis during strong El Nino drought in a Central Amazon forest. Glob. Chang. Biol. 2018, 24, 4266–4279. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Stockle, C.O. Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Gimenez, C.; Mitchell, V.J.; Lawlor, D.W. Regulation of Photosynthetic Rate of Two Sunflower Hybrids under Water Stress. Plant Physiol. 1992, 98, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Gunasekera, D.; Berkowitz, G.A. Use of Transgenic Plants with Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Antisense DNA to Evaluate the Rate Limitation of Photosynthesis under Water Stress. Plant Physiol. 1993, 103, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Shirke, P.A.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef]
- Ma, P.; Bai, T.-h.; Ma, F.-w. Effects of progressive drought on photosynthesis and partitioning of absorbed light in apple trees. J. Integr. Agric. 2015, 14, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Zaefyzadeh, M.; Quliyev, R.A.; Babayeva, S.M.; Abbasov, M.A. The Effect of the Interaction between Genotypes and Drought Stress on the Superoxide Dismutase and Chlorophyll Content in Durum Wheat Landraces. Turk. J. Biol. 2009, 33, 1–7. [Google Scholar]
- Wu, M.; Zhang, W.H.; Ma, C.; Zhou, J.Y. Changes in morphological, physiological, and biochemical responses to different levels of drought stress in Chinese cork oak (Quercus variabilis Bl.) seedlings. Russ. J. Plant Physiol. 2013, 60, 681–692. [Google Scholar] [CrossRef]
- Azzeme, A.M.; Abdullah, S.N.A.; Aziz, M.A.; Wahab, P.E.M. Oil palm leaves and roots differ in physiological response, antioxidant enzyme activities and expression of stress-responsive genes upon exposure to drought stress. Acta Physiol. Plant. 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Dastborhan, S.; Ghassemi-Golezani, K. Influence of seed priming and water stress on selected physiological traits of borage. Folia Hortic. 2015, 27, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Huber, W.; Sankhla, N. C4 Pathway and Regulation of the Balance Between C4 and C3 Metabolism; Springer: Berlin/Heidelberg, Germany, 1976. [Google Scholar]
- Silvera, K.; Santiago, L.S.; Winter, K. Distribution of crassulacean acid metabolism in orchids of Panama: Evidence of selection for weak and strong modes. Funct. Plant Biol. 2005, 32, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Holtum, J.A.M. The effects of salinity, crassulacean acid metabolism and plant age on the carbon isotope composition of Mesembryanthemum crystallinum L., a halophytic C3-CAM species. Planta 2005, 222, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Holtum, J.A. Environment or development? Lifetime net CO2 exchange and control of the expression of Crassulacean acid metabolism in Mesembryanthemum crystallinum. Plant Physiol. 2007, 143, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Winter, K.; Garcia, M.; Holtum, J.A.M. Drought-stress-induced up-regulation of CAM in seedlings of a tropical cactus, Opuntia elatior, operating predominantly in the C3 mode. J. Exp. Bot. 2011, 62, 4037–4042. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wan, C.; Wang, Y.; Chen, H.; Zhou, Z.; Fu, H.; Sosebee, R. The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa Desert, China. J. Arid Environ. 2004, 56, 525–539. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Jones, R.W.; Storey, R. Salt Stress and Comparative Physiology in the Gramineae. II. Glycinebetaine and Proline Accumulation in Two Salt- and Water-Stressed Barley Cultivars. Funct. Plant Biol. 1978, 5, 817–829. [Google Scholar] [CrossRef]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous application of glycinebetaine and potassium for improving water relations and grain yield of wheat under drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Cao, F.; Richmond, M.E.A.; Qiu, C.; Wu, F. Foliar application of betaine improves water-deficit stress tolerance in barley (Hordeum vulgare L.). Plant Growth Regul. 2019, 89, 109–118. [Google Scholar] [CrossRef]
- Korkmaz, A.; Değer, Ö.; Kocaçınar, F. Alleviation of water stress effects on pepper seedlings by foliar application of glycinebetaine. N. Z. J. Crop Hortic. Sci. 2015, 43, 18–31. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, Z. Abscisic Acid and Glycine Betaine Mediated Tolerance Mechanisms under Drought Stress and Recovery in Axonopus compressus: A New Insight. Sci. Rep. 2020, 10, 6942. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.L.; Wang, Y.J.; Xie, S.L.; Wang, C.; Wang, W. Glycinebetaine application ameliorates negative effects of drought stress in tobacco. Russ. J. Plant Physiol. 2007, 54, 472. [Google Scholar] [CrossRef]
- Bies-Ethève, N.; Gaubier-Comella, P.; Debures, A.; Lasserre, E.; Jobet, E.; Raynal, M.; Cooke, R.; Delseny, M. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.; Wolff, M.; Thalhammer, A.; Hincha, D.K. Folding of intrinsically disordered plant LEA proteins is driven by glycerol-induced crowding and the presence of membranes. Febs J. 2017, 284, 919–936. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant. 2010, 97, 795–803. [Google Scholar] [CrossRef]
- Soulages, J.L.; Kim, K.; Arrese, E.L.; Walters, C.; Cushman, J.C. Conformation of a group 2 late embryogenesis abundant protein from soybean. Evidence of poly (L-proline)-type II structure. Plant Physiol. 2003, 131, 963–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, M.; Fujinaga, M.; Kuboi, T. Radical scavenging activity and oxidative modification of citrus dehydrin. Plant Physiol. Biochem. 2004, 42, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Koag, M.C.; Fenton, R.D.; Wilkens, S.; Close, T.J. The binding of maize DHN1 to lipid vesicles. Gain of structure and lipid specificity. Plant Physiol. 2003, 131, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Terashima, S.; Kuboi, F.T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 2003, 217, 290–298. [Google Scholar] [CrossRef]
- Thalhammer, A.; Hundertmark, M.; Popova, A.V.; Seckler, R.; Hincha, D.K. Interaction of two intrinsically disordered plant stress proteins (COR15A and COR15B) with lipid membranes in the dry state. Bba Biomembr. 2010, 1798, 1812–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vítámvás, P.; Kosová, K.; Prá Ilová, P.; Prášil, I.T. Accumulation of WCS120 protein in wheat cultivars grown at 9 °C or 17 °C in relation to their winter survival. Plant Breed. 2010, 129, 611–616. [Google Scholar] [CrossRef]
- Puhakainen, T.; Hess, M.W.; Mäkelä, P.; Svensson, J.; Palva, E.T. Overexpression of Multiple Dehydrin Genes Enhances Tolerance to Freezing Stress in Arabidopsis. Plant Mol. Biol. 2004, 54, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Chaga, G.S. Twenty-five years of immobilized metal ion affinity chromatography: Past, present and future. J. Biochem. Biophys. Methods 2001, 49, 313–334. [Google Scholar] [CrossRef]
- Kruger, C.; Berkowitz, O.; Stephan, U.W.; Hell, R. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J. Biol. Chem. 2002, 277, 25062–25069. [Google Scholar] [CrossRef] [Green Version]
- Ingram, J.; Bartels, D. The Molecular Basis Of Dehydration Tolerance In Plants. Annu. Rev. Plant Biol. 1996, 47, 377–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Z.; Wang, L.; Wu, R.; Phillips, J.; Deng, X. LEA 4 group genes from the resurrection plant Boea hygrometrica confer dehydration tolerance in transgenic tobacco. Plant Sci. 2009, 176, 90–98. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, S.; Liu, G. Enhanced drought tolerance in transgenic Leymus chinensis plants with constitutively expressed wheat TaLEA3. Biotechnol. Lett. 2009, 31, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh, M.K. Ion Binding Properties of the Dehydrin ERD14 Are Dependent upon Phosphorylation. J. Biol. Chem. 2003, 278, 40882–40889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroy, A.F.; Castonguay, Y.; Laberge, S.; Sarhan, F.; Dhindsa, L.P.V.A. A new cold-induced alfalfa gene is associated with enhanced hardening at subzero temperature. Plant Physiol. 1993, 102, 873–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, M.; Terashima, S.; Kuboi, T. Characterization and cryoprotective activity of cold-responsive dehydrin from Citrus unshiu. J. Plant Physiol. 2001, 158, 1333–1339. [Google Scholar] [CrossRef]
- Allagulova, C.R.; Gimalov, F.R.; Shakirova, F.M.; Vakhitov, V.A. The Plant Dehydrins: Structure and Putative Functions. Biochem. Biokhimiia 2003, 68, 945–951. [Google Scholar] [CrossRef]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Kjellbom, P.J. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rougé, P.; Barre, A. A molecular modeling approach defines a new group of Nodulin 26-like aquaporins in plants. Biochem. Biophys. Res. Commun. 2008, 367, 60–66. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.J.; Jian, F.M. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, F.; Suga, S.; Uemura, T.; Sato, M.H.; Maeshima, M. Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett. 2005, 579, 5814–5820. [Google Scholar] [CrossRef] [Green Version]
- Frigerio, L. Mapping of Tonoplast Intrinsic Proteins in Maturing and Germinating Arabidopsis Seeds Reveals Dual Localization of Embryonic TIPs to the Tonoplast and Plasma Membrane. Mol. Plant 2011, 4, 180–189. [Google Scholar]
- Muto, Y.; Segami, S.; Hayashi, H.; Sakurai, J.; Murai-Hatano, M.; Hattori, Y.; Ashikari, M.; Maeshima, M. Vacuolar proton pumps and aquaporins involved in rapid internode elongation of deepwater rice. Biosci. Biotechnol. Biochem. 2011, 75, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javot, H. Role of a Single Aquaporin Isoform in Root Water Uptake. Plant Cell 2003, 15, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Heinen, R.B.; Ye, Q.; François, C. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 2971–2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bots, M. Aquaporins of the PIP2 Class Are Required for Efficient Anther Dehiscence in Tobacco. Plant Physiol. 2005, 137, 1049–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Tu, L.; Wang, L.; Li, Y.; Zhu, L.; Zhang, X. Characterization and expression of plasma and tonoplast membrane aquaporins in elongating cotton fibers. Plant Cell Rep. 2008, 27, 1385–1394. [Google Scholar] [CrossRef]
- Wudick, M.M.; Luu, D.T.; Maurel, C. A look inside: Localization patterns and functions of intracellular plant aquaporins. New Phytol. 2010, 184, 289–302. [Google Scholar] [CrossRef]
- Maurel, C.; Santoni, V.; Luu, D.T.; Wudick, M.M.; Verdoucq, L. The cellular dynamics of plant aquaporin expression and functions. Curr. Opin. Plant Biol. 2009, 12, 690–698. [Google Scholar] [CrossRef]
- Fotiadis, D.; Jeno, P.; Mini, T.; Wirtz, S.; Muller, S.A.; Fraysse, L.; Kjellbom, P.; Engel, A. Structural Characterization of Two Aquaporins Isolated from Native Spinach Leaf Plasma Membranes. J. Biol. Chem. 2001, 276, 1707–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, B.; Kaldenhoff, R. Cell-specific expression of the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 2000, 211, 167–172. [Google Scholar] [CrossRef]
- Yamada, S. A family of transcripts encoding water channel proteins: Tissue-specific expression in the common ice plant. Plant Cell 1995, 7, 1129–1142. [Google Scholar]
- Kaldenhoff, R. The blue light-responsive AthH2 gene of Arabidopsis thaliana is primarily expressed in expanding as well as in differentiating cells and encodes a putative channel protein of the plasmalemma. Plant J. Cell Mol. Biol. 2010, 7, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Netting, A.G. pH, abscisic acid and the integration of metabolism in plants under stressed and non-stressed conditions: Cellular responses to stress and their implication for plant water relations. J. Exp. Bot. 2000, 51, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.-J. Thiol-Based Peroxidases and Ascorbate Peroxidases: Why Plants Rely on Multiple Peroxidase Systems in the Photosynthesizing Chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [PubMed] [Green Version]
- Pospía il, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 2012, 1817, 218–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dröse, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012, 748, 145–169. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadtman, E.R.; Moskovitz, J.; Levine, R.L. Oxidation of methionine residues of proteins: Biological consequences. Antioxid Redox Signal 2003, 5, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtaszek, P. Oxidative burst: An early plant response to pathogen infection. Biochem. J. 1997, 322, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxygen Stress and Superoxide Dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Slooten, L. Factors Affecting the Enhancement of Oxidative Stress Tolerance in Transgenic Tobacco Overexpressing Manganese Superoxide Dismutase in the Chloroplasts. Plant Physiol. 1995, 107, 737–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van Montagu, M.; Inzé, D.; Camp, W. Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. Embo J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassinen, V.H.; Tervahauta, A.I.; Schat, H.; K Renlampi, S.O. Plant metallothioneins--metal chelators with ROS scavenging activity? Plant Biol. 2011, 13, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wigoda, N.; Ben-Nissan, G.; Granot, D.; Schwartz, A.; Weiss, D. The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. Plant J. 2010, 48, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, W.B.; Yi, H.; Kline, D.; Cameron, J.C.; Wignes, J.; Dey, S.; Pakrasi, H.B.; Jez, J.M. Probing the origins of glutathione biosynthesis through biochemical analysis of glutamate-cysteine ligase and glutathione synthetase from a model photosynthetic prokaryote. Biochem. J. 2013, 450, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Barth, C. The Timing of Senescence and Response to Pathogens Is Altered in the Ascorbate-Deficient Arabidopsis Mutant vitamin c-1. Plant Physiol. 2004, 134, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Veljovic-Jovanovic, S.D.; Pignocchi, C.; Noctor, G.; Foyer, C.H. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 2001, 127, 426–435. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbate biosynthesis and function in photoprotection. Philos. Trans. R. Soc. Lond. 2000, 355, 1455–1464. [Google Scholar] [CrossRef]
- Liebler, D.C.; Kling, D.S.; Reed, D.J. Antioxidant protection of phospholipid bilayers by alpha-tocopherol. Control of alpha-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J. Biol. Chem. 1986, 261, 12114–12119. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V. Plant L-ascorbic acid: Chemistry, function, metabolism, biovailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; Damatta, F.M.; Chaves, A.R.M.; Fontes, E.P.B.; Loureiro, M.E. Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant Sci. 2004, 167, 1307–1314. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Enzymatic responses of the ascorbate-glutathione cycle to drought in sorghum and sunflower plants. Plant Sci. 1996, 113, 139–147. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J.H. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Chazen, O.; Neumann, P.M. Hydraulic Signals from the Roots and Rapid Cell-Wall Hardening in Growing Maize (Zea mays L.) Leaves Are Primary Responses to Polyethylene Glycol-Induced Water Deficits. Plant Physiol. 1994, 104, 1385–1392. [Google Scholar] [CrossRef] [Green Version]
- Fromm, J.; Fei, H. Electrical signaling and gas exchange in maize plants of drying soil—ScienceDirect. Plant Sci. 1998, 132, 203–213. [Google Scholar] [CrossRef]
- Walker, J.C.; Willows, D.R. Mechanism and regulation of Mg-chelatase. Biochem. J. 1997, 327, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, N.; Brusslan, J.A.; Larkin, R. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA 2001, 98, 2053–2058. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yue, Y.; Li, B.; Nie, Y.; Li, W.; Wu, W.H.; Ma, L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science 2007, 315, 1712–1716. [Google Scholar] [CrossRef]
- Yin, P.; Fan, H.; Hao, Q.; Yuan, X.; Wu, D.; Pang, Y.; Yan, C.; Li, W.; Wang, J.; Yan, N. Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nat. Struct. Mol. Biol. 2009, 16, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Yi, X.Q.; Han, A.D.; Liu, T.W.; Chen, J.; Wu, F.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Calcium-sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis. J. Exp. Bot. 2012, 63, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling system. Cell Calcium 2007, 42, 345–350. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Assmann, S.M.; Albert, R. Predicting essential components of signal transduction networks: A dynamic model of guard cell abscisic acid signaling. Plos Biol. 2006, 4, e312. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J. Separating active and passive influences on stomatal control of transpiration. Plant Physiol. 2014, 164, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Schroeder, J.I. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol. 2004, 135, 702–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Tsuichihara, N.; Etoh, T.; Iwai, S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007, 30, 1320–1325. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hirt, H. Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci. Trends Plant Sci 1997, 2, 11–15. [Google Scholar] [CrossRef]
- Payne, D.M.; Rossomando, A.J.; Martino, P.; Erickson, A.K.; Sturgill, T.W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). Embo J. 1991, 10, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. Cell Mol. Biol. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Hua, X.J.; van de Cotte, B.; Van Montagu, M.; Verbruggen, N. Developmental regulation of pyrroline-5-carboxylate reductase gene expression in Arabidopsis. Plant Physiol. 1997, 114, 1215–1224. [Google Scholar] [CrossRef] [Green Version]
- Savouré, A.; Jaoua, S.; Hua, X.-J.; Ardiles, W.; Van Montagu, M.; Verbruggen, N. Isolation, characterization, and chromosomal location of a gene encoding the ” 1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Lett. 1995, 372, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.A.; Delauney, A.J.; Verma, D.P. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA 1992, 89, 9354–9358. [Google Scholar] [CrossRef] [Green Version]
- Kishor, P.; Hong, Z.; Miao, G.H.; Hu, C.; Verma, D. Overexpression of [delta]-Pyrroline-5-Carboxylate Synthetase Increases Proline Production and Confers Osmotolerance in Transgenic Plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Larosa, P.C.; Rhodes, D.; Rhodes, J.C.; Bressan, R.A.; Csonka, L.N. Elevated Accumulation of Proline in NaCl-Adapted Tobacco Cells Is Not Due to Altered Delta-Pyrroline-5-Carboxylate Reductase. Plant Physiol 1991, 96, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Su, J.; Chang, M.; Verma, D.P.S.; Fan, Y.-L.; Wu, R. Overexpression of a Δ1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water- and salt-stress in transgenic rice. Plant Sci. 1998, 139, 41–48. [Google Scholar] [CrossRef]
- Yamchi, A.; Rastgar Jazii, F.; Mousavi, A.; Karkhane, A.A.; Renu. Proline Accumulation in Transgenic Tobacco as a Result of Expression of Arabidopsis ” 1-Pyrroline-5-carboxylate synthetase (P5CS) During Osmotic Stress. J. Plant Biochem. Biotechnol. 2007, 16, 9–15. [Google Scholar] [CrossRef]
- Hmida-Sayari, A.; Gargouri-Bouzid, R.; Bidani, A.; Jaoua, L.; Savouré, A.; Jaoua, S. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci. 2005, 169, 746–752. [Google Scholar] [CrossRef]
- Guerzoni, J.T.S.; Belintani, N.G.; Moreira, R.M.P.; Hoshino, A.A.; Domingues, D.S.; Filho, J.O.C.B.; Vieira, L.G.E. Stress-induced Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene confers tolerance to salt stress in transgenic sugarcane. Acta Physiol. Plant. 2014, 36, 2309–2319. [Google Scholar] [CrossRef]
- Zhang, G.-C.; Zhu, W.-L.; Gai, J.-Y.; Zhu, Y.-L.; Yang, L.-F. Enhanced salt tolerance of transgenic vegetable soybeans resulting from overexpression of a novel Δ1-pyrroline-5-carboxylate synthetase gene from Solanum torvum Swartz. Hortic. Environ. Biotechnol. 2015, 56, 94–104. [Google Scholar] [CrossRef]
- Hervieu, F.; Dily, F.L.; Billard, J.-P.; Huault, C. Effects of water-stress on proline content and ornithine aminotransferase activity of radish cotyledons. Phytochemistry 1994, 37, 1227–1231. [Google Scholar] [CrossRef]
- Roosens, N.H.; Bitar, F.A.; Loenders, K.; Angenon, G.; Jacobs, M. Overexpression of ornithine-δ-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol. Breed. 2002, 9, 73–80. [Google Scholar] [CrossRef]
- Wu, L.; Fan, Z.; Guo, L.; Li, Y.; Zhang, W.; Qu, L.J.; Chen, Z. Over-expression of an Arabi-dopsisd δ-OAT gene enhances salt and drought tolerance in transgenic rice. Chin. Sci. Bull. 2003, 48, 2594–2600. [Google Scholar] [CrossRef]
- Kiyosue, T.; Yoshiba, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 1996, 8, 1323–1335. [Google Scholar]
- Rentsch, D.; Hirner, B.; Frommer, S.W.B. Salt Stress-Induced Proline Transporters and Salt Stress-Repressed Broad Specificity Amino Acid Permeases Identified by Suppression of a Yeast Amino Acid Permease-Targeting Mutant. Plant Cell 1996, 8, 1437–1446. [Google Scholar] [PubMed] [Green Version]
- Ueda, A.; Shi, W.; Shimada, T.; Miyake, H.; Takabe, T. Altered expression of barley proline transporter causes different growth responses in Arabidopsis. Planta 2007, 227, 277–286. [Google Scholar] [CrossRef]
- Nuccio, M.L.; McNeil, S.D.; Ziemak, M.J.; Hanson, A.D.; Jain, R.K.; Selvaraj, G. Choline import into chloroplasts limits glycine betaine synthesis in tobacco: Analysis of plants engineered with a chloroplastic or a cytosolic pathway. Metab. Eng. 2000, 2, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Brendza, K.M.; Haakenson, W.; Cahoon, R.E.; Hicks, L.M.; Palavalli, L.H.; Chiapelli, B.J.; McLaird, M.; McCarter, J.P.; Williams, D.J.; Hresko, M.C.; et al. Phosphoethanolamine N-methyltransferase (PMT-1) catalyses the first reaction of a new pathway for phosphocholine biosynthesis in Caenorhabditis elegans. Biochem. J. 2007, 404, 439–448. [Google Scholar] [CrossRef] [Green Version]
- McNeil, S.D.; Nuccio, M.L.; Ziemak, M.J.; Hanson, A.D. Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proc. Natl. Acad. Sci. USA 2001, 98, 10001–10005. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, N.H.; Akira, H.; Nana, Y.; Vandna, R.; Takashi, H.; Teruhiro, T. Regulation of betaine synthesis by precursor supply and choline monooxygenase expression in Amaranthus tricolor. J. Exp. Bot. 2007, 4203–4212. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. PlantCell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rathinasabapathi, B.; Burnet, M.; Russell, B.L. Choline Monooxygenase, an Unusual Iron-Sulfur Enzyme Catalyzing the First Step of Glycine Betaine Synthesis in Plants: Prosthetic Group Characterization and cDNA Cloning. Proc. Natl. Acad. Sci. USA 1997, 94, 3454–3458. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P.J. Salinity, Osmolytes and Compatible Solutes; Springer: Dordrecht, The Netherlands, 2002; pp. 181–204. [Google Scholar]
- Fitzgerald, T.L.; Waters, D.L.E.; Henry, R.J. Betaine aldehyde dehydrogenase in plants. Plant Biol. 2009, 11, 119–130. [Google Scholar] [CrossRef]
- Fujiwara, T.; Hori, K.; Ozaki, K.; Yokota, Y.; Mitsuya, S.; Ichiyanagi, T.; Hattori, T.; Takabe, T. Enzymatic characterization of peroxisomal and cytosolic betaine aldehyde dehydrogenases in barley. Physiol. Plant. 2008, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance—Role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.-G.; Du, B.-X.; Zhang, W.-K.; Zhang, J.-S.; Chen, S.-Y. AhCMO, regulated by stresses in Atriplex hortensis, can improve drought tolerance in transgenic tobacco. Tag. Theor. Appl. Genet. Theor. Und Angew. Genet. 2002, 105, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, S.; Kuwahara, J.; Ozaki, K.; Saeki, E.; Fujiwara, T.; Takabe, T. Isolation and characterization of a novel peroxisomal choline monooxygenase in barley. Planta 2011, 234, 1215–1226. [Google Scholar] [CrossRef]
- Wu, S.; Su, Q.; An, L.J. Isolation of choline monooxygenase (CMO) gene from salicornia europaea and enhanced salt tolerance of transgenic tobacco with CMO genes. Indian J. Geo-Mar. Sci. 2010, 47, 298–305. [Google Scholar]
- Ishitani, M.; Nakamura, T.; Han, S.Y.; Takabe, T. Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Mol. Biol. 1995, 27, 307–315. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, M.; Zhang, H.; Zhang, P. Improved Tolerance to Various Abiotic Stresses in Transgenic Sweet Potato (Ipomoea batatas) Expressing Spinach Betaine Aldehyde Dehydrogenase. PLoS ONE 2012, 7, e37344. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, Z.; Li, S.; Guo, S. Genetic Engineering of Glycine Betaine Biosynthesis Reduces Heat-Enhanced Photoinhibition by Enhancing Antioxidative Defense and Alleviating Lipid Peroxidation in Tomato. Plant Mol. Biol. Rep. 2014, 32, 42–51. [Google Scholar] [CrossRef]
- Cho, M.H.; Jang, A.; Bhoo, S.H.; Jeon, J.S.; Hahn, T.R. Manipulation of triose phosphate/phosphate translocator and cytosolic fructose-1,6-bisphosphatase, the key components in photosynthetic sucrose synthesis, enhances the source capacity of transgenic Arabidopsis plants. Photosynth. Res. 2012, 111, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Krause, K.e.; Apel, P.; Sonnewald, U. Reduction of the cytosolic fructose-1,6-bisphosphatase in transgenic potato plants limits photosynthetic sucrose biosynthesis with no impact on plant growth and tuber yield. Plant J. 2010, 9, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.C.; Huber, J.L. Role and regulation of sucrose-phosphate synthase in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef]
- Park, J.Y.; Canam, T.; Kang, K.Y.; Ellis, D.D.; Mansfield, S.D. Over-expression of an arabidopsis family A sucrose phosphate synthase (SPS) gene alters plant growth and fibre development. Transgenic Res. 2008, 17, 181–192. [Google Scholar] [CrossRef]
- Nguyen-Quoc, B.; N’Tchobo, H.; Foyer, C.H.; Yelle, S. Overexpression of sucrose phosphate synthase increases sucrose unloading in transformed tomato fruit. J. Exp. Bot. 1999, 50, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, K.; Hirotsu, N.; Kashiwagi, T.; Madoka, Y.; Nagasuga, K.; Ono, K.; Ohsugi, R. Overexpression of a Maize SPS Gene Improves Yield Characters of Potato under Field Conditions. Plant Prod. Sci. 2008, 11, 104–107. [Google Scholar] [CrossRef]
- Strand, Å.; Foyer, C.H.; Gustafsson, P.; Gardeström, P.; Hurry, V. Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant Cell Environ. 2010, 26, 523–535. [Google Scholar] [CrossRef]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA proteins during water stress: Not just for plants anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Sivamani, E.; Bahieldin, A.; Wraith, J.M.; Al-Niemi, T.; Dyer, W.E.; Ho, T.D.; Qu, R. Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. Int. J. Exp. Plant Biol. 2000, 155, 1–9. [Google Scholar] [CrossRef]
- Poku, S.A.; Chukwurah, P.N.; Aung, H.H.; Nakamura, I. Over-Expression of a Melon Y3SK2-Type LEA Gene Confers Drought and Salt Tolerance in Transgenic Tobacco Plants. Plants 2020, 9, 1749. [Google Scholar] [CrossRef]
- Luo, D.; Hou, X.; Zhang, Y.; Meng, Y.; Zhang, H.; Liu, S.; Wang, X.; Chen, R. CaDHN5, a Dehydrin Gene from Pepper, Plays an Important Role in Salt and Osmotic Stress Responses. Int. J. Mol. Sci. 2019, 20, 1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poku, S.A.; Seçgin, Z.; Kavas, M. Overexpression of Ks-type dehydrins gene OeSRC1 from Olea europaea increases salt and drought tolerance in tobacco plants. Mol. Biol. Rep. 2019, 46, 5745–5757. [Google Scholar] [CrossRef] [PubMed]
- Kaldenhoff, R.; Fischer, M. Aquaporins in plants. Acta Physiol. 2006, 187, 169–176. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, M.; Chen, W.; Zhou, X.; Lu, J.; Wang, Y.; Li, Y.; Jiang, C.Z. In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat. Plants 2019, 5, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lin, R.; Pu, L.; Wang, Z.; Jian, S. Ectopic Expression of CrPIP2;3, a Plasma Membrane Intrinsic Protein Gene from the Halophyte Canavalia rosea, Enhances Drought and Salt-Alkali Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 565. [Google Scholar] [CrossRef]
- Khan, K.; Agarwal, P.; Shanware, A.; Sane, V.A. Heterologous Expression of Two Jatropha Aquaporins Imparts Drought and Salt Tolerance and Improves Seed Viability in Transgenic Arabidopsis thaliana. PLoS ONE 2015, 10, e0128866. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, W.; Cai, W.; Arora, R. Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 2007, 226, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.P.S.; Neves, D.M.; Cidade, L.C.; Mendes, A.F.S.; Silva, D.C.; Almeida, A.-A.F.; Coelho-Filho, M.A.; Gesteira, A.S.; Soares-Filho, W.S.; Costa, M.G.C. Expression of the citrus CsTIP2;1 gene improves tobacco plant growth, antioxidant capacity and physiological adaptation under stress conditions. Planta 2017, 245, 951–963. [Google Scholar] [CrossRef]
- Seo, M. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid, a key in abscisic acid biosynthesis in Arabidopsis. Plant J. Cell Mol. Biol. 2001, 27, 325. [Google Scholar] [CrossRef] [Green Version]
- Awan, S.Z.; Chandler, J.O.; Harrison, P.J.; Sergeant, M.J.; Bugg, T.D.H.; Thompson, A.J. Promotion of Germination Using Hydroxamic Acid Inhibitors of 9-cis-Epoxycarotenoid Dioxygenase. Front. Plant Sci. 2017, 8, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.S. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, A.C.; Gribskov, M.; Gubrium, E.; Harper, J.F. The CDPK superfamily of protein kinases. New Phytol. 2001, 151, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Botella, J.R.; Arteca, J.M.; Somodevilla, M.; Arteca, R.N. Calcium-dependent protein kinase gene expression in response to physical and chemical stimuli in mungbean (Vigna radiata). Plant Mol. Biol. 1996, 30, 1129–1137. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Cushman, J.C. A stress-induced calcium-dependent protein kinase from Mesembryanthemum crystallinum phosphorylates a two-component pseudo-response regulator. Plant J. Cell Mol. Biol. 2000, 24, 679–691. [Google Scholar] [CrossRef]
- Urao, T.; Katagiri, T.; Mizoguchi, T.; Yamaguchi-Shinozaki, K.; Hayashida, N.; Shinozaki, K. Two genes that encode Ca(2+)-dependent protein kinases are induced by drought and high-salt stresses in Arabidopsis thaliana. Mol. Gen. Genet. MGG 1994, 244, 331–340. [Google Scholar] [CrossRef]
- Sheen, J. Ca2+-Dependent Protein Kinases and Stress Signal Transduction in Plants. Science 1997, 274, 1900–1902. [Google Scholar] [CrossRef] [Green Version]
- Saijo, Y. A Ca2+-Dependent Protein Kinase that Endows Rice Plants with Cold- and Salt-Stress Tolerance Functions in Vascular Bundles. Plant Cell Physiol. 2001, 42, 1228–1233. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, T.; Irie, K.; Hirayama, T.; Hayashida, N.; Yamaguchi-Shinozaki, K.; Matsumoto, K.; Shinozaki, K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Chitlaru, E.; Seger, R.; Pick, U. Activation of a 74 kDa plasma membrane protein kinase by hyperosmotic shocks in the halotolerant alga Dunaliella salina—ScienceDirect. J. Plant Physiol. 1997, 151, 429–436. [Google Scholar] [CrossRef]
- Xiong, L. Disease Resistance and Abiotic Stress Tolerance in Rice Are Inversely Modulated by an Abscisic Acid Inducible Mitogen-Activated Protein Kinase. Plant Cell 2003, 15, 745–759. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Zhang, J.; Li, H.; Yang, C.; Zhang, C.; Zhang, X.; Khurram, Z.; Zhang, Y.; Wang, T.; Fei, Z. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot. 2010, 61, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Degenkolbe, T.; Do, P.T.; Zuther, E.; Repsilber, D.; Walther, D.; Hincha, D.K.; K Hl, K.I. Expression profiling of rice cultivars differing in their tolerance to long-term drought stress. Plant Mol. Biol. 2009, 69, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, I.K. Transcriptional Regulatory Networks in Response to Abiotic Stresses in Arabidopsis and Grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.C.; Hrmova, M.; Lopato, S.; Langridge, P. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytol. 2011, 190, 823–837. [Google Scholar] [CrossRef]
- Agalou, A.; Purwantomo, S.; Overnäs, E.; Johannesson, H.; Zhu, X.; Estiati, A.; de Kam, R.J.; Engström, P.; Slamet-Loedin, I.H.; Zhu, Z.; et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol. Biol. 2008, 66, 87–103. [Google Scholar] [CrossRef]
- Deng, X.; Phillips, J.; Bräutigam, A.; Engström, P.; Johannesson, H.; Ouwerkerk, P.B.F.; Ruberti, I.; Salinas, J.; Vera, P.; Iannacone, R.; et al. A homeodomain leucine zipper gene from Craterostigma plantagineum regulates abscisic acid responsive gene expression and physiological responses. Plant Mol. Biol. 2006, 61, 469–489. [Google Scholar] [CrossRef] [Green Version]
- Manavella, P.A.; Arce, A.L.; Dezar, C.A.; Bitton, F.; Renou, J.-P.; Crespi, M.; Chan, R.L. Cross-talk between ethylene and drought signalling pathways is mediated by the sunflower Hahb-4 transcription factor. Plant J. Cell Mol. Biol. 2006, 48, 125–137. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Zou, J.; Zhang, X.; Jiang, L.; Liu, K.; Lü, P.; Gao, J.; Zhang, C. The RhHB1/RhLOX4 module affects the dehydration tolerance of rose flowers (Rosa hybrida) by fine-tuning jasmonic acid levels. Hortic. Res. 2020, 7, 74. [Google Scholar] [CrossRef]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Zhang, J.; Zhang, H.; Zhang, Z.; Quan, R.; Zhou, S.; Huang, R. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, K.; Takeda, M.; Kidokoro, S.; Yamada, K.; Sakuma, Y.; Urano, K.; Fujita, M.; Yoshiwara, K.; Matsukura, S.; Morishita, Y.; et al. Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcripts regulated by DREB1A and DREB2A. Plant Physiol. 2009, 150, 1972–1980. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Guo, X.; Zhang, M.; Wang, X.; Zhao, Y.; Yin, Z.; Zhang, Z.; Wang, Y.; Xiong, H.; Zhang, H. OsERF71 confers drought tolerance via modulating ABA signaling and proline biosynthesis. Plant Sci. 2018, 270, 131–139. [Google Scholar] [CrossRef]
- Li, M.-R.; Li, Y.; Li, H.-Q.; Wu, G.-J. Ectopic expression of FaDREB2 enhances osmotic tolerance in paper mulberry. J. Integr. Plant Biol. 2011, 53, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, W.; Wan, L.; Li, F.; Dai, L.; Li, D.; Zhang, Z.; Huang, R. Functional analyses of ethylene response factor JERF3 with the aim of improving tolerance to drought and osmotic stress in transgenic rice. Transgenic Res. 2010, 19, 809–818. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, M.; Li, L.; Xu, Z.; Chen, X.; Guo, J.; Ma, Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009, 60, 3781–3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Zhang, Z.; Zhang, H.; Wang, X.-C.; Huang, R. Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol. 2008, 148, 1953–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef]
- Liu, J.; Osbourn, A.; Ma, P. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [Green Version]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L.C. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Dai, X.; Xu, Y.; Ma, Q.; Xu, W.; Wang, T.; Xue, Y.; Chong, K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007, 143, 1739–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, H.I.; Yang, Z.; Gong, Q.; Chen, E.; Wang, X.; Zhao, G.; Ge, X.; Zhang, X.; Li, F. GaMYB85, an R2R3 MYB gene, in transgenic Arabidopsis plays an important role in drought tolerance. BMC Plant Biol. 2017, 17, 142. [Google Scholar] [CrossRef]
- Chen, T.; Li, W.; Hu, X.; Guo, J.; Liu, A.; Zhang, B. A Cotton MYB Transcription Factor, GbMYB5, is Positively Involved in Plant Adaptive Response to Drought Stress. Plant Cell Physiol. 2015, 56, 917–929. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, W.; Qin, M.; Li, S.; Qiao, M.; Liu, Z.; Xiang, F. Drought Tolerance Conferred in Soybean (Glycine max. L) by GmMYB84, a Novel R2R3-MYB Transcription Factor. Plant Cell Physiol. 2017, 58, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef] [Green Version]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 Enhance Drought Tolerance by Modulating Root Vessels and Cell Walls in Apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef] [Green Version]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Wang, C.; Deng, P.; Chen, L.; Wang, X.; Ma, H.; Hu, W.; Yao, N.; Feng, Y.; Chai, R.; Yang, G.; et al. A wheat WRKY transcription factor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco. PLoS ONE 2013, 8, e65120. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jia, H.; Chen, X.; Hao, L.; An, H.; Guo, X. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Wang, C.; Wang, F.; Liu, S.; Li, G.; Guo, X. GhWRKY68 reduces resistance to salt and drought in transgenic Nicotiana benthamiana. PLoS ONE 2015, 10, e0120646. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Zhang, Y.; Han, L.; Guan, Z.; Chai, T. A novel WRKY transcriptional factor from Thlaspi caerulescens negatively regulates the osmotic stress tolerance of transgenic tobacco. Plant Cell Rep. 2008, 27, 795–803. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Maruyama, K.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Tran, L.-S.P.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. Cell Mol. Biol. 2004, 39, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Zhang, Y.; Lyu, Y. A Stress-Responsive NAC Transcription Factor from Tiger Lily (LlNAC2) Interacts with LlDREB1 and LlZHFD4 and Enhances Various Abiotic Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 3225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cui, X.; Guo, Y.; Luo, C.; Zhang, L. Picea wilsonii transcription factor NAC2 enhanced plant tolerance to abiotic stress and participated in RFCP1-regulated flowering time. Plant Mol. Biol. 2018, 98, 471–493. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, C.; Lü, P.; Jiang, G.; Liu, X.; Dai, F.; Gao, J. RhNAC3, a stress-associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress-related genes in rose petals. Plant Biotechnol. J. 2014, 12, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.-L.; Yue, X.-F.; Min, Z.; Wang, X.-H.; Fang, Y.-L.; Zhang, J.-X. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 98–111. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. https://doi.org/10.3390/horticulturae7030050
Yang X, Lu M, Wang Y, Wang Y, Liu Z, Chen S. Response Mechanism of Plants to Drought Stress. Horticulturae. 2021; 7(3):50. https://doi.org/10.3390/horticulturae7030050
Chicago/Turabian StyleYang, Xinyi, Meiqi Lu, Yufei Wang, Yiran Wang, Zhijie Liu, and Su Chen. 2021. "Response Mechanism of Plants to Drought Stress" Horticulturae 7, no. 3: 50. https://doi.org/10.3390/horticulturae7030050
APA StyleYang, X., Lu, M., Wang, Y., Wang, Y., Liu, Z., & Chen, S. (2021). Response Mechanism of Plants to Drought Stress. Horticulturae, 7(3), 50. https://doi.org/10.3390/horticulturae7030050





