Biochemical Responses and Leaf Gas Exchange of Fig (Ficus carica L.) to Water Stress, Short-Term Elevated CO2 Levels and Brassinolide Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Greenhouse Conditions
2.2. Experimental Design
2.3. Application of Treatments
2.3.1. Brassinolide
2.3.2. Elevated CO2
2.3.3. Field Capacity Determination
2.4. Measurements
2.4.1. Leaf Gas Exchange
Determination of Photosynthetic Rate (A), Stomatal Conductance (gs), Transpiration Rate (E), Intercellular CO2 (Ci), and Vapour Pressure Deficit (VPD)
Determination of Water-Use Efficiency (WUE) and Intrinsic Water-Use Efficiency (Int-WUE)
Determination of Chlorophyll Fluorescence
2.4.2. Biochemical Response
Determination of Total Chlorophyll Content (T-Chl)
Determination of Proline Content
Determination of Starch
Determination of Malondialdehyde (MDA) and Soluble Sugar Content (SSC)
Determination of Protein
Determination of Peroxidase and Catalase
2.5. Statistical Anakysis
3. Results
3.1. Leaf Gas Exchange
3.1.1. Photosynthetic Rate, Stomatal Conductance, Transpiration Rate, Intercellular CO2, and Vapour Pressure Deficit
3.1.2. Water-Use-Efficiency, Intrinsic WUE, and Chlorophyll Fluorescence
3.2. Biochemical Responses
3.2.1. Chlorophyll Content, Lipid Peroxidation, Osmolyte Accumulation, Protein, and Starch Content
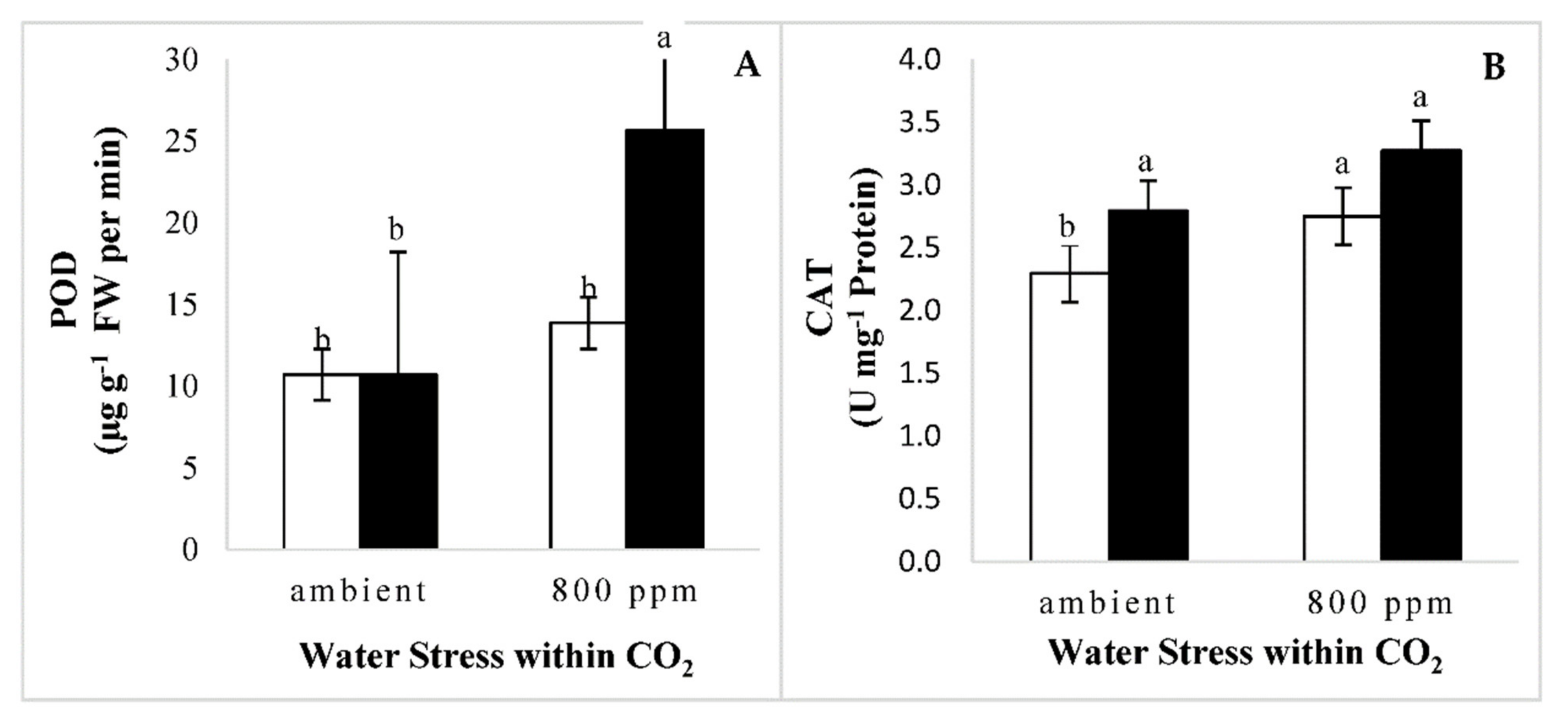
3.2.2. Activation of Antioxidant Defense Systems
3.3. Correlation Analysis
4. Discussion
4.1. Leaf Gas Exchange
4.2. Biochemical Responses

5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjum, S.; Xie, X.-Y.; Wang, L.-C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Rasool, F.; Habib, R.; Javed, T.; Sardar, K.; Mohsin Ayub, M.; Ahsin Ayub, M.; Rasool, A. A Review of Morphological, Physiological and Biochemical Responses of Plants under Drought Stress Conditions. Imp. J. Interdiscip. Res. (IJIR) 2016, 2, 1600–1606. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Review article Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.J.; Farquhar, G.D. The Mechanical Diversity of Stomata and Its Significance in Gas-Exchange Control. PLANT Physiol. 2006, 143, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. Water Stress Crop Plants A Sustain. Approach 2016, 1–2, 24–40. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Marusig, D.; Tombesi, S. Abscisic acid mediates drought and salt stress responses in vitis vinifera—A review. Int. J. Mol. Sci. 2020, 21, 8648. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of Agricultural Crops to Free-Air CO2 Enrichment. Adv. Agron 2002, 77, 293–368. [Google Scholar] [CrossRef]
- IPCC 2013 Summary for Policymakers. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Proceedings of the CEUR Workshop Proceedings, Geneva, Switzerland, 17 September 2013; Volume 1542, pp. 33–36.
- Makino, A.; Tadahiko, M. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Amthor, J.S. Respiration in a future, higher-CO2 world. Plant. Cell Environ. 1991, 14, 13–20. [Google Scholar] [CrossRef]
- Kazemi, S.; Eshghizadeh, H.R.; Zahedi, M. Responses of Four Rice Varieties to Elevated CO2 and Different Salinity Levels. Rice Sci. 2018, 25, 142–151. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Wang, Y.; Zhu, J.; Huang, J.; Liu, G.; Dong, G.; Wang, Y. Impact of elevated CO2 concentration on inter-subspecific hybrid rice cultivar Liangyoupeijiu under fully open-air field conditions. Field Crop. Res. 2009, 112, 7–15. [Google Scholar] [CrossRef]
- Dahal, K.; Knowles, V.L.; Plaxton, W.C.; Hüner, N.P.A. Enhancement of photosynthetic performance, water use efficiency and grain yield during long-term growth under elevated CO2 in wheat and rye is growth temperature and cultivar dependent. Environ. Exp. Bot. 2014, 106, 207–220. [Google Scholar] [CrossRef]
- Hamim, H. Photosynthesis of C3 and C4 Species in Response to Increased CO 2. Hayati 2005, 12, 131–138. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Katny, M.A.C.; Hoffmann-Thoma, G.; Schrier, A.A.; Fangmeier, A.; Jäger, H.J.; Van Bel, A.J.E. Increase of photosynthesis and starch in potato under elevated CO 2 is dependent on leaf age. J. Plant Physiol. 2005, 162, 429–438. [Google Scholar] [CrossRef]
- Pfister, B.; Zeeman, S.C. Formation of starch in plant cells. Cell. Mol. Life Sci. 2016, 73, 2781–2807. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Muramatsu, S.; Makino, A.; Mae, T. Relationship between the suppression of photosynthesis and starch accumulation in the pod-removed bean. Funct. Plant Biol. 2002, 27, 167. [Google Scholar] [CrossRef]
- Caemmerer, S.; Evans, J. Determination of the Average Partial Pressure of CO2 in Chloroplasts From Leaves of Several C3 Plants. Funct. Plant Biol. 2006, 18, 287. [Google Scholar] [CrossRef]
- Singh, A.; Prakash, J.; Meghwal, P.R. Fig(Ficus carica L.). In Breeding of Underutilized Fruit Crops; Jaya Publishing House: New Delhi, India, 2015; pp. 149–174. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Edible Med. Non-Med. Plants 2016, 10, 1–659. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management; Springer: New York, NY, USA, 2009; ISBN 9789048126651. [Google Scholar]
- Li, L.; Van Staden, J. Effects of plant growth regulators on drought resistance of two maize cultivars. S. Afr. J. Bot. 1998, 64, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Ahmad, A. Brassinosteroids: A Class of Plant Hormone; Springer: New York, NY, USA, 2011; ISBN 9789400701892. [Google Scholar]
- Jager, C.E.; Symons, G.M.; Ross, J.J.; Reid, J.B. Do brassinosteroids mediate the water stress response? Physiol. Plant. 2008, 133, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Zulkarnaini, Z.M.; Zaharah, S.S.; Mohamed, M.T.M.; Jaafar, H.Z.E. Effect brassinolide aplication on growth and physiologicalchanges in two cultivars of fig (ficus carica l.). Pertanika J. Trop. Agric. Sci. 2019, 42, 333–346. [Google Scholar]
- Sinamo, V.; Hanafi, N.D.; Wahyuni, T.H. Legume Plant Growth at Various Levels of Drought Stress Treatment Veronica. Indones. J. Agric. Res. 2018, 01, 9–19. [Google Scholar] [CrossRef]
- Evans, J.R.; Santiago, L.S. Prometheus wiki gold leaf protocol: Gas exchange using LI-COR 6400. Funct. Plant Biol. 2014, 41, 223–226. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bort, J.; Araus, J.L. Water use efficiency in C3 cereals under Mediterranean conditions: A review of physiological aspects. Ann. Appl. Biol. 2007, 150, 307–321. [Google Scholar] [CrossRef]
- Wassink, E.C. Chlorophyll fluorescence and photosynthesis. In Advances in Enzymology and Related Areas of Molecular Biology; Wiley: Hoboken, NJ, USA, 2006; ISBN 9780470122563. [Google Scholar]
- Harris, G.A.; Coombs, J.; Hall, D.D.; Long, S.P.; Scurlock, J.M.O. Techniques in Bioproductivity and Photosynthesis. J. Range Manag. 2007, 40, 480. [Google Scholar] [CrossRef]
- Pesci, P.; Beffagna, N. Inhibiting effect of fusicoccin on abscisic acid-induced proline accumulation in barley leaves. Plant Sci. Lett. 1984, 36, 7–12. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Analysis of Malondialdehyde, Chlorophyll Proline, Soluble Sugar, and Glutathione Content in Arabidopsis seedling. BIO-PROTOCOL 2016, 3, 817. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Alici, E.; Arabaci, G. Determination of SOD, POD, PPO and CAT Enzyme Activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Rampino, P.; Pataleo, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Akitha Devi, M.K.; Giridhar, P. Variations in Physiological Response, Lipid Peroxidation, Antioxidant Enzyme Activities, Proline and Isoflavones Content in Soybean Varieties Subjected to Drought Stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 35–44. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Huang, Q.C.; Qin, G.Y.; Zhao, S.P.; Zhou, J.G. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 2014, 52, 193–202. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Wang, F.; Liu, Y.; Zahoor, R.; Jiang, D.; Dai, T. Adaptation to and recovery from drought stress at vegetative stages in wheat (Triticum aestivum) cultivars. Funct. Plant Biol. 2016, 43, 1159–1169. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chai, L.; Yang, Z.; Mubarak, H.; Tang, C. Chlorophyll Fluorescence in Leaves of Ficus tikoua Under Arsenic Stress. Bull. Environ. Contam. Toxicol. 2016, 97, 576–581. [Google Scholar] [CrossRef]
- Hossain, A.B.M.S.; Salleh, A.; Mekhled, M.A.; Al-Saif, A.M. Chlorophyll fluorescence intensity, photosynthetic yield and flowering in fig fruit trees as affected by phloem stress. Bulg. J. Agric. Sci. 2010, 16, 547–552. [Google Scholar]
- Zheng, L.; Van Labeke, M.C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Jee, G. Advances in photosynthesis and respiration, Volume 19: ‘Chlorophyll a Fluorescence: A signature of photosynthesis’. Photosynth. Res. 2005, 83, 101–105. [Google Scholar] [CrossRef]
- Kumari, S. Temperature, Vapour Pressure Deficit and Water Stress Interaction on Transpiration in Wheat Temperature, Vapour Pressure Deficit and Water Stress Interaction on Transpiration in Wheat. Int. J. Sci. Res 2014, 2, 375–376. [Google Scholar] [CrossRef]
- Usuda, H.; Shimogawara, K. The effects of increased atmospheric carbon dioxide on growth, carbohydrates, and photosynthesis in radish, Raphanus sativus. Plant Cell Physiol. 1998, 39, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sage, R.F.; Sharkey, T.D.; Seemann, J.R. Acclimation of Photosynthesis to Elevated CO2 in Five C3 Species. PLANT Physiol. 2008, 89, 590–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh, K.; Yonetani, K.; Murota, T.; Kuroda, T. Effects of Flag Leaves and Panicles on Light Interception and Canopy Photosynthesis in High-Yielding Rice Cultivars. Plant Prod. Sci. 2009, 5, 275–280. [Google Scholar] [CrossRef]
- Sasek, T.W.; Delucia, E.H.; Strain, B.R. Reversibility of Photosynthetic Inhibition in Cotton after Long-Term Exposure to Elevated CO2 Concentrations. PLANT Physiol. 1985, 78, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Tuyen, D.D. Genetic studies on saline and sodic tolerances in soybean. Breed. Sci. 2012, 61, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulkarnaini, Z.M.; Sakimin, S.Z.; Mohamed, M.T.M.; Jaafar, H.Z.E. Relationship between chlorophyll content and soil plant analytical development values in two cultivars of fig (Ficus carica L.) as brassinolide effect at an open field. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Malang City, Indonesia, 12–13 March 2019; Volume 250, pp. 1–7. [Google Scholar]
- Zulkarnaini, Z.M.; Sakimin, S.Z.; Mohamed, M.T.M.; Jaafar, H.Z.E. Changes in leaf area index, leaf mass ratio, net assimilation rate, relative growth rate and specific leaf area two cultivars of fig (Ficus carica L.) treated under different concentrations of brassinolide. Agrivita 2019, 41, 158–165. [Google Scholar] [CrossRef]
- Sasek, T.W.; Strain, B.R. Effects of Carbon Dioxide Enrichment on the Expansion and Size of Kudzu ( Pueraria lobata ) Leaves. Weed Sci. 1989, 78, 619–622. [Google Scholar] [CrossRef]
- Sicher, R.C.; Kremer, D.F. Responses of Nicotiana tabacum to CO2 enrichment at low-photon flux density. Physiol. Plant. 1994, 92, 383–388. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO 2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Taïbi, K.; del Campo, A.D.; Vilagrosa, A.; Bellés, J.M.; López-Gresa, M.P.; Pla, D.; Calvete, J.J.; López-Nicolás, J.M.; Mulet, J.M. Drought Tolerance in Pinus halepensis Seed Sources As Identified by Distinctive Physiological and Molecular Markers. Front. Plant Sci. 2017, 8, 1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Rowley, K.G.; Itsiopoulos, C.; O’Dea, K. Identification and quantification of major carotenoids in selected components of the Mediterranean diet: Green leafy vegetables, figs and olive oil. Eur. J. Clin. Nutr. 2002, 56, 1149–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inanc, A.L. Chlorophyll: Structural Properties, Health Benefits and Its Occurrence in Virgin Olive Oils. Akad. Gida 2011, 9, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Gagné-Bourque, F.; Bertrand, A.; Claessens, A.; Aliferis, K.A.; Jabaji, S. Alleviation of Drought Stress and Metabolic Changes in Timothy (Phleum pratense L.) Colonized with Bacillus subtilis B26. Front. Plant Sci. 2016, 7, 584. [Google Scholar] [CrossRef] [Green Version]
- Türkan, I.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Sultan, M.A.R.F.; Hui, L.; Yang, L.J.; Xian, Z.H. Assessment of drought tolerance of some triticum l. species through physiological indices. Czech J. Genet. Plant Breed. 2012, 48, 178–184. [Google Scholar] [CrossRef]
- Nakano, H.; Makino, A.; Mae, T. The Effect of Elevated Partial Pressures of CO2 on the Relationship between Photosynthetic Capacity and N Content in Rice Leaves. Plant Physiol. 1997, 115, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO 2 on the protein concentration of food crops: A meta-analysis. Glob. Chang. Biol. 2007, 14, 565–575. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Lisar, S.Y.S.; Motafakkerazad, R.; Hossain, M.M.; Ismail, M.; Rahman, M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; InTech: Rijeka, Croatia, 2012; pp. 1–15. [Google Scholar]
- Zhang, C.; Shi, S.; Wu, F. Effects of drought stress on root and physiological responses of different drought-tolerant alfalfa varieties. Sci. Agric. Sin. 2018, 51, 868–882. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [Green Version]
- Sairam, R.K.; Rao, K.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Xu, L.; Han, L.; Huang, B. Antioxidant Enzyme Activities and Gene Expression Patterns in Leaves of Kentucky Bluegrass in Response to Drought and Post-drought Recovery. J. Am. Soc. Hortic. Sci. 2019, 136, 247–255. [Google Scholar] [CrossRef] [Green Version]



| A | gs | E | Ci | VPD | WUE | Int-WUE | Fv/Fm | T_Chl | Proline | MDA | SSC | Protein | POD | CAT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1 | ||||||||||||||
| gs | 0.132 | 1 | |||||||||||||
| E | 0.235 | 0.206 | 1 | ||||||||||||
| Ci | 0.443 ** | −0.011 | 0.192 | 1 | |||||||||||
| VPD | −0.155 | 0.444 ** | −0.122 | −0.351 * | 1 | ||||||||||
| WUE | 0.188 | 0.114 | −0.675 ** | −0.067 | 0.335 * | 1 | |||||||||
| Int-WUE | 0.226 | −0.619 ** | 0.016 | 0.101 | −0.271 | −0.061 | 1 | ||||||||
| Fv/Fm | 0.176 | 0.333 * | −0.130 | −0.090 | 0.539 ** | 0.400 ** | −0.032 | 1 | |||||||
| T_Chl | −0.012 | 0.436 ** | −0.032 | −0.133 | 0.512 ** | 0.244 | −0.216 | 0.262 | 1 | ||||||
| Proline | 0.006 | −0.438 ** | 0.248 | −0.063 | −0.419 ** | −0.236 | 0.324 * | −0.074 | −0.303 * | 1 | |||||
| MDA | −0.241 | −0.656 ** | −0.157 | −0.044 | −0.377 ** | −0.187 | 0.257 | 0.018 | −0.428 ** | 0.462 ** | 1 | ||||
| SSC | −0.161 | −0.583 ** | 0.076 | −0.003 | −0.413 ** | −0.357 * | 0.269 | 0.037 | −0.279 | 0.362 * | 0.642 ** | 1 | |||
| Protein | 0.370 ** | 0.651 ** | 0.265 | 0.243 | 0.252 | 0.131 | −0.436 ** | −0.429 ** | 0.149 | −0.234 | −0.520 ** | −0.506 ** | 1 | ||
| POD | −0.064 | −0.210 | 0.118 | −0.016 | −0.308 * | −0.140 | 0.196 | −0.040 | −0.100 | 0.315 * | 0.436 ** | 0.425 ** | −0.132 | 1 | |
| CAT | −0.131 | −0.584 ** | −0.078 | 0.078 | −0.589 ** | −0.202 | 0.234 | −0.117 | −0.498 ** | 0.522 ** | 0.769 ** | 0.746 ** | −0.403 ** | 0.563 ** | 1 |
| Starch | 0.432 ** | 0.740 ** | 0.208 | 0.216 | 0.396 ** | 0.226 | −0.342 * | −0.475 ** | 0.224 | −0.304 * | −0.637 ** | −0.649 ** | 0.866 ** | −0.249 | −0.556 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardinata, Z.; Edy Sabli, T.; Ulpah, S. Biochemical Responses and Leaf Gas Exchange of Fig (Ficus carica L.) to Water Stress, Short-Term Elevated CO2 Levels and Brassinolide Application. Horticulturae 2021, 7, 73. https://doi.org/10.3390/horticulturae7040073
Mardinata Z, Edy Sabli T, Ulpah S. Biochemical Responses and Leaf Gas Exchange of Fig (Ficus carica L.) to Water Stress, Short-Term Elevated CO2 Levels and Brassinolide Application. Horticulturae. 2021; 7(4):73. https://doi.org/10.3390/horticulturae7040073
Chicago/Turabian StyleMardinata, Zulias, Tengku Edy Sabli, and Saripah Ulpah. 2021. "Biochemical Responses and Leaf Gas Exchange of Fig (Ficus carica L.) to Water Stress, Short-Term Elevated CO2 Levels and Brassinolide Application" Horticulturae 7, no. 4: 73. https://doi.org/10.3390/horticulturae7040073
APA StyleMardinata, Z., Edy Sabli, T., & Ulpah, S. (2021). Biochemical Responses and Leaf Gas Exchange of Fig (Ficus carica L.) to Water Stress, Short-Term Elevated CO2 Levels and Brassinolide Application. Horticulturae, 7(4), 73. https://doi.org/10.3390/horticulturae7040073






