Phenotypic Characterization and Differential Gene Expression Analysis Reveal That Dwarf Mutant dwf Dwarfism Is Associated with Gibberellin in Eggplant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
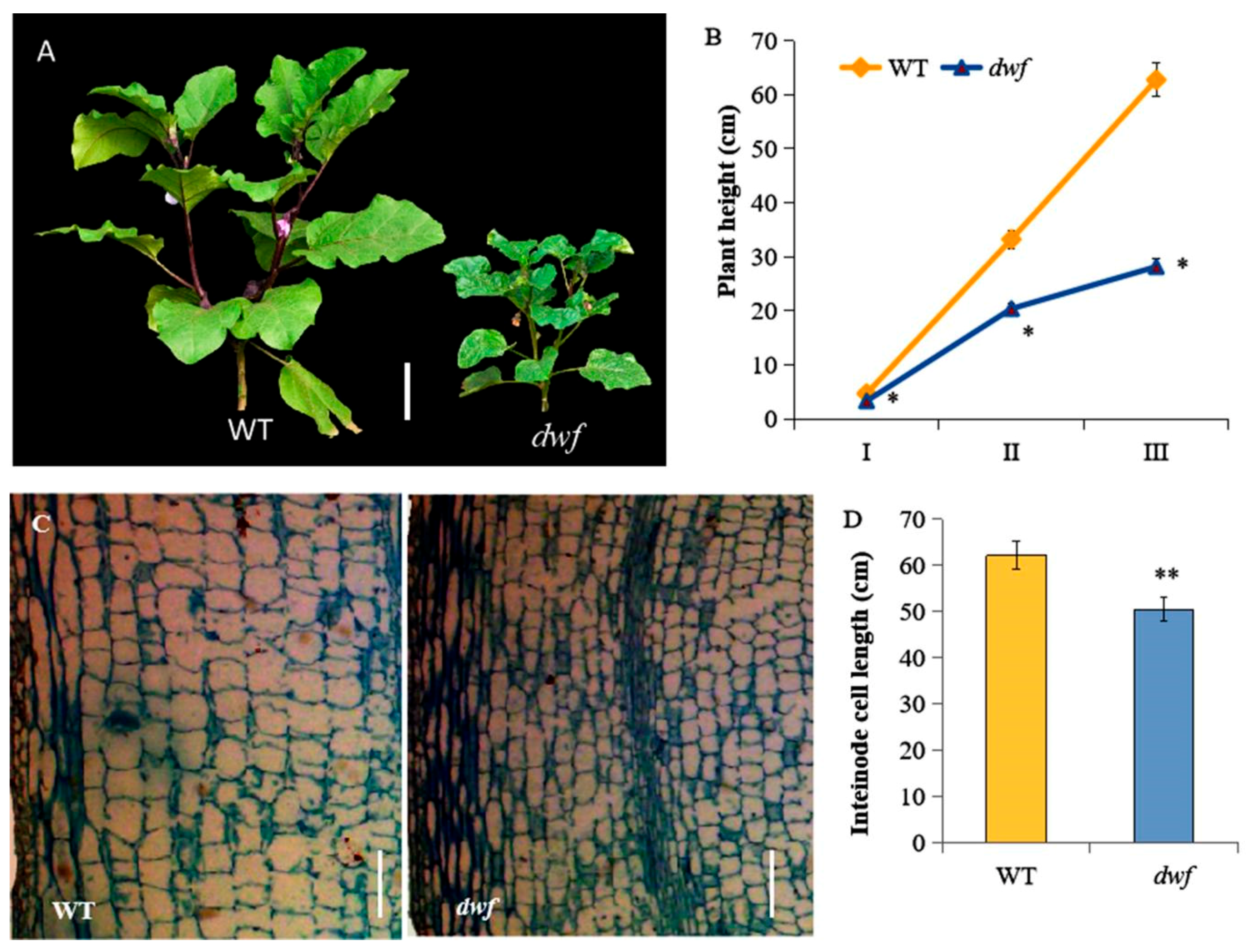
2.2. Plant Height Measurement
2.3. Histological Analysis
2.4. Hormone Assay
2.5. RNA-seq Analysis
2.6. Validation of Selected DEGs Using Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
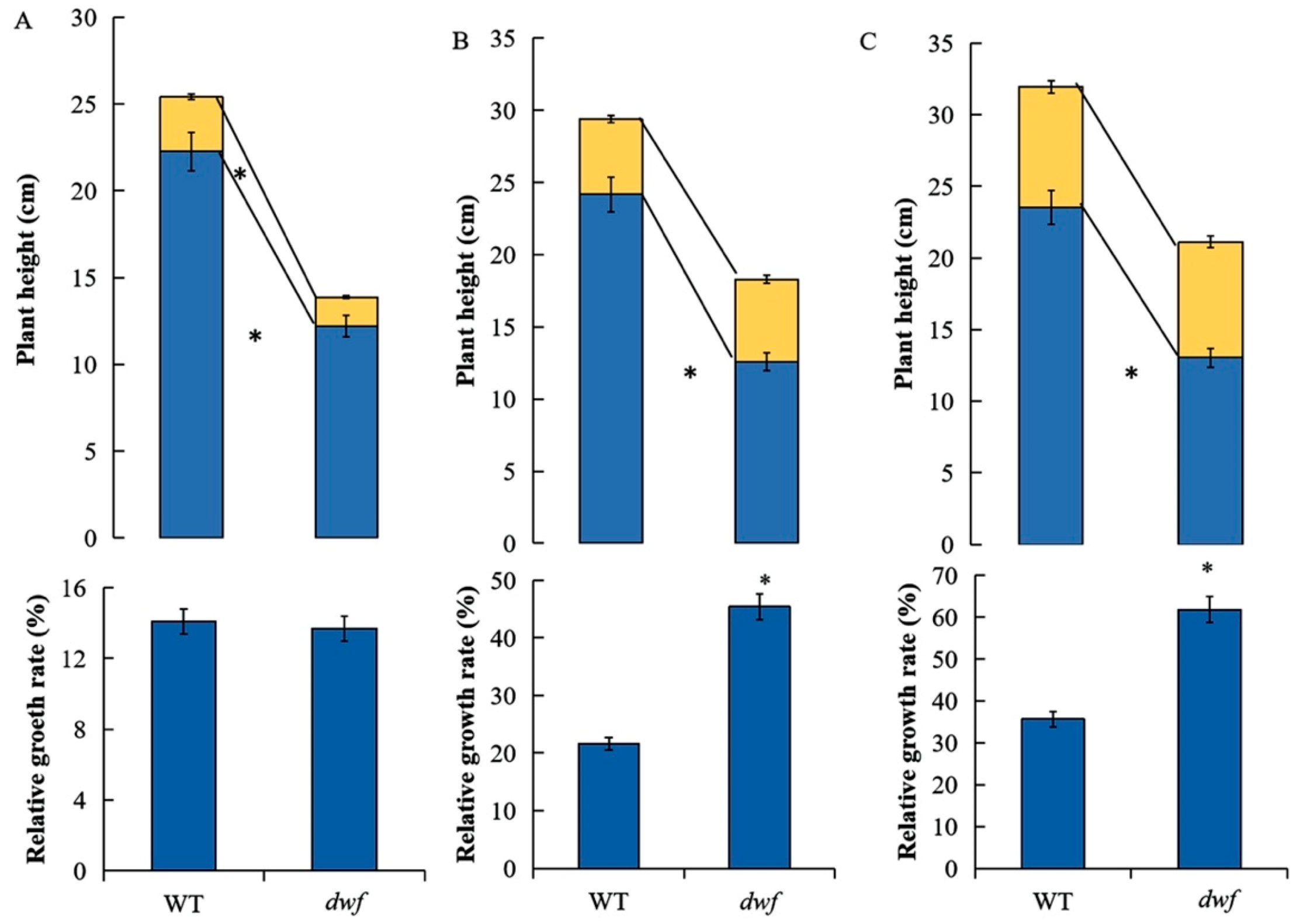
2.7. Exogenous GA3 Treatments
2.8. Statistical Analysis
3. Results
3.1. Phenotypic Comparison of the dwf Mutant and WT
3.2. Analysis of the Contents of GA3, IAA, BR, and ZR
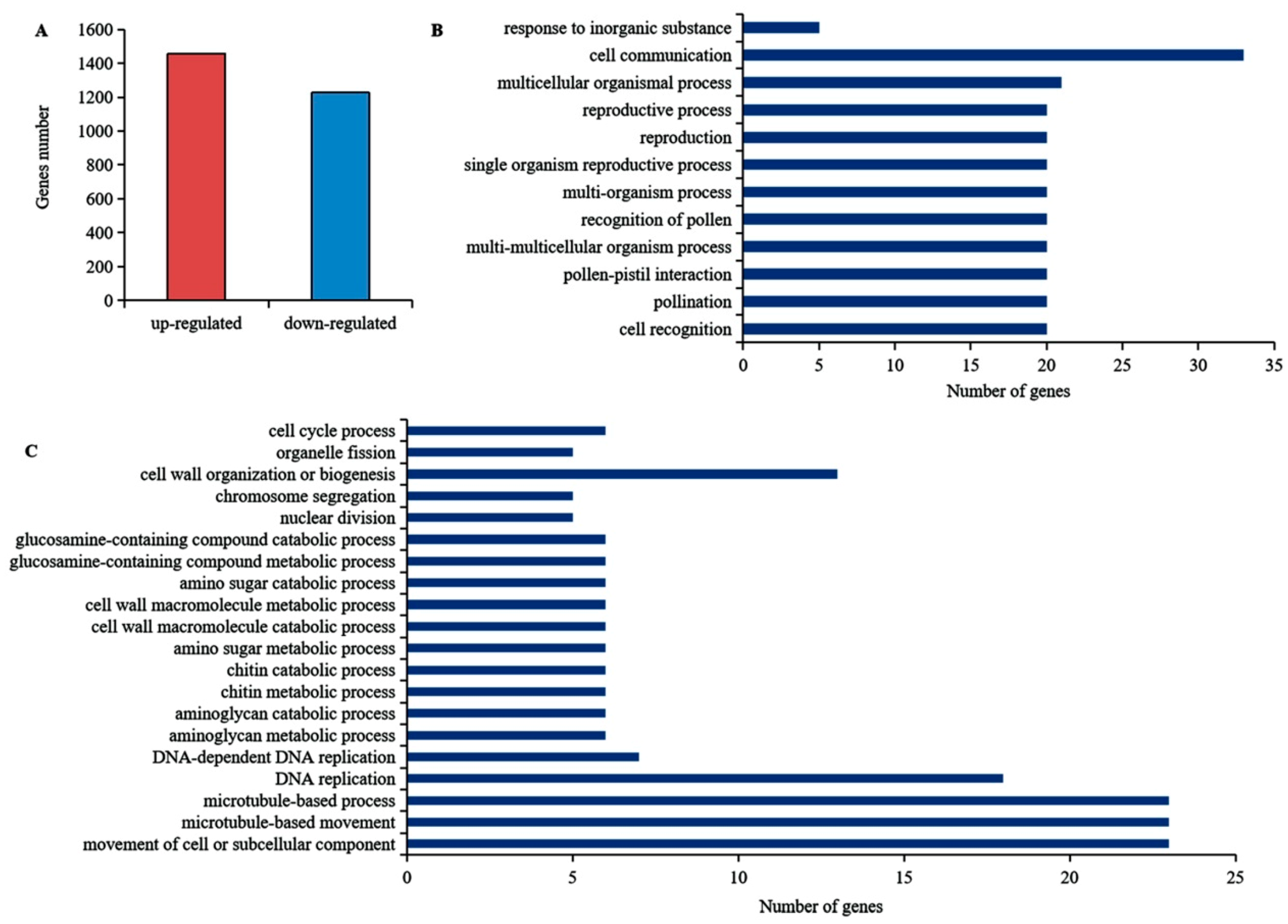
3.3. DEGs and GO Enrichment Analysis
3.4. DEGs in GA Biosynthesis and Signal Transduction Pathways
3.5. The Effects of Exogenous GA3 on dwf and WT Plant Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: http://www.fao.org/faostat/en/#data (accessed on 10 December 2020).
- Hedden, P. The genes of the green revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Peng, J.R.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. A mutant gibberellin-synthesis gene in rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.A.; Busov, V.B.; Kosola, K.R.; Ma, C.; Etherington, E.; Shevchenko, O.; Gandhi, H.; Pearce, D.W.; Rood, S.B.; Strauss, S.H. Green Revolution Trees: Semi-dwarfism transgenes modify gibberellins, promote root growth, enhance morphological diversity, and reduce competitiveness in hybrid poplar. Plant Physiol. 2012, 160, 1130–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Q.J.; Li, C.D.; Shang, Y.; Zhu, J.H.; Hua, W.; Wang, J.M.; Yang, J.M.; Zhang, G.P. Molecular characterization and functional analysis of barley semi-dwarf mutant Riso no. 9265. BMC Genom. 2015, 16, 927–938. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.R.; Liu, L.; Zhi, X.N.; Bai, J.R.; Cui, Y.N.; Shu, J.S.; Li, J.M. Genetic analysis of tomato internode length via mixed major gene plus polygene inheritance model. Sci. Hortic. 2019, 246, 759–764. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Depuydt, S.; Hardtke, C.S. Hormone signaling crosstalk in plant growth regulation. Curr. Biol. 2011, 21, 365–373. [Google Scholar] [CrossRef]
- Hu, S.L.; Wang, C.L.; Sanchez, D.L.; Lipka, A.E.; Liu, P.; Yin, Y.H.; Blanco, M.; Lübberstedt, T. Gibberellins promote brassinosteroids action and both increase heterosis for plant height in maize (Zea mays L.). Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M.M.; Su, H.N.; Liu, X.; Yang, L.M.; Zhang, Y.Y.; Wang, Y.; Fang, Z.Y.; Lv, H.H. Morphological, transcriptomics and phytohormone analysis shed light on the development of a novel dwarf mutant of cabbage (Brassica oleracea). Plant Sci. 2020, 290, 110283. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Tatsumi, T.; Sakamoto, T.; Otomo, K.; Toyomasu, T.; Kitano, H.; Ashikari, M.; Ichihara, S.; Matsuoka, M. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol. Biol. 2004, 54, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Koshioka, M.; Kojima, K.; Yoshida, H.; Kawata, M. A role of OsGA20ox1, encoding an isform of gibberellin 20-oxidase, for regulation of plant stature in rice. Plant Mol. Biol. 2004, 55, 687–700. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, M.M.; Liu, L.J.; Wu, S.; Shen, Y.; Ishiyama, K.; Kobayashi, M.; McCarty, D.R.; Tan, B.C. The maize DWARF1 encodes a gibberellin 3-oxidase and is dual localized to the nucleus and cytosol. Plant Physiol. 2014, 166, 2028–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, H.; Ueguchi-Tanaka, M.; Sentoku, N.; Kitano, H.; Matsuoka, M.M.K. Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 8909–8914. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.X.; Tao, Y.B.; Xu, Z.F. Overexpression of jatropha gibberellin 2-oxidase 6 GA2ox6) induces dwarfism and smaller leaves, flowers and fruits in Arabidopsis and Jatropha. Front. Plant Sci. 2017, 8, 2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.X.; Yang, W.L.; Li, S.J.; Liu, D.C.; Guo, X.L.; Sun, J.Z.; Zhang, A.M. Molecular characterization of three GIBBERELLIN-INSENSITIVE DWARF1 homologous genes in hexaploid wheat. J. Plant Physiol. 2013, 170, 432–443. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.M.; Tan, B.; Jiang, Y.J.; Zheng, X.B.; Ye, X.; Guo, Z.J.; Xiong, T.T.; Wang, W.; Li, J.D.; et al. A signal nucleotide mutation in GID1c disrupts its interaction with DELLA1 and causes a GA-insensitive dwarf phenotype in peach. Plant Biotechnol. J. 2019, 17, 1723–1735. [Google Scholar] [CrossRef] [Green Version]
- Piao, R.H.; Chu, S.H.; Jiang, W.Z.; Yu, Y.Y.; Jin, Y.M.; Woo, M.; Lee, J.; Kim, S.; Koh, H. Isolation and characterization of a dominant dwarf gene, d-h, in Rice. PLoS ONE 2014, 9, e86210. [Google Scholar] [CrossRef]
- Achard, P.; Gusti, A.; Cheminant, S.; Alioua, M.; Dhondt, S.; Coppens, F.; Beemster, G.T.S.; Genschik, P. Gibberellin signaling control cell proliferation rate in Arabidopsis. Curr. Boil. 2009, 19, 1188–1193. [Google Scholar] [CrossRef]
- Arro, J.; Yang, Y.Z.; Song, G.Q.; Zhong, G.Y. RNA-Seq reveals new DELLA targets and regulation in transgenic GA-insensitive grapevines. BMC Plant Biol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Harberd, N.P.; Belfifield, E.; Yasumura, Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flflexible response to flfluctuating environments. Plant Cell 2009, 21, 1328–1339. [Google Scholar] [CrossRef] [Green Version]
- Itoh, H.; Sasaki, A.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Hasegawa, Y.; Minami, E.; Ashikari, M.; Matsuoka, M. Dissection of the phosphorylation of rice DELLA protein, SLENDER RICE1. Plant Cell Physiol. 2005, 46, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Richards, D.E.; Ait-Ali, T.; Hynes, L.W.; Ougham, H.; Peng, J.; Harberd, N.P. Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 2002, 14, 3191–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera, E.; Ruiz-Rivero, O.; Peres, L.E.P.; Atares, A.; Garcia-Martinez, J.L. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012, 160, 1581–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, H.M.; Li, C.; Duan, Y.H.; Wei, L.B.; Ju, M.; Zhang, H.Y. Identification of a Sidwf1 gene controlling short internode length trait in the sesame dwarf mutant dw607. Theor. Appl. Genet. 2020, 133, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Zhang, H.Q.; Fan, M.; He, Y.J.; Guo, P.A. A mutation in the intron splice acceptor site of a GA3ox gene confers dwarf architecture in watermelon (Citrullus lanatus L.). Sci. Rep. 2020, 10, 14915. [Google Scholar] [CrossRef]
- Hirakawa, H.; Shirasawa, K.; Miyatake, K.; Nunome, T.; Negoro, S.; Ohyama, A.; Yamaguchi, H.; Sato, S.; Isobe, S.; Tabata, S.; et al. Draft genome sequence of eggplant (Solanum melongena L.): The representative solanum species indigenous to the old world. DNA Res. 2014, 21, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Deng, A.X.; Tan, W.M.; He, S.P.; Liu, W.; Nan, T.G.; Li, Z.H.; Wang, B.M.; Li, Q.X. Monoclonal antibody-based enzyme linked immunosorbent assay for the analysis of jasmonates in plants. J. Integr. Plant Biol. 2008, 50, 1046–1052. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomicfeatures. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Tarazona, S.; García-Alcalde, F.; Dopazo, J.; Ferrer, A.; Conesa, A. Differential expression in RNA-seq: A matter of depth. Genome Res. 2011, 21, 2213–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbierato, V.; Sala, T.; Rinaldi, P.; Bassolino, L.; Barchi, L.; Rotino, G.L.; Toppino, L. A spiking strategy facilitates housekeeping selection for RT-qPCR analysis under different biotic stresses in eggplant. Protoplasma 2017, 254, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Annu. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Yang, H.L.; Fang, Y.S.; Guo, W.; Chen, H.F.; Zhang, X.J.; Dai, W.J.; Chen, S.L.; Hao, Q.N.; Yuan, S.L.; et al. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef]
- Li, C.; Tang, J.; Hu, Z.Y.; Wang, J.W.; Yu, T.; Yi, H.Y.; Cao, M.J. A novel maize dwarf mutant generated by Ty1-copia LTR-retrotransposon insertion in Brachytic2 after spaceflight. Plant Cell Rep. 2020, 39, 393–408. [Google Scholar] [CrossRef]
- Sun, L.H.; Yang, W.L.; Li, Y.F.; Shan, Q.Q.; Ye, X.B.; Wang, D.Z.; Yu, K.; Lu, W.W.; Xin, P.Y.; Pei, Z.; et al. A wheat dominant dwarfing line with Rht12, which reduces stem cell length and affects gibberellic acid synthesis, is a 5AL terminal deletion line. Plant J. 2019, 97, 887–900. [Google Scholar] [CrossRef]
- Lee, J.; Moon, S.; Jang, S.; Lee, S.; An, S.; Jung, K.-H.; Park, S.K. OsbHLH073 Negatively Regulates Internode Elongation and Plant Height by Modulating GA Homeostasis in Rice. Plants 2020, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Zhao, J.; Lu, W.J.; Deng, D.X. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.L.; Xu, N.; Wu, Q.; Yu, B.; Li, X.X.; Chen, R.R.; Huang, J.L. Rice transcription factor OsMADS57 regulates plant height by modulating gibberellin catabolism. Rice 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomburg, F.M.; Bizzell, C.M.; Lee, D.J.; Zeevaart, J.A.D.; Amasino, R.M. Overexpression of a novel class of gibberellin 2-Oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 2002, 15, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magome, H.; Yamaguchi, S.; Hanada, A.; Kamiya, Y.; Oda, K. The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J. 2008, 56, 613–626. [Google Scholar] [CrossRef]
- Huang, J.; Tang, D.; Shen, Y.; Qin, B.X.; Hong, L.L.; You, A.Q.; Li, M.; Wang, X.; Yu, H.X.; Gu, M.H.; et al. Activation of gibberellin 2-oxidase 6 decreases active gibberellin levels and creates a dominant semi-dwarf phenotype in rice (Oryza sativa L.). JGG 2010, 37, 23–36. [Google Scholar] [CrossRef]
- Sun, X.R.; Shu, J.S.; Mohamed, A.M.A.; Deng, X.B.; Zhi, X.N.; Bai, J.R.; Cui, Y.N.; Lu, X.X.; Du, Y.C.; Wang, X.X.; et al. Identification and characterization of EI (Elongated Internode) gene in tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2019, 20, 2204. [Google Scholar] [CrossRef] [Green Version]
- Bishop, G.J.; Nomura, T.; Yokota, T.; Harrison, K.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Jones, J.D.G.; Kamiya, Y. The tomato DWARF enzyme catalyzes C-6 oxidation in brassinosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1761–1766. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.H.; Gururani, M.A.; Lee, J.W.; Ahn, B.O.; Chun, S.C. Isolation and characterisation of a dwarf rice mutant exhibiting defective gibberellins biosynthesis. Plant Biol. 2014, 16, 428–443. [Google Scholar] [CrossRef]
- Chen, X.S.; Di, J.C.; Xu, N.Y.; Xiao, S.H.; Liu, J.G. The inheritance of an ultra -dwarf plant mutant from upland cotton. Hereditas 2007, 29, 471–498. [Google Scholar] [CrossRef]
- Willige, B.C.; Ghosh, S.; Nill, C.; Zourelidou, M.; Dohmann, E.M.N.; Maier, A.; Schwechheimer, C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 2007, 19, 1209–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawit, S.J.; Wych, H.M.; Xu, D.P.; Kundu, S.; Tomes, D.T. Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development. Plant Cell Physiol. 2010, 51, 1854–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, T.; Miura, K.; Itoh, H.; Tatsumi, T.; Ueguchi-tanaka, M.; Ishiyama, K.; Kobayashi, M.; Agrawal, G.K.; Takeda, S.; Abe, K.; et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004, 134, 1642–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.G.; Tang, D.; Liu, K.; Miao, C.B.; Zhuo, X.X.; Li, Y.F.; Tan, X.L.; Sun, M.F.; Luo, Q.; Cheng, Z.K. Characterization of a new semi-dominant dwarf allele of SLR1 and its potential application in hybrid rice breeding. J. Exp. Bot. 2018, 69, 4703–4713. [Google Scholar] [CrossRef] [PubMed]
- Davière, J.M.; Lucas, M.D.; Prat, S. Transcriptional factor interaction: A central step in DELLA function. Curr. Opin. Genet. Dev. 2008, 18, 295–303. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [Green Version]


| Sample Name | dwf-1 | dwf-2 | dwf-3 | WT-1 | WT-2 | WT-3 |
|---|---|---|---|---|---|---|
| Raw reads | 58,570,416 | 56,724,560 | 55,828,514 | 48,299,032 | 48,653,906 | 49,359,746 |
| Clean reads | 57,610,860 | 54,994,770 | 54,936,474 | 47,392,084 | 47,418,858 | 48,711,482 |
| Mapped reads | 52,295,231 | 49,904,306 | 49,978,934 | 42,883,337 | 42,975,644 | 44,637,625 |
| Ratio of mapped reads (%) | 90.77 | 90.74 | 90.98 | 90.49 | 90.63 | 91.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Luo, S.; Li, Q.; Li, N.; Du, W.; Yu, P.; Wang, X.; Zhang, W.; Xuan, S.; Zhou, X.; et al. Phenotypic Characterization and Differential Gene Expression Analysis Reveal That Dwarf Mutant dwf Dwarfism Is Associated with Gibberellin in Eggplant. Horticulturae 2021, 7, 114. https://doi.org/10.3390/horticulturae7050114
Lu Y, Luo S, Li Q, Li N, Du W, Yu P, Wang X, Zhang W, Xuan S, Zhou X, et al. Phenotypic Characterization and Differential Gene Expression Analysis Reveal That Dwarf Mutant dwf Dwarfism Is Associated with Gibberellin in Eggplant. Horticulturae. 2021; 7(5):114. https://doi.org/10.3390/horticulturae7050114
Chicago/Turabian StyleLu, Yang, Shuangxia Luo, Qiang Li, Na Li, Wenchao Du, Ping Yu, Xing Wang, Weiwei Zhang, Shuxin Xuan, Xuan Zhou, and et al. 2021. "Phenotypic Characterization and Differential Gene Expression Analysis Reveal That Dwarf Mutant dwf Dwarfism Is Associated with Gibberellin in Eggplant" Horticulturae 7, no. 5: 114. https://doi.org/10.3390/horticulturae7050114





