Basil as Secondary Crop in Cascade Hydroponics: Exploring Salinity Tolerance Limits in Terms of Growth, Amino Acid Profile, and Nutrient Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Plant Growth
2.3. Nutrient Element Analysis
2.4. Ascorbic Acid Content
2.5. Amino Acids
2.6. Statistics
3. Results and Discussion
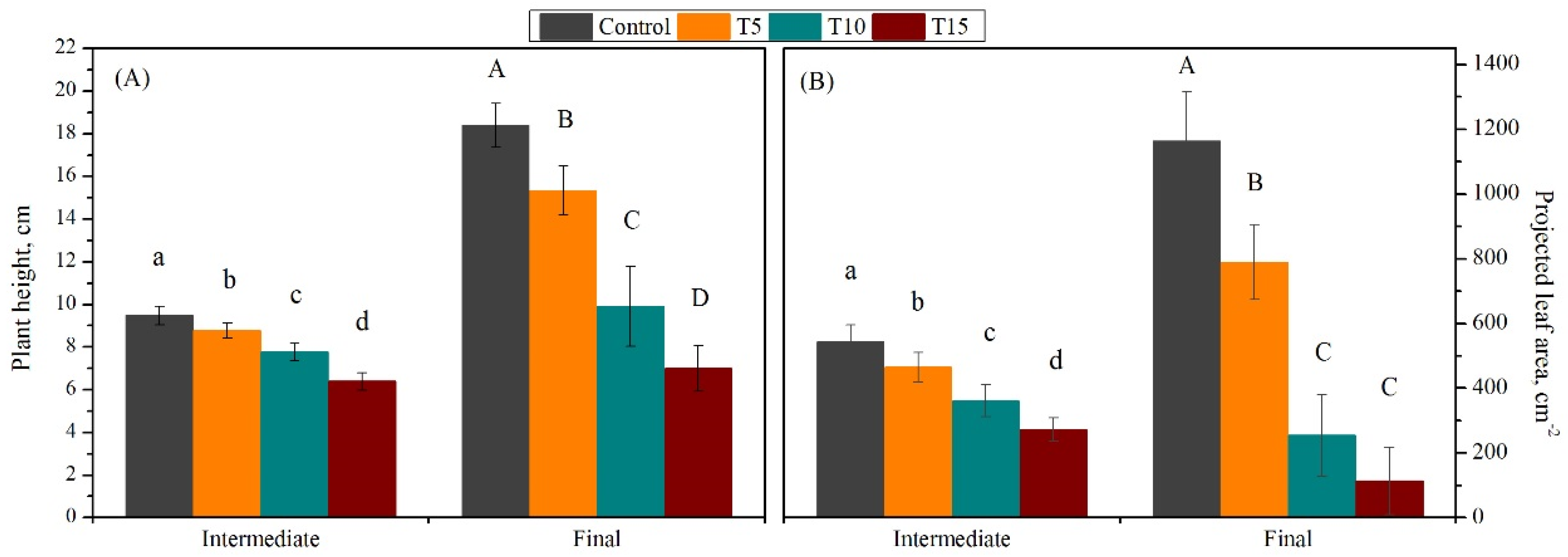
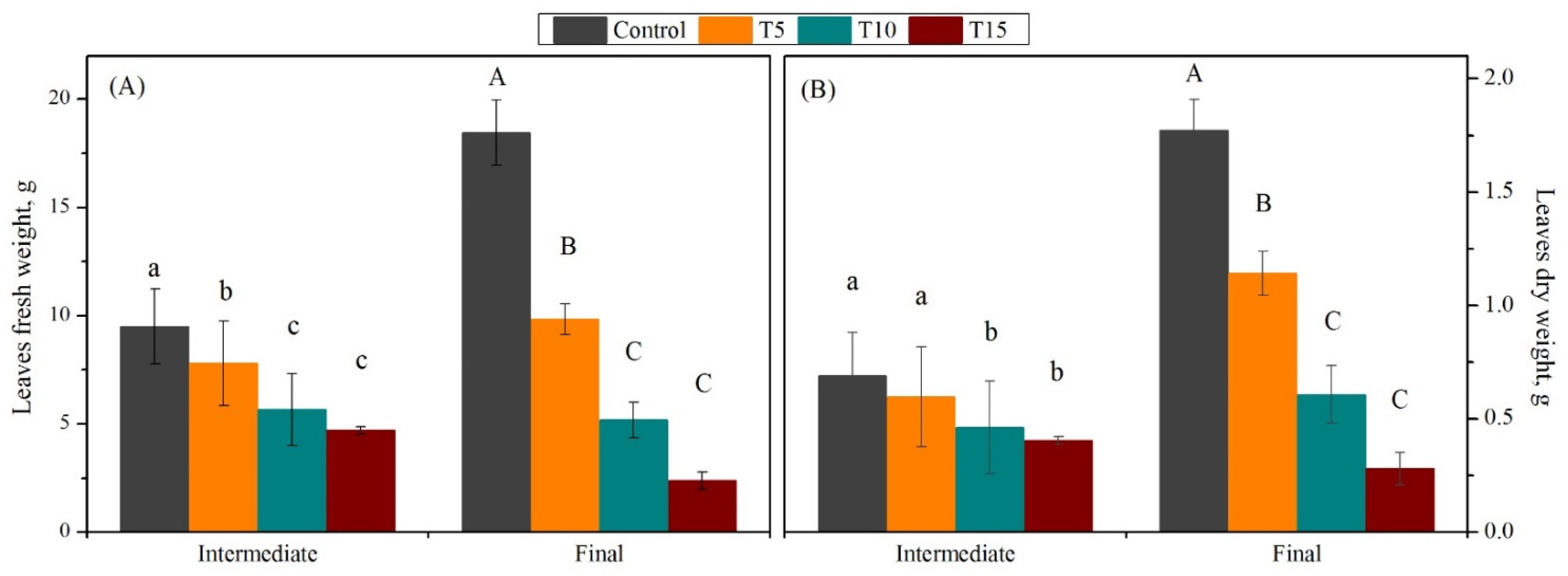
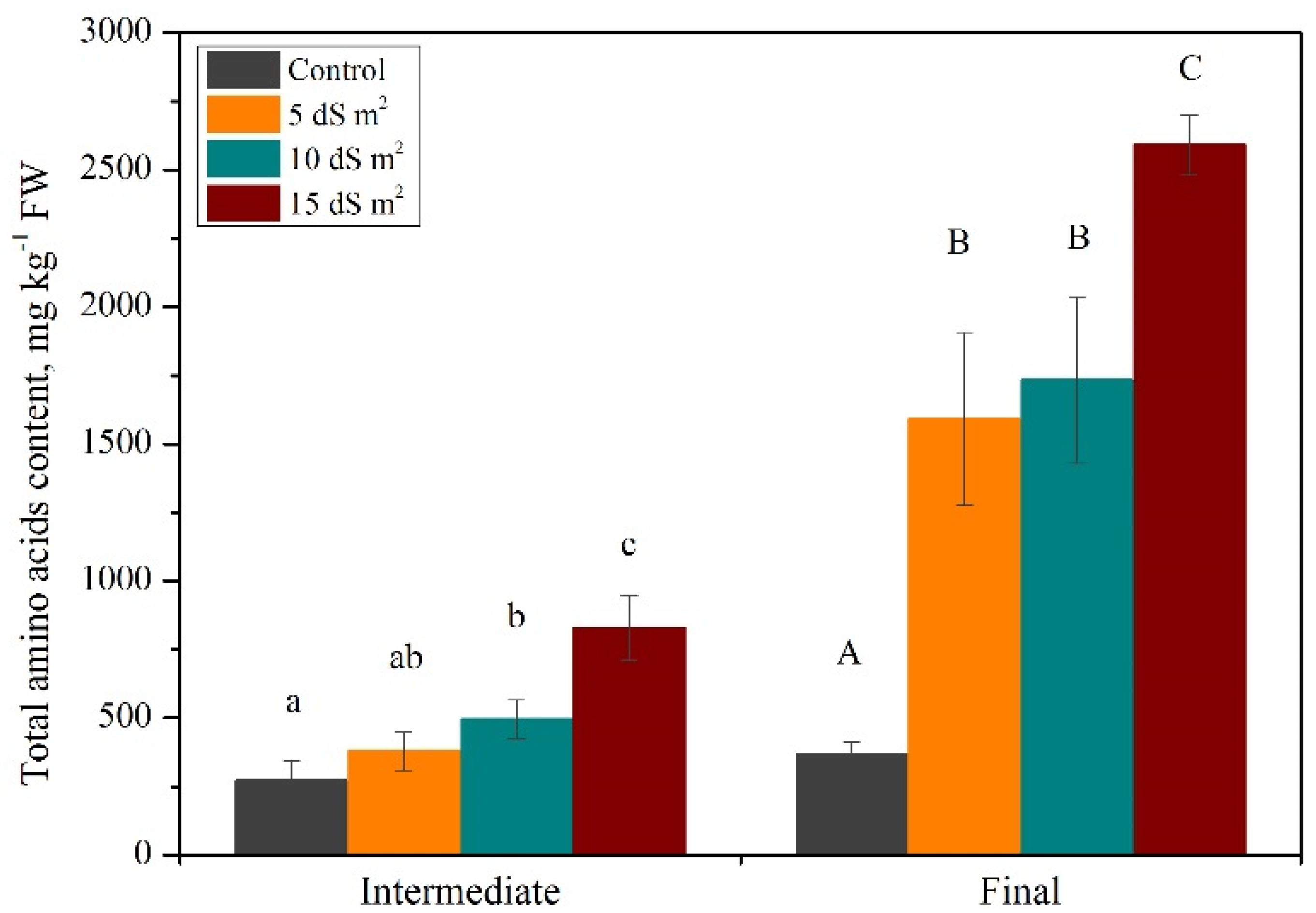
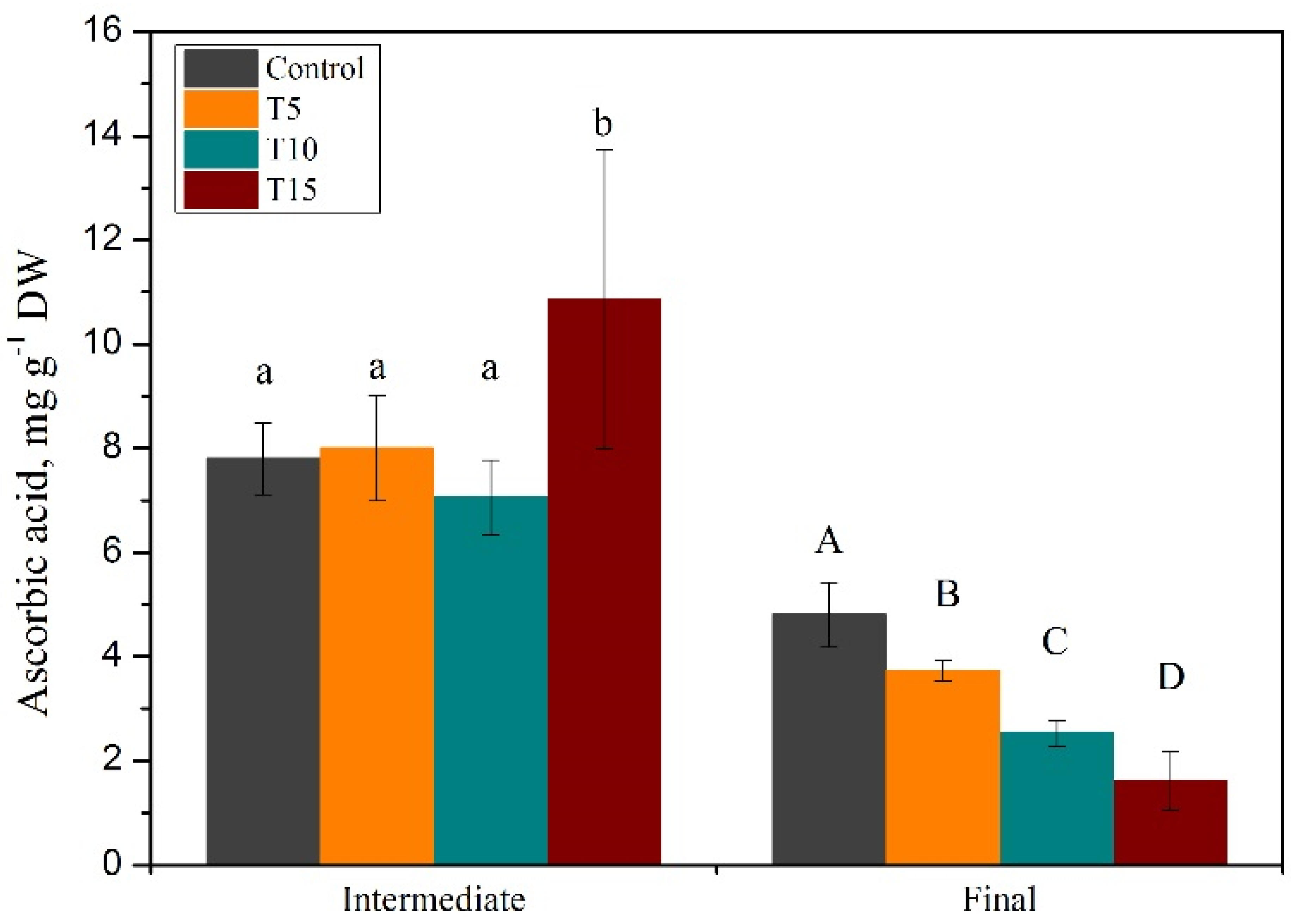
3.1. Exploration of Salinity-Tolerance Thresholds of Basil
3.2. Is Basil Suitable as a Secondary Crop in a Cascade Hydroponics System?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Plaut, Z.; Edelstein, M.; Ben-Hur, M. Overcoming Salinity Barriers to Crop Production Using Traditional Methods. Crit. Rev. Plant Sci. 2013, 32, 250–291. [Google Scholar] [CrossRef]
- Katsoulas, N.; Voogt, W. Recent trends in salinity control for soilless growing systems managemenT. Acta Hortic. 2014, 433–442. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M.; Al-Qurainy, F.; Harris, P.J.C. Salt Tolerance in Selected Vegetable Crops. Crit. Rev. Plant Sci. 2012, 31, 303–320. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables—A review. Eur. J. Hortic. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Elvanidi, A.; Reascos, C.B.; Gourzoulidou, E.; Kunze, A.; Max, J.; Katsoulas, N. Implementation of the Circular Economy Concept in Greenhouse Hydroponics for Ultimate Use of Water and Nutrients. Horticulturae 2020, 6, 83. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; Llanderal, A.; Maksimovic, I.; Lao, M.T. Cascade Cropping System with Horticultural and Ornamental Plants under Greenhouse Conditions. Water 2018, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Katsoulas, N.; Elvanidi, A.; Zagari, E.; Bartzanas, T.; Kalaitzis, P.; Kittas, C. Detection of salinity stress in soilless tomato based on crop reflectance. Acta Hortic. 2019, 1242, 723–728. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from crop plants struggling with salinity. Plant Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Tarchoune, I.; Degl’Innocenti, E.; Kaddour, R.; Guidi, L.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Effects of NaCl or Na2SO4 salinity on plant growth, ion content and photosynthetic activity in Ocimum basilicum L. Acta Physiol. Plant. 2011, 34, 607–615. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M. Enhancing Quality of Fresh Vegetables Through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Bahcesular, B. Seed Priming with Melatonin Effects on Growth, Essential Oil Compounds and Antioxidant Activity of Basil (Ocimum Basilicum L.) under Salinity Stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crop. Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Papenbrock, J. Changes in secondary metabolites in the halophytic putative crop species Crithmum maritimum L., Triglochin maritima L. and Halimione portulacoides (L.) Aellen as reaction to mild salinity. PLoS ONE 2017, 12, e0176303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruda, N. Do Soilless Culture Systems Have an Influence on Product Quality of Vegetables? J. Appl. Bot. Food Qual. 2009, 82, 141–147. [Google Scholar]
- Tanaka, H.; Yamada, S.; Masunaga, T.; Yamamoto, S.; Tsuji, W.; Murillo-Amador, B. Comparison of nutrient uptake and antioxidative response among four Labiatae herb species under salt stress condition. Soil Sci. Plant Nutr. 2018, 64, 589–597. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scagel, C.F.; Lee, J.; Mitchell, J.N. Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind. Crop. Prod. 2019, 127, 119–128. [Google Scholar] [CrossRef]
- Neto, A.D.D.A.; Menezes, R.V.; Gheyi, H.R.; Silva, P.C.C.; Cova, A.M.W.; Ribas, R.F.; Ribeiro, M.D.O. Salt-induced changes in solutes, pigments and essential oil of two basil (Ocimum basilicum L.) genotypes under hydroponic cultivation. Aust. J. Crop. Sci. 2019, 13. [Google Scholar] [CrossRef]
- Pegg, R.B.; Amarowicz, R.; Naczk, M.; Shahidi, F. PHOTOCHEM® for Determination of Antioxidant Capacity of Plant Extracts. In Antioxidant Measurement and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; Volume 956, pp. 140–158. ISBN 978-0-8412-7428-0. [Google Scholar]
- Elhindi, K.M.; Al-Amri, S.M.; Abdel-Salam, E.; Al-Suhaibani, N.A. Effectiveness of salicylic acid in mitigating salt-induced adverse effects on different physio-biochemical attributes in sweet basil (Ocimum basilicum L.). J. Plant Nutr. 2017, 40, 908–919. [Google Scholar] [CrossRef]
- Gohari, G.; Alavi, Z.; Esfandiari, E.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming ofOcimum basilicumL. against salt stress. Physiol. Plant. 2019, 168, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, H.; Ouhibi, C.; Ellili, A.; Msilini, N.; Bouzaïen, G.; Karray, N.; Lachaâl, M. Analysis of salinity effects on basil leaf surface area, photosynthetic activity, and growth. Acta Physiol. Plant. 2010, 33, 823–833. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Ouerghi, Z.; Navari-Izzo, F. Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. 2010, 48, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, O.; Kurt, D.; Temizel, K.E.; Odabas, M.S. Effect of Salt Stress and Irrigation Water on Growth and Development of Sweet Basil (Ocimum basilicum L.). Open Agric. 2017, 2, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Berger, B.; de Regt, B.; Tester, M. Trait Dissection of Salinity Tolerance with Plant Phenomics. In Plant Salt Tolerance: Methods and Protocols; Shabala, S., Cuin, T.A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 399–413. ISBN 978-1-61779-986-0. [Google Scholar]
- Awlia, M.; Nigro, A.; Fajkus, J.; Schmoeckel, S.M.; Negrao, S.; Santelia, D.; Trtílek, M.; Tester, M.; Julkowska, M.M.; Panzarová, K. High-Throughput Non-destructive Phenotyping of Traits that Contribute to Salinity Tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morano, G.; Amalfitano, C.; Sellitto, M.; Cuciniello, A.; Maiello, R.; Caruso, G. Effects of Nutritive Solution Electrical Conductivity and Plant Density on Growth, Yield and Quality of Sweet Basil Grown in Gullies by Subirrigation. Adv. Hortic. Sci. 2017, 31, 25–30. [Google Scholar] [CrossRef]
- Barbieri, G.; Vallone, S.; Orsini, F.; Paradiso, R.; De Pascale, S.; Zakharov, F.; Maggio, A. Stomatal density and metabolic determinants mediate salt stress adaptation and water use efficiency in basil (Ocimum basilicum L.). J. Plant Physiol. 2012, 169, 1737–1746. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Bastías, E.I.; González-Murua, C.; González-Moro, M.B. Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea. Plants 2020, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Oddy, J.; Raffan, S.; Wilkinson, M.; Elmore, J.S.; Halford, N.G. Stress, nutrients and genotype: Understanding and managing asparagine accumulation in wheat grain. CABI Agric. Biosci. 2020, 1, 1–14. [Google Scholar] [CrossRef]
- Neocleous, D.; Ntatsi, G. Seasonal variations of antioxidants and other agronomic features in soilless production of selected fresh aromatic herbs. Sci. Hortic. 2018, 234, 290–299. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.; Grieve, C. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bryla, D.; Lee, J. Salt Exclusion and Mycorrhizal Symbiosis Increase Tolerance to NaCl and CaCl2 Salinity in ‘Siam Queen’ Basil. HortScience 2017, 52, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Maathuis, F.J.M. The role of monovalent cation transporters in plant responses to salinity. J. Exp. Bot. 2005, 57, 1137–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and Seed Application of Amino Acids Affects the Antioxidant Metabolism of the Soybean Crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Joshi, M.; DiPiazza, J.; Joshi, V. Functional Relevance of Citrulline in the Vegetative Tissues of Watermelon During Abiotic Stresses. Front. Plant Sci. 2020, 11, 512. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Espino, C.; Lao, M. Comparative Behavior of Dracaena marginata Plants Integrated into a Cascade Cropping System with the Addition of Hydrogen Peroxide. Agronomy 2021, 11, 218. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Velasquez, C.; Lao, M. Water and Nutrient Balance in an Ornamental Cascade Cropping System. Agronomy 2021, 11, 1251. [Google Scholar] [CrossRef]
- Plaza, B.M.; Soriano, F.; Jiménez-Becker, S.; Lao, M.T. Nutritional responses of Cordyline fruticosa var. ‘Red Edge’ to fertigation with leachates vs. conventional fertigation: Chloride, nitrogen, phosphorus and sulphate. Agric. Water Manag. 2016, 173, 61–66. [Google Scholar] [CrossRef]



| Control | T5 | T10 | T15 | |
|---|---|---|---|---|
| NO3− | 13.6 | 47.4 | 93.4 | 140.8 |
| NH4+ | 5.4 | 14.8 | 29.2 | 44.0 |
| Ca2+ | 1.7 | 11.8 | 23.3 | 35.2 |
| P | 0.2 | 1.2 | 2.4 | 3.6 |
| K+ | 1.7 | 5.4 | 10.6 | 15.9 |
| Mg2+ | 0.4 | 1.9 | 3.7 | 5.6 |
| S | 0.4 | 2.4 | 4.7 | 7.0 |
| Fe | 12.3 | 31.6 | 62.3 | 94.0 |
| Cu | 1.5 | 4.0 | 7.8 | 11.8 |
| Mn | 7.1 | 18.3 | 36.2 | 54.5 |
| Zn | 3.7 | 9.6 | 19.0 | 28.6 |
| B | 22.7 | 58.3 | 115.0 | 173.4 |
| Mo | 0.4 | 1.1 | 2.1 | 3.1 |
| Intermediate Harvest | Final Harvest | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | T5 | T10 | T15 | Control | T5 | T10 | T15 | |
| alanine | 15.18 ± 2.43 a | 19.54 ± 0.40 ab | 22.47 ± 3.41 b | 23.49 ± 3.19 b | 13.30 ± 2.9 AB | 17.41 ± 3.77 A | 13.31 ± 2.71 A | 9.87 ± 1.00 B |
| arginine | 48.55 ± 22.03 a | 81.53 ± 41.92 a | 96.92 ± 37.05 a | 258.26 ± 65.20 b | 85.02 ± 24.38 A | 742.44 ± 186.1 B | 790.47 ± 144.2 B | 1107.78 ± 37.4 C |
| asparagine | 15.02 ± 9.93 a | 14.80 ± 8.30 a | 9.07 ± 2.91 a | 31.50 ± 18.98 a | 51.87 ± 7.69 A | 171.59 ± 51.02 B | 220.80 ± 81.25 B | 428.67 ± 72.07 C |
| aspartic acid | 9.29 ± 2.08 a | 11.41 ± 1.41 a | 17.82 ± 1.58 b | 19.82 ± 1.99 b | 11.74 ± 1.14 A | 17.90 ± 3.40 B | 16.25 ± 2.9 AB | 15.04 ± 0.9 AB |
| b-alanine | 0.32 ± 0.12 ab | 0.31 ± 0.08 a | 0.24 ± 0.03 a | 0.55 ± 0.18 b | 0.43 ± 0.10 A | 1.34 ± 0.34 B | 1.50 ± 0.42 B | 2.47 ± 0.31 C |
| b-amino-isobutyric acid | 0.30 ± 0.07 ab | 0.28 ± 0.11 a | 0.51 ± 0.08 ab | 0.57 ± 0.24 b | 0.11 ± 0.02 A | 0.26 ± 0.06 B | 0.33 ± 0.19 C | 0.54 ± 0.22 D |
| citrulline | 19.58 ± 5.33 a | 26.55 ± 2.14 ab | 30.75 ± 2.08 b | 46.11 ± 7.56 c | 12.94 ± 3.69 A | 68.15 ± 24.34 B | 73.16 ± 16.20 B | 100.24 ± 2.00 B |
| g-aminobutyric acid | 5.14 ± 0.39 a | 10.71 ± 3.74 ab | 11.72 ± 4.17 b | 7.51 ± 1.89 ab | 9.19 ± 2.04 A | 13.43 ± 4.37 A | 8.86 ± 2.22 A | 6.60 ± 2.18 A |
| glutamic acid | 38.29 ± 5.87 a | 41.59 ± 4.05 a | 47.34 ± 8.24 a | 44.58 ± 7.38 a | 43.96 ± 5.08 A | 45.22 ± 11.56 A | 37.61 ± 7.18 A | 28.95 ± 2.37 A |
| glutamine | 71.27 ± 11.95 a | 117.16 ± 12.50 a | 190.17 ± 42.23 b | 292.82 ± 53.92 c | 66.09 ± 13.01 A | 343.46 ± 78.23 B | 387.21 ± 69.89 B | 654.43 ± 106.3 C |
| glycine | 4.60 ± 0.87 a | 7.53 ± 1.28 ab | 8.95 ± 0.46 ab | 13.91 ± 6.37 b | 1.29 ± 0.25 A | 7.62 ± 2.94 B | 8.50 ± 1.47 B | 3.10 ± 1.75 A |
| histidine | 8.93 ± 4.27 a | 10.19 ± 2.93 a | 15.22 ± 2.03 a | 27.52 ± 3.83 b | 18.56 ± 6.01 aA | 70.36 ± 15.64 B | 73.57 ± 13.66 B | 112.98 ± 6.42 C |
| isoleucine | 1.75 ± 0.61 a | 1.10 ± 0.75 a | 1.12 ± 0.15 a | 1.82 ± 0.32 a | 4.24 ± 0.37 A | 6.70 ± 0.91 B | 6.04 ± 1.7 AB | 4.81 ± 0.5 AB |
| leucine | 1.78 ± 0.68 ab | 1.46 ± 0.28 ab | 1.25 ± 0.21 a | 2.12 ± 0.24 b | 4.13 ± 0.45 A | 7.22 ± 0.93 B | 6.59 ± 1.78 B | 5.98 ± 0.8 AB |
| lysine | 3.03 ± 0.99 a | 3.51 ± 0.77 a | 3.20 ± 0.42 a | 5.71 ± 0.69 b | 4.89 ± 1.07 A | 19.49 ± 3.33 B | 19.96 ± 3.70 B | 29.02 ± 0.85 C |
| methionine | 0.13 ± 0.04 a | 0.10 ± 0.01 a | 0.19 ± 0.06 b | 0.21 ± 0.02 b | 0.13 ± 0.04 A | 0.21 ± 0.12 B | 0.25 ± 0.12 B | 0.77 ± 0.22 B |
| ornithine | 1.61 ± 0.38 a | 2.18 ± 0.99 b | 2.57 ± 0.67 b | 4.82 ± 0.82 b | 1.56 ± 0.27 A | 10.10 ± 2.66 B | 10.75 ± 3.53 B | 14.67 ± 2.35 B |
| phenylalanine | 2.54 ± 1.57 a | 1.71 ± 0.38 a | 1.25 ± 0.17 a | 1.70 ± 0.33 a | 5.23 ± 2.13 A | 5.94 ± 0.44 A | 5.88 ± 2.27 A | 7.07 ± 1.44 A |
| proline | 0.91 ± 0.23 a | 1.36 ± 0.37 a | 2.18 ± 0.64 a | 4.83 ± 1.36 b | 0.96 ± 0.70 A | 3.61 ± 1.3 B | 2.48 ± 0.9 AB | 2.63 ± 0.9 AB |
| serine | 10.30 ± 1.83 a | 13.21 ± 1.54 a | 19.81 ± 1.29 b | 23.45 ± 2.75 b | 8.92 ± 1.57 A | 22.47 ± 2.66 B | 17.51 ± 3.14 C | 20.30 ± 1.31 BC |
| threonine | 5.17 ± 1.43 a | 5.28 ± 0.31 a | 5.61 ± 0.47 ab | 7.01 ± 0.48 ab | 6.88 ± 0.22 A | 10.41 ± 0.95 B | 9.36 ± 1.82 B | 8.72 ± 0.8 AB |
| tryptophan | 4.44 ± 1.97 a | 3.29 ± 0.95 a | 3.01 ± 0.37 a | 4.00 ± 0.81 a | 7.85 ± 2.88 A | 9.50 ± 0.76 A | 7.97 ± 2.35 A | 10.33 ± 2.87 A |
| tyrosine | 1.33 ± 0.64 a | 1.04 ± 0.15 ab | 0.31 ± 0.13 b | 0.51 ± 0.18 b | 2.56 ± 0.35 A | 3.22 ± 0.52 A | 2.42 ± 0.91 A | 1.97 ± 0.11 A |
| valine | 3.56 ± 1.56 a | 3.04 ± 0.50 a | 3.37 ± 0.29 a | 5.54 ± 0.43 b | 7.66 ± 0.88 A | 13.77 ± 1.54 B | 13.11 ± 3.23 B | 14.19 ± 1.20 B |
| Intermediate Harvest | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | Fe (mg/kg) | Zn (mg/kg) | Mn (mg/kg) | Cu (mg/kg) |
| Control | 5.18 ± 0.16 a | 0.94 ± 0.05 a | 5.31 ± 0.29 a | 2.32 ± 0.16 a | 0.47 ± 0.04 a | 136.39 ± 13.59 a | 97.50 ± 4.98 a | 293.29 ± 23.55 a | 12.05 ± 1.12 ab |
| T5 | 5.62 ± 0.06 b | 0.88 ± 0.03 b | 5.12 ± 0.12 a | 2.62 ± 0.08 a | 0.43 ± 0.03 a | 143.01 ± 16.17 a | 100.49 ± 8.60 a | 323.06 ± 24.20 a | 13.54 ± 1.78 a |
| T10 | 5.56 ± 0.18 b | 0.70 ± 0.05 c | 4.23 ± 0.25 b | 4.02 ± 0.50 b | 0.57 ± 0.11 b | 132.82 ± 22.12 a | 100.69 ± 11.62 a | 462.32 ± 54.44 b | 11.23 ± 2.18 b |
| T15 | 5.71 ± 0.14 b | 0.58 ± 0.04 d | 4.09 ± 0.26 b | 4.76 ± 0.35 c | 0.51 ± 0.07 ab | 89.24 ± 7.42 b | 108.18 ± 13.89 a | 540.10 ± 90.89 c | 9.46 ± 1.66 b |
| Final Harvest | |||||||||
| Treatment | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | Fe (mg/kg) | Zn (mg/kg) | Mn (mg/kg) | Cu (mg/kg) |
| Control | 5.16 ± 0.14 A | 0.77 ± 0.02 A | 5.04 ± 0.28 A | 3.04 ± 0.18 A | 0.64 ± 0.05 A | 152.46 ± 11.62 A | 93.76 ± 4.80 A | 234.17 ± 19.15 A | 14.77 ± 2.32 A |
| T5 | 6.16 ± 0.18 B | 0.69 ± 0.03 B | 5.18 ± 0.21 A | 2.91 ± 0.17 A | 0.55 ± 0.04 B | 109.91 ± 14.74 B | 75.82 ± 6.58 B | 182.71 ± 17.37 B | 13.44 ± 1.79 A |
| T10 | 6.40 ± 2.86 B | 0.66 ± 0.30 B | 5.33 ± 2.38 A | 3.31 ± 1.48 A | 0.57 ± 0.26 AB | 108.20 ± 48.30 B | 90.81 ± 4.69 AC | 223.65 ± 100.18 AC | 13.49 ± 6.04 A |
| T15 | 6.49 ± 0.30 B | 0.67 ± 0.02 B | 4.92 ± 0.25 A | 3.28 ± 0.69 A | 0.59 ± 0.09 AB | 94.98 ± 28.05 B | 80.47 ± 2.52 BC | 186.65 ± 17.24 BC | 13.38 ± 0.53 A |
| Intermediate Harvest | Final Harvest | |||
|---|---|---|---|---|
| Control | Treated | Control | Treated | |
| alanine | 13.27 ± 1.88 a | 13.13 ± 3.39 a | 9.14 ± 0.71 A | 12.83 ± 2.69 B |
| arginine | 2.44 ± 0.64 a | 1.56 ± 0.64 b | 13.07 ± 3.65 A | 9.48 ± 16.25 A |
| asparagine | 2.48 ± 0.68 a | 1.97 ± 0.53 a | 11.41 ± 3.84 A | 8.86 ± 5.29 A |
| aspartic acid | 3.92 ± 0.40 a | 4.29 ± 1.30 a | 8.34 ± 1.44 A | 10.44 ± 2.54 A |
| b-alanine | 0.04 ± 0.00 a | 0.05 ± 0.02 a | 0.09 ± 0.04 A | 0.10 ± 0.04 A |
| b-amino-isobutyric acid | 0.03 ± 0.02 a | 0.13 ± 0.08 b | 0.05 ± 0.02 A | 0.09 ± 0.05 A |
| citrulline | 3.16 ± 0.51 a | 3.09 ± 0.96 a | 0.82 ± 0.22 A | 1.80 ± 1.32 A |
| g-aminobutyric acid | 9.18 ± 1.87 a | 6.88 ± 2.49 a | 8.72 ± 2.04 A | 6.61 ± 1.32 B |
| glutamic acid | 31.31 ± 1.29 a | 38.05 ± 6.52 b | 28.91 ± 3.74 A | 48.20 ± 7.30 B |
| glutamine | 25.49 ± 3.61 a | 22.67 ± 7.62 a | 30.87 ± 6.19 A | 31.28 ± 8.60 A |
| glycine | 2.09 ± 0.33 a | 1.83 ± 0.48 a | 0.84 ± 0.22 A | 1.80.51 A |
| histidine | 0.84 ± 0.20 a | 0.79 ± 0.16 a | 1.67 ± 0.47 A | 1.49 ± 0.74 A |
| isoleucine | 0.33 ± 0.05 a | 0.34 ± 0.08 a | 0.87 ± 0.21 A | 0.87 ± 0.37 A |
| leucine | 0.37 ± 0.04 a | 0.34 ± 0.07 a | 0.90 ± 0.23 A | 0.93 ± 0.41 A |
| lysine | 2.08 ± 0.48 a | 2.20 ± 0.98 a | 2.57 ± 0.54 A | 2.60 ± 0.68 A |
| methionine | 0.05 ± 0.01 a | 0.03 ± 0.02 b | 0.02 ± 0.02 A | 0.03 ± 0.02 A |
| ornithine | 0.30 ± 0.10 a | 0.39 ± 0.17 a | 0.13 ± 0.05 A | 0.19 ± 0.10 A |
| phenylalanine | 0.30 ± 0.03 a | 0.42 ± 0.09 b | 0.68 ± 0.18 A | 0.85 ± 0.38 A |
| proline | 0.37 ± 0.07 a | 0.38 ± 0.20 a | 0.27 ± 0.16 A | 0.42 ± 0.17 A |
| serine | 5.09 ± 0.64 a | 5.16 ± 1.37 a | 4.07 ± 0.63 A | 5.07 ± 1.31 A |
| threonine | 1.68 ± 0.10 a | 1.74 ± 0.36 a | 2.66 ± 0.35 A | 3.23 ± 0.76 A |
| tryptophan | 0.33 ± 0.13 a | 0.69 ± 0.35 b | 0.51 ± 0.16 A | 0.56 ± 0.29 A |
| tyrosine | 0.07 ± 0.04 a | 0.12 ± 0.10 a | 0.35 ± 0.18 A | 0.32 ± 0.15 A |
| valine | 1.11 ± 0.10 a | 1.27 ± 0.41 a | 1.73 ± 0.27 A | 1.93 ± 0.50 A |
| Intermediate Harvest | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | Fe (mg/kg) | Zn (mg/kg) | Mn (mg/kg) | Cu (mg/kg) |
| Control | 5.54 ± 0.64 a | 0.91 ± 0.21 a | 5.97 ± 0.45 a | 2.66 ± 0.68 a | 0.44 ± 0.13 a | 132.62 ± 30.72 a | 87.11 ± 7.02 a | 175.75 ± 17.69 a | 17.49 ± 2.93 a |
| Treated | 4.82 ± 0.32 b | 0.55 ± 0.05 b | 4.87 ± 0.65 b | 2.74 ± 0.23 a | 0.59 ± 0.09 b | 146.36 ± 25.92 a | 57.27 ± 8.31 b | 46.36 ± 10.19 b | 16.92 ± 2.87 a |
| Final Harvest | |||||||||
| Treatment | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | Fe (mg/kg) | Zn (mg/kg) | Mn (mg/kg) | Cu (mg/kg) |
| Control | 4.74 ± 0.13 A | 1.06 ± 0.05 A | 7.11 ± 0.46 A | 2.23 ± 0.13 A | 0.33 ± 0.02 A | 172.69 ± 30.86 A | 91.49 ± 5.46 A | 198.76 ± 10.19 A | 19.22 ± 2.21 A |
| Treated | 4.75 ± 0.17 A | 0.50 ± 0.05 B | 3.89 ± 0.46 B | 2.95 ± 0.14 B | 0.69 ± 0.06 B | 148.13 ± 17.79 B | 58.24 ± 4.61 B | 46.36 ± 12.01 B | 14.69 ± 2.42 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdouli, D.; Max, J.F.J.; Katsoulas, N.; Levizou, E. Basil as Secondary Crop in Cascade Hydroponics: Exploring Salinity Tolerance Limits in Terms of Growth, Amino Acid Profile, and Nutrient Composition. Horticulturae 2021, 7, 203. https://doi.org/10.3390/horticulturae7080203
Avdouli D, Max JFJ, Katsoulas N, Levizou E. Basil as Secondary Crop in Cascade Hydroponics: Exploring Salinity Tolerance Limits in Terms of Growth, Amino Acid Profile, and Nutrient Composition. Horticulturae. 2021; 7(8):203. https://doi.org/10.3390/horticulturae7080203
Chicago/Turabian StyleAvdouli, Denisa, Johannes F. J. Max, Nikolaos Katsoulas, and Efi Levizou. 2021. "Basil as Secondary Crop in Cascade Hydroponics: Exploring Salinity Tolerance Limits in Terms of Growth, Amino Acid Profile, and Nutrient Composition" Horticulturae 7, no. 8: 203. https://doi.org/10.3390/horticulturae7080203
APA StyleAvdouli, D., Max, J. F. J., Katsoulas, N., & Levizou, E. (2021). Basil as Secondary Crop in Cascade Hydroponics: Exploring Salinity Tolerance Limits in Terms of Growth, Amino Acid Profile, and Nutrient Composition. Horticulturae, 7(8), 203. https://doi.org/10.3390/horticulturae7080203







