Reliability of a Handheld Bluetooth Colourimeter and Its Application to Measuring the Effects of Time from Harvest, Row Orientation and Training System on Nectarine Skin Colour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of the Reliability of a Portable Colourimeter
2.1.1. Instrument Description
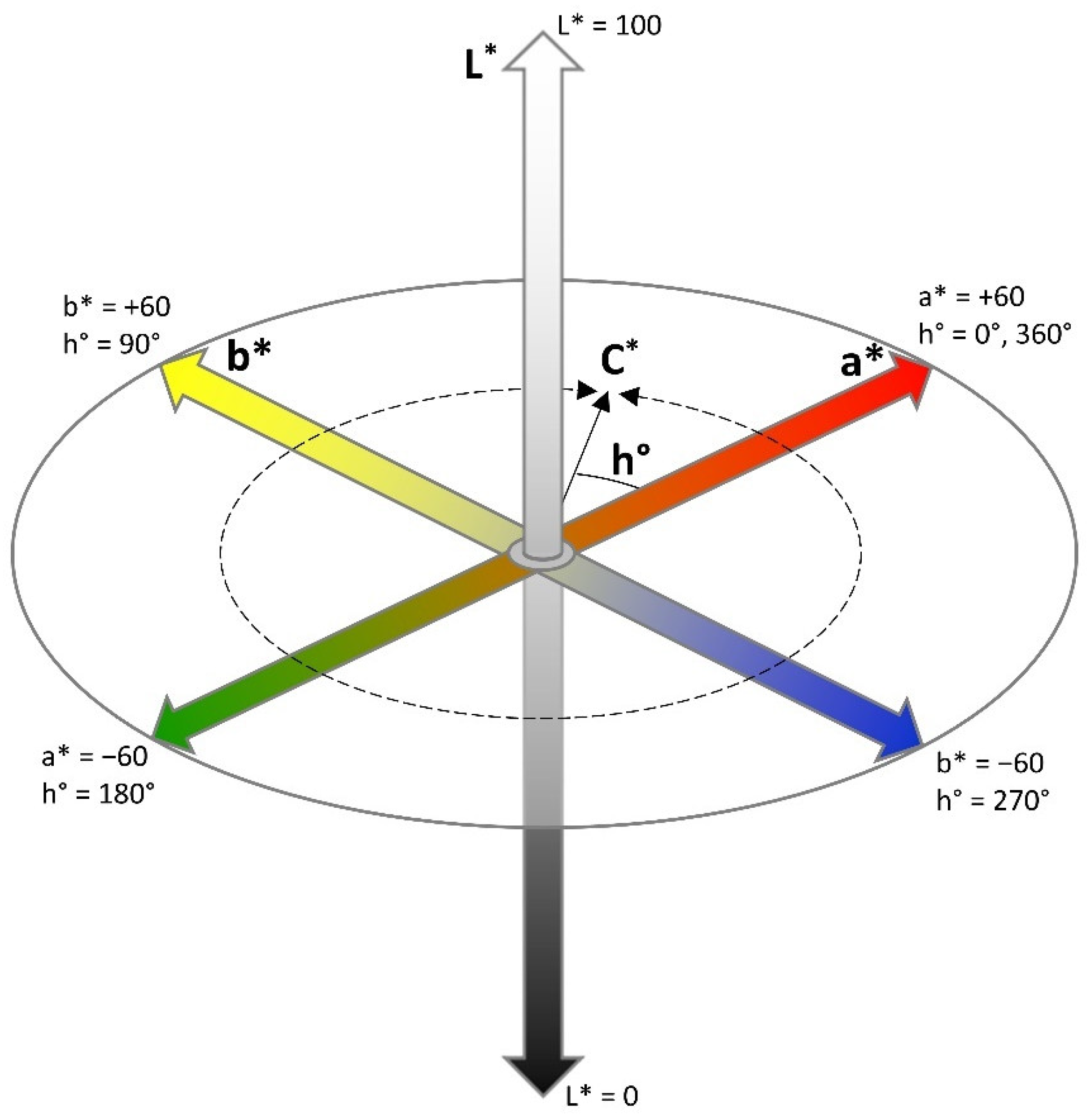
2.1.2. Calibration of Colour Attributes
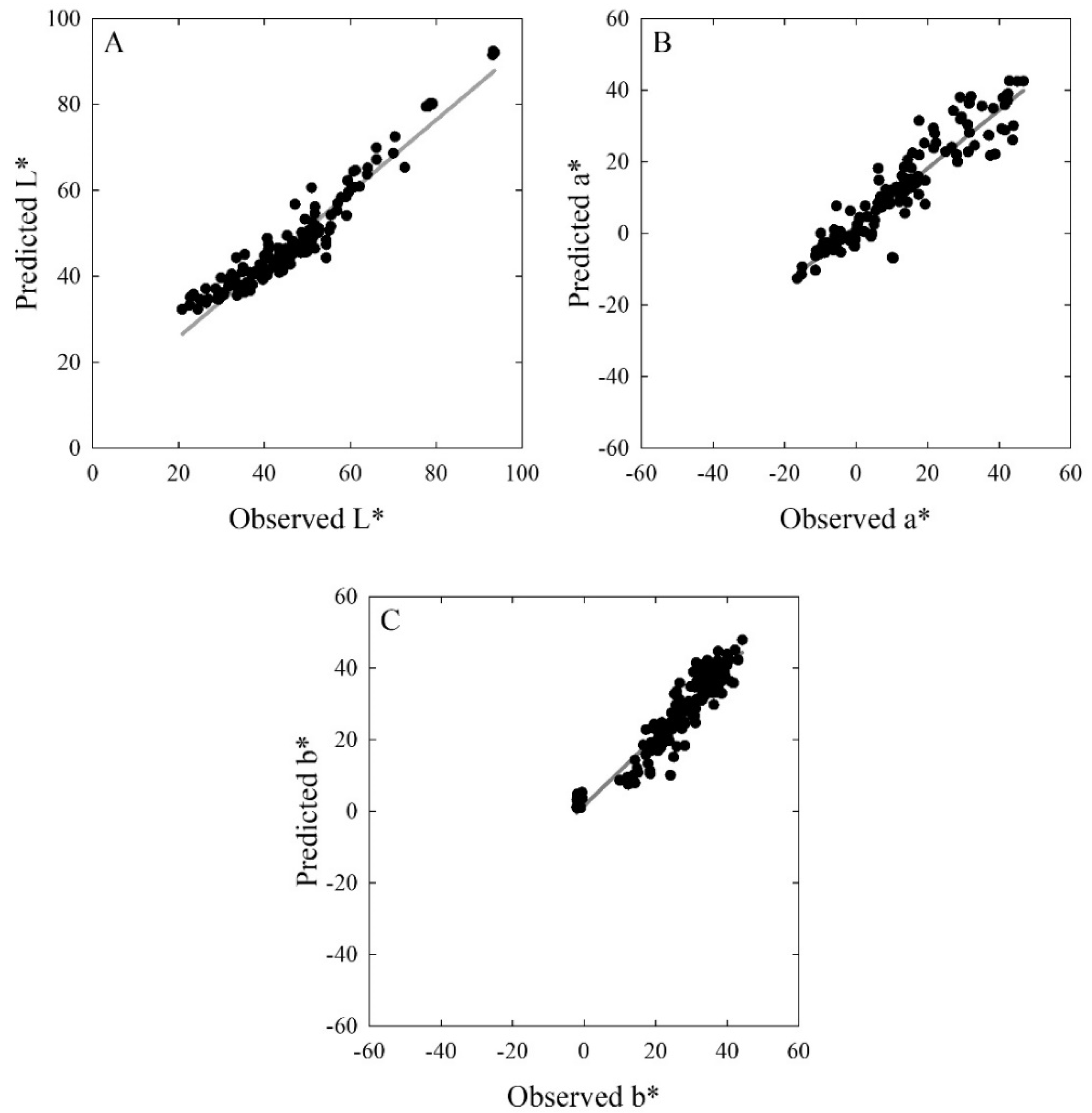
2.1.3. Validation of Colour Predictions
- A calibration greyscale set (QPcard 102) with four greyscale colours: dark grey (L* = 25, a* = 0 and b* = 0), medium grey (L* = 48, a* = 0 and b* = 0), light grey (L* = 80, a* = 0 and b* = 0) and white (L* = 95, a* = 0 and b* = 0). Five measurements were taken on each greyscale colour (n = 20).
- 20 ‘Angeleno’ plums (Prunus salicina L.).
- 20 ‘Golden May’ apricots (P. armeniaca L.).
- 20 ‘Snow Flame’ white peaches (P. persica L. Batsch).
- 20 ‘September Sun’ yellow peaches (P. persica L. Batsch).
- 20 ‘Rose Bright’ yellow nectarines (P. persica L. Batsch).
- 20 ‘Autumn Bright’ yellow nectarines (P. persica L. Batsch).
- 20 ‘Majestic Pearl’ white nectarines (P. persica L. Batsch).
2.2. The Effects of Time, Row Orientation and Training System on Nectarine Skin Colour
2.2.1. Study Site and Experimental Design
2.2.2. Colour Measurements
2.3. Data Analysis
3. Results
3.1. Accuracy and Precision of a Portable Colourimeter
3.1.1. Calibration of Colour Attributes
3.1.2. Validation of Colour Attributes
3.2. Colour Development over Time and the Effects of Row Orientation and Training Systems
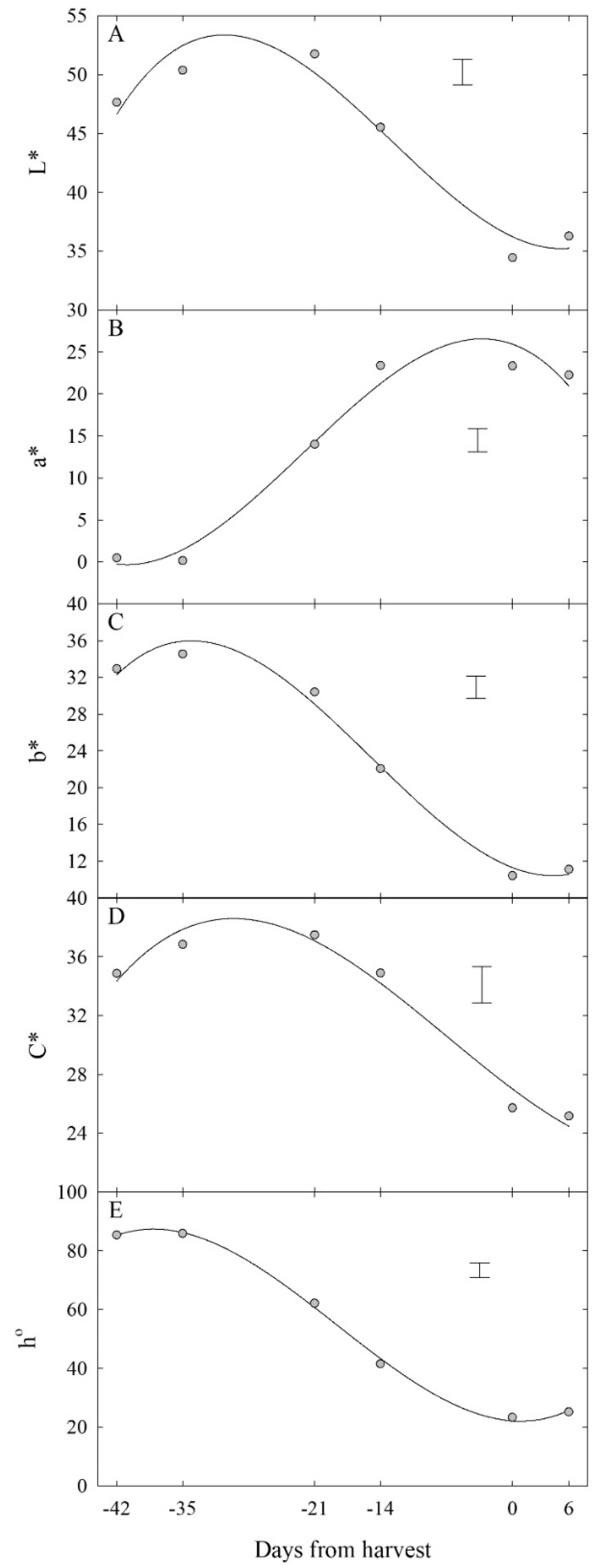
3.2.1. Colour Development over Time
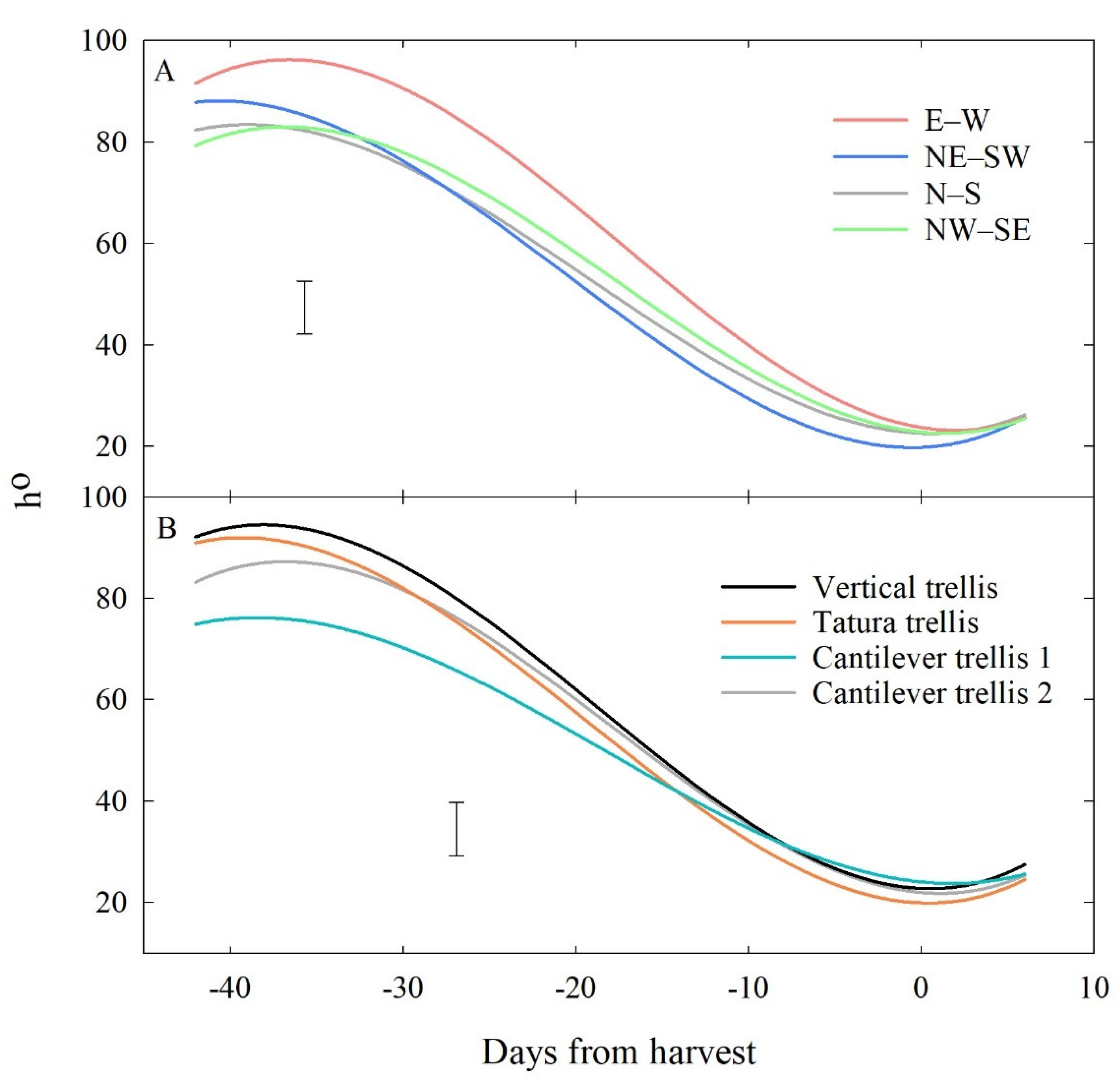
3.2.2. Effects of Row Orientation and Training System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravaglia, D.; Espley, R.V.; Henry-Kirk, A.R.; Andreotti, C.; Ziosi, V.; Hellens, R.P.; Costa, G.; Allan, A.C. Transcriptional Regulation of Flavonoid Biosynthesis in Nectarine (Prunus Persica) by a Set of R2R3 MYB Transcription Factors. BMC Plant Biol. 2013, 13, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelly, M.; Recasens, I.; Mata, M.; Arbones, A.; Rufat, J.; Girona, J.; Marsal, J. Effects of Water Deficit during Stage II of Peach Fruit Development and Postharvest on Fruit Quality and Ethylene Production. J. Hortic. Sci. Biotechnol. 2003, 78, 324–330. [Google Scholar] [CrossRef]
- O’Connell, M.; Scalisi, A. Sensing Fruit and Tree Performance under Deficit Irrigation in ‘September Bright’ Nectarine. Acta Hortic. 2021, 9–16. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Johnson, R.S.; Day, K.R.; Beede, B.; Andris, H. Contaminants and Injury induce Inking on Peaches and nectarines. Calif. Agric. 1999, 53, 19–23. [Google Scholar] [CrossRef]
- Nunes, M.C.d.N. Color Atlas of Postharvest Quality of Fruits and Vegetables; Wiley-Blackwell: Ames, IA, USA, 2008. [Google Scholar]
- Crisosto, C.H. Stone Fruit Maturity Indices: A Descriptive Review. Postharvest News Inf. 1994, 5, 65N–68N. [Google Scholar]
- Greer, D.H. Non-Destructive Chlorophyll Fluorescence and Colour Measurements of ‘Braeburn’ and ‘Royal Gala’ Apple (Malus Domestica) Fruit Development throughout the Growing Season. N. Z. J. Crop. Hortic. Sci. 2005, 33, 413–421. [Google Scholar] [CrossRef]
- International Organisation for Standardisation CIE 1976 L*a*b* Colour Space 1976. Available online: https://www.iso.org/standard/52497.html (accessed on 20 March 2021).
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Peavey, M.; Goodwin, I.; McClymont, L.; Chandra, S. Effect of Shading on Red Colour and Fruit Quality in Blush Pears “ANP-0118” and “ANP-0131”. Plants 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, D.H.; Nikolic, A.N.; Burns, E.E. Variability in Sugars, Acids, Firmness, and Color Characteristics of 12 Peach Genotypes. J. Am. Soc. Hortic. Sci. 1991, 116, 1004–1006. [Google Scholar] [CrossRef] [Green Version]
- Luchsinger, L.; Walsh, C. Development of an Objective and Non-Destructive Harvest Maturity Index for Peaches and Nectarines. Acta Hortic. 1998, 679–688. [Google Scholar] [CrossRef]
- Robertson, J.A.; Meredith, F.I.; Horvat, R.J.; Senter, S.D. Effect of Cold Storage and Maturity on the Physical and Chemical Characteristics and Volatile Constituents of Peaches (cv. Cresthaven). J. Agric. Food Chem. 1990, 38, 620–624. [Google Scholar] [CrossRef]
- Ferrer, A.; Remón, S.; Negueruela, A.I.; Oria, R. Changes during the Ripening of the very Late Season Spanish Peach Cultivar Calanda: Feasibility of Using CIELAB Coordinates as Maturity indices. Sci. Hortic. Amst. 2005, 105, 435–446. [Google Scholar] [CrossRef]
- Scalisi, A.; Pelliccia, D.; O’Connell, M.G. Maturity Prediction in Yellow Peach (Prunus Persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors 2020, 20, 6555. [Google Scholar] [CrossRef] [PubMed]
- Loreti, F.; Morini, S.; Stefanelli, D. Observations on Sunlight Interception and Penetration into the Canopy of Peach Trees at Different Planting Densities. Riv. Della Ortoflorofruttic. Ital. 1980, 64, 315–323. [Google Scholar]
- Gullo, G.; Motisi, A.; Zappia, R.; Dattola, A.; Diamanti, J.; Mezzetti, B. Rootstock and Fruit Canopy Position Affect Peach [Prunus Persica (L.) Batsch] (cv. Rich May) Plant Productivity and Fruit Sensorial and Nutritional Quality. Food Chem. 2014, 153, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Sharma, O.C.; Singh, D.B. Effect of Tree Architecture on Fruit Quality and Yield Attributes of Nectarine (Prunus Persica var. Nectarina) cv. Fantasia under Temperate Condition. Indian J. Agric. Sci. 2017, 87, 1008–1012. [Google Scholar]
- Scalisi, A.; O’Connell, M.; Bianco, R.L. Field Non-Destructive Determination of Nectarine Quality under Deficit Irrigation. Acta Hortic. 2021, 91–98. [Google Scholar] [CrossRef]
- Instruments & Data Tools Pty, L. IDT Data Logger. 2021. Available online: https://apps.apple.com/app/id1555614905 (accessed on 15 April 2021).
- Pelliccia, D. RGB to Lab Conversion. 2021. Available online: https://github.com/nevernervous78/rgb_to_Lab (accessed on 10 May 2021).
- Agriculture Victoria Stonefruit Sundial Experiments. Available online: http://www.hin.com.au/networks/profitable-stonefruit-research/stonefruit-orchard-technology/stonefruit-sundial-experiments (accessed on 3 August 2021).
- Lin, L.I.-K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Palapol, Y.; Ketsa, S.; Stevenson, D.; Cooney, J.; Allan, A.; Ferguson, I. Colour Development and Quality of Mangosteen (Garcinia Mangostana L.) Fruit during Ripening and after Harvest. Postharvest Biol. Technol. 2009, 51, 349–353. [Google Scholar] [CrossRef]
- Tadesse, T.; Hewett, E.W.; Nichols, A.M.; Fisher, K.J. Changes in Physicochemical Attributes of Sweet Pepper cv. Domino during Fruit Growth and Development. Sci. Hortic. Amst. 2002, 93, 91–103. [Google Scholar] [CrossRef]
- Bron, I.U.; Jacomino, A.P. Ripening and Quality of ‘Golden’ Papaya Fruit Harvested at Different Maturity Stages. Braz. J. Plant Physiol. 2006, 18, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Trentacoste, E.; Connor, D.J.; Gomez-Del-Campo, M. Row Orientation: Applications to Productivity and Design of Hedgerows in Horticultural and Olive Orchards. Sci. Hortic. Amst. 2015, 187, 15–29. [Google Scholar] [CrossRef]
- DeJong, T.; Doyle, J. The Effect of Row Orientation on Light Distribution in Hedgerow Peach Tree Canopies. Acta Hortic. 1985, 159–166. [Google Scholar] [CrossRef]


| Colour Attribute | Equation | R2 | p | n |
|---|---|---|---|---|
| L* | L* = 1.58 + 3.92 L*u − 0.0423 L*u2 | 0.947 | <0.001 | 115 |
| a* | a* = 5.87 + 22.9 a*u − 1.87 a*u2 | 0.976 | <0.001 | 115 |
| b* | b* = − 12.5 + 26.2 b*u + 2.90 b*u2 | 0.983 | <0.001 | 115 |
| Colour Parameters | n | rc | RMSE |
|---|---|---|---|
| L* | 160 | 0.930 (0.040) | 5.158 |
| a* | 160 | 0.924 (0.043) | 5.747 |
| b* | 160 | 0.946 (0.033) | 4.373 |
| Colour Attribute | Equation | Adj. R2 x | y0 y | % RMSE z |
|---|---|---|---|---|
| L* | y = 36.2 − 0.375 x + 0.0304 x2 + 0.000797 x3 | 0.805 | 36.2 (0.748) | 20.2 |
| a* | y = 25.9 − 0.399 x − 0.0664 x2 − 0.0100 x3 | 0.846 | 25.9 (1.16) | 20.5 |
| b* | y = 11.3 − 0.398 x + 0.0403 x2 + 0.000903 x3 | 0.897 | 11.3 (0.946) | 16.0 |
| C* | y = 27.0 − 0.480 x + 0.00721 x2 + 0.000345 x3 | 0.754 | 27.0 (0.683) | 25.8 |
| h° | y = 22.2 − 0.225 x + 0.123 x2 + 0.00220 x3 | 0.931 | 22.2 (2.19) | 13.3 |
| Colour Attribute | Row Orientation (RO) | Days from Harvest (D) | Mean (RO) | |||||
|---|---|---|---|---|---|---|---|---|
| −42 | −35 | −21 | −14 | 0 | 6 | |||
| L* | E–W | 49.59 | 52.34 | 54.62 | 49.31 | 33.68 | 36.40 | 45.99 |
| NE–SW | 48.01 | 50.83 | 50.49 | 42.15 | 34.68 | 36.60 | 43.79 | |
| N–S | 47.13 | 48.88 | 51.03 | 44.67 | 34.68 | 36.63 | 43.84 | |
| NW–SE | 45.83 | 49.44 | 50.88 | 45.94 | 34.73 | 35.44 | 43.71 | |
| Mean (D) | 47.64 | 50.37 | 51.76 | 45.52 | 34.44 | 36.27 | - | |
| p: 0.106 (RO), <0.001 (D), 0.351 (RO × D); LSD (5%): 2.138 (RO), 2.176 (D), 4.469 (RO × D). | ||||||||
| a* | E–W | −3.17 | −3.68 | 7.52 | 20.51 | 23.64 | 21.96 | 11.13 |
| NE–SW | −0.47 | 0.84 | 15.99 | 26.78 | 23.96 | 24.78 | 15.31 | |
| N–S | 1.81 | 1.82 | 17.35 | 23.97 | 22.35 | 21.68 | 14.83 | |
| NW–SE | 3.73 | 1.63 | 15.09 | 22.22 | 23.35 | 20.59 | 14.43 | |
| Mean (D) | 0.47 | 0.15 | 13.99 | 23.37 | 23.33 | 22.25 | - | |
| p: 0.053 (RO), <0.001 (D), 0.173 (RO × D); LSD (5%): 3.128 (RO), 2.761 (D), 5.851 (RO × D). | ||||||||
| b* | E–W | 36.16 | 37.73 | 34.23 | 26.09 | 9.90 | 11.48 | 25.93 |
| NE–SW | 33.72 | 34.40 | 28.65 | 18.51 | 10.57 | 11.84 | 22.95 | |
| N–S | 32.20 | 32.81 | 29.01 | 21.30 | 10.23 | 11.11 | 22.78 | |
| NW–SE | 29.66 | 33.25 | 29.78 | 22.37 | 10.92 | 9.92 | 22.65 | |
| Mean (D) | 32.94 | 34.55 | 30.41 | 22.07 | 10.41 | 11.09 | - | |
| p: 0.044 (RO), <0.001 (D), 0.289 (RO × D); LSD (5%): 2.502 (RO), 2.430 (D), 5.041 (RO × D). | ||||||||
| C* | E–W | 37.49 | 39.48 | 38.65 | 36.90 | 25.68 | 25.01 | 33.87 |
| NE–SW | 34.97 | 36.75 | 36.68 | 33.50 | 26.33 | 27.80 | 32.67 | |
| N–S | 34.96 | 35.58 | 37.03 | 34.65 | 24.78 | 24.61 | 31.93 | |
| NW–SE | 32.05 | 35.59 | 37.59 | 34.57 | 26.13 | 23.23 | 31.53 | |
| Mean (D) | 34.87 | 36.85 | 37.49 | 34.90 | 25.73 | 25.17 | - | |
| p: 0.109 (RO), <0.001 (D), 0.558 (RO × D); LSD (5%): 2.004 (RO), 2.455 (D), 4.910 (RO × D). | ||||||||
| h° | E–W | 92.9 | 92.96 | 73.15 | 49.55 | 22.05 | 26.59 | 59.53 |
| NE–SW | 87.59 | 84.6 | 57.59 | 33.61 | 22.84 | 24.19 | 51.74 | |
| N–S | 82.06 | 82.23 | 57.39 | 40.14 | 23.73 | 25.55 | 51.85 | |
| NW–SE | 78.71 | 83.73 | 60.41 | 42.75 | 24.57 | 24.43 | 52.43 | |
| Mean (D) | 85.31 | 85.88 | 62.14 | 41.51 | 23.3 | 25.19 | - | |
| p: 0.019 (RO), <0.001 (D), 0.167 (RO × D); LSD (5%): 5.094 (RO), 5.032 (D), 10.440 (RO × D). | ||||||||
| Colour Attribute | Training System (TS) | Days from Harvest (D) | Mean (TS) | |||||
|---|---|---|---|---|---|---|---|---|
| −42 | −35 | −21 | −4 | 0 | 6 | |||
| L* | Cantilever trellis 1 | 45.62 | 46.22 | 49.08 | 45.51 | 34.13 | 35.87 | 42.74 |
| Cantilever trellis 2 | 46.38 | 49.66 | 51.5 | 45.00 | 34.82 | 34.65 | 43.67 | |
| Tatura Trellis | 50.89 | 54.19 | 53.12 | 45.83 | 34.00 | 36.04 | 45.68 | |
| Vertical Trellis | 47.68 | 51.40 | 53.32 | 45.73 | 34.83 | 38.5 | 45.24 | |
| Mean (D) | 47.64 | 50.37 | 51.76 | 45.52 | 34.44 | 36.27 | - | |
| p: 0.041 (TS), <0.001 (D), 0.277 (TS × D); LSD (5%): 2.138 (TS), 2.176 (D), 4.469 (TS × D). | ||||||||
| a* | Cantilever trellis 1 | 5.06 | 5.34 | 17.46 | 23.63 | 23.12 | 21.36 | 16.00 |
| Cantilever trellis 2 | 1.83 | −0.65 | 12.42 | 21.42 | 22.26 | 21.23 | 13.09 | |
| Tatura Trellis | −2.21 | −2.10 | 15.60 | 24.73 | 23.62 | 23.32 | 13.83 | |
| Vertical Trellis | −2.80 | −1.98 | 10.46 | 23.68 | 24.30 | 23.10 | 12.79 | |
| Mean (D) | 0.47 | 0.15 | 13.99 | 23.37 | 23.33 | 22.25 | - | |
| p: 0.158 (TS), <0.001 (D), 0.122 (TS × D); LSD (5%): 3.128 (TS), 2.761 (D), 5.851 (TS × D). | ||||||||
| b* | Cantilever trellis 1 | 29.25 | 29.64 | 27.58 | 22.32 | 10.31 | 10.86 | 21.66 |
| Cantilever trellis 2 | 31.59 | 34.50 | 30.80 | 21.60 | 10.45 | 9.88 | 23.14 | |
| Tatura Trellis | 36.41 | 37.38 | 30.34 | 21.72 | 9.67 | 10.66 | 24.36 | |
| Vertical Trellis | 34.50 | 36.66 | 32.94 | 22.64 | 11.19 | 12.94 | 25.15 | |
| Mean (D) | 32.94 | 34.55 | 30.41 | 22.07 | 10.41 | 11.09 | - | |
| p: 0.052 (TS), <0.001 (D), 0.267 (TS × D); LSD (5%): 2.502 (TS), 2.430 (D), 5.041 (TS × D). | ||||||||
| C* | Cantilever trellis 1 | 32.28 | 33.54 | 36.14 | 35.12 | 25.42 | 24.29 | 31.13 |
| Cantilever trellis 2 | 34.09 | 36.72 | 37.14 | 32.98 | 24.98 | 23.52 | 31.57 | |
| Tatura Trellis | 37.77 | 38.79 | 38.36 | 36.16 | 25.55 | 25.84 | 33.75 | |
| Vertical Trellis | 35.32 | 38.36 | 38.31 | 35.36 | 26.96 | 27.00 | 33.55 | |
| Mean (D) | 34.87 | 36.85 | 37.49 | 34.90 | 25.73 | 25.17 | - | |
| p: 0.033 (TS), <0.001 (D), 0.734 (TS × D); LSD (5%): 2.004 (TS), 2.455 (D), 4.910 (TS × D). | ||||||||
| h° | Cantilever trellis 1 | 74.92 | 75.01 | 55.23 | 41.66 | 23.95 | 25.60 | 49.40 |
| Cantilever trellis 2 | 82.93 | 87.01 | 63.99 | 42.24 | 23.93 | 24.37 | 54.08 | |
| Tatura Trellis | 90.42 | 90.48 | 60.51 | 39.97 | 21.71 | 23.55 | 54.44 | |
| Vertical Trellis | 92.98 | 91.03 | 68.81 | 42.17 | 23.60 | 27.23 | 57.64 | |
| Mean (D) | 85.31 | 85.88 | 62.14 | 41.51 | 23.30 | 25.19 | - | |
| p: 0.033 (TS), <0.001 (D), 0.114 (TS × D); LSD (5%): 5.094 (TS), 5.032 (D), 10.440 (TS × D). | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalisi, A.; O’Connell, M.G.; Pelliccia, D.; Plozza, T.; Frisina, C.; Chandra, S.; Goodwin, I. Reliability of a Handheld Bluetooth Colourimeter and Its Application to Measuring the Effects of Time from Harvest, Row Orientation and Training System on Nectarine Skin Colour. Horticulturae 2021, 7, 255. https://doi.org/10.3390/horticulturae7080255
Scalisi A, O’Connell MG, Pelliccia D, Plozza T, Frisina C, Chandra S, Goodwin I. Reliability of a Handheld Bluetooth Colourimeter and Its Application to Measuring the Effects of Time from Harvest, Row Orientation and Training System on Nectarine Skin Colour. Horticulturae. 2021; 7(8):255. https://doi.org/10.3390/horticulturae7080255
Chicago/Turabian StyleScalisi, Alessio, Mark G. O’Connell, Daniele Pelliccia, Tim Plozza, Christine Frisina, Subhash Chandra, and Ian Goodwin. 2021. "Reliability of a Handheld Bluetooth Colourimeter and Its Application to Measuring the Effects of Time from Harvest, Row Orientation and Training System on Nectarine Skin Colour" Horticulturae 7, no. 8: 255. https://doi.org/10.3390/horticulturae7080255
APA StyleScalisi, A., O’Connell, M. G., Pelliccia, D., Plozza, T., Frisina, C., Chandra, S., & Goodwin, I. (2021). Reliability of a Handheld Bluetooth Colourimeter and Its Application to Measuring the Effects of Time from Harvest, Row Orientation and Training System on Nectarine Skin Colour. Horticulturae, 7(8), 255. https://doi.org/10.3390/horticulturae7080255







