Abstract
Salinity eustress is one of the pre-harvest factors that can be used to improve the phytochemical profile and the quality attributes of horticultural species, and most of the studies are carried out using NaCl. In this work, we compared the effect of three mildly saline iso-osmotic nutrient solutions (NS) differing in the cation employed (either K, Na, or Ca) in baby lettuce cultivated in a floating system. Specifically, we analyzed the impact on key morphological traits and polyphenol composition in leaves in a completely randomized design experiment with the following experimental factors and levels: two differently pigmented lettuce varieties (green and full red), three NSs (20 mM KCl, 20 mM NaCl, 13.3 mM CaCl2, each with a final ionic concentration of 40 mM), and two successive harvests. The lettuce response to mild salinity was multifaceted and with a marked role for the cultivar factor and its interactions, as also indicated by multivariate analysis. The morphological response of baby lettuce to the saline solutions was predominantly affected by the osmolarity, and ion-specific alleviating or detrimental effects were not observed. The phytochemical analysis revealed significant interactions among all tested factors, with ion-specific differences over some of the detected phenolics. This is consistent with the high sensitivity of this class of bioactive compounds to environmental factors. This work indicated that certain combinations of the experimental factors tested can be exploited to improve the biochemical profile and possibly the nutraceutical quality of baby lettuce in a floating system.
1. Introduction
Lettuce (Lactuca sativa L., Asteraceae) is increasingly popular around the world, mainly because it can satisfy the rising consumer demand for healthy, crisp, fresh, and easily prepared plant food [1]. In addition to being a central element for weight loss diet programs, the consumption of lettuce is also expanding because of its nutritional value, characterized by a high ratio of bioactive phytochemicals (such as vitamins and phenolic compounds) to calories [2]. Another reason for the commercial success of lettuce is that plant breeding has produced L. sativa varieties with a remarkable morphological and compositional variability, for example, for the tint, anthocyanin pigmentation pattern, and intensity of the color of the leaf, as well as head compactness and leaf margin [1].
In view of the myriads of classifications, main traditional horticultural types of lettuce are probably butterhead (cabbage), crisphead, Latin, leaf, lollo, romaine (cos), and stem (stalk). In recent years, the vegetable market in high-income countries has seen a steady increase in the fresh-cut food sector (e.g., baby leaves), especially for commercial products that include leafy vegetables with different colors and shapes [3]. The rise of baby leaf salad mixes is closely dependent on the affordability of indoor, greenhouse, and plant factories’ cultivation systems based on a variety of soilless techniques (such as hydroponics, aquaponics, and aeroponics), and on the ease of access to reliable temperature-controlled supply chains [4,5]. Although a universal definition may not be possible considering the multitude of plant species, baby leaf vegetables are broadly defined as those harvested between the development of the true leaves and the eighth true-leaf stage [6,7]. In particular, baby leaf lettuce is typically sowed at high density and harvested when leaves surpass the size of 8 cm. Another important feature is that baby-lettuce also offers the possibility to have multiple harvests (cut-and-come again strategy). Plants are mechanically trimmed a few centimeters from the substrate and then let to grow up to three or four times.
The phytochemical profile in vegetables is rapidly modified following exposure to stress [8]. This flexible metabolic response has been exploited to activate pathways that may increase the quality of the vegetables [9,10]. A controlled application of the dose and duration of a chemical, physical, or biological stressor may cause a positive effect on the nutritional value of the edible product, thus leading to the definition of eustress [11]. The majority of studies have exploited NaCl [12,13], probably because of the ecological and agronomic relevance of this salt and the related wealth of knowledge [14]. Although it is long known that salinity causes detrimental effects at high concentrations (typically, above 4 dS/m), salt is a widely employed chemical (eu)stressor in horticulture because it can be precisely manipulated and automatically controlled in soilless cultivation systems by simply managing the nutrient solution [15,16]. In several crops, the response to a mild to moderate salinity may lead to a relative increase of useful compounds, without occurring in large yield penalties [11,17,18]. For instance, a reduced amount of NaCl can increase primary metabolites (typically measured by total soluble content and/or organic acids) in major horticultural crops such as tomato, eggplant, pepper, melon, watermelon, and cauliflower [19,20,21,22,23,24]. Similarly, eustress can promote bioactive secondary compounds. Limiting our attention to a few leafy vegetables, NaCl can increase vitamin content in spiny chicory [25], carotenoids in cos lettuce [26], polyphenols in broccoli [27], and anthocyanins in red leaf lettuce [28]. Nonetheless, the phytochemical response to NaCl is also affected by the variety, with conflicting results in the literature in relation to the effect of mild-to-moderate salt-stress [29,30,31,32,33].
Previous studies on lettuce growing in hydroponics indicated that a mild NaCl salinity (i.e., between 5 and 30 mM) could improve the nutritional profile, often without a large yield loss [34,35,36]. In this work, we aimed to understand whether the phytochemical responses to chemical eustress in baby lettuce vary according to different salts. Specifically, we employed three iso-osmotic nutrient solutions that included NaCl, KCl, or CaCl2, and monitored the accumulation of the main polyphenols and anthocyanins by HPLC in two differently pigmented (green and red) baby lettuce varieties. In addition, we also analyzed plants in two consecutive harvests, not only because baby lettuce is a typical cut-and-come again crop in professional horticulture, but also considering that the effects of the exposure to suboptimal saline conditions may be more evident over a longer period of time [33].
2. Materials and Methods
2.1. Plant Material, Growing Condition, and Experimental Design
This work was carried out using two Lactuca sativa L. cultivars, namely, ‘Green Salad Bowl’ (hereafter GSB) and ‘Red Salad Bowl’ (hereafter RSB) from the Società Agricola Italiana Sementi (Cesena, Italy), with green and red leaves, respectively. The experiment was carried out in the spring cycle (21 March to 4 May), in an unheated greenhouse at the “Azienda Agraria e Zootecnica Universitaria Torre Lama” (Bellizzi, Salerno, Italy) of the Department of Agricultural Sciences (Federico II University of Naples) [35]. Seeds were germinated in polystyrene trays, providing a density of 1149 plants per square meter. Eight days after sowing (DAS), trays were moved to a hydroponic floating system. Polystyrene tanks were filled with 150 L of nutrient solution (NS), and replenished when necessary to maintain a constant volume. NSs were changed weekly. Each experimental unit (see below) relied on an independent tank equipped with an immersion pump to ensure a dissolved oxygen level above 6 mg/L. Lettuce plants were grown under natural light conditions. Inside the glasshouse, the mean air temperatures was 22 °C, varying between 17 and 26 °C, and the relative humidity was around 55%/70% during day/night, respectively. We used a completely randomized design with three experimental factors (i.e., categorical variables): the cultivar (two levels: GSB and RSB), the salt (four levels: basal NS as a control, NS plus NaCl, NS plus KCl, and NS plus CaCl2), and the harvest (two levels: first and second). Each experimental thesis (i.e., block) was replicated three times and randomly distributed in the greenhouse. The composition and the electric conductivity of the basal NS (no salt added) and of the three saline treatments were as reported in [35]. Briefly, the basal NS is a modified Hoagland’s solution (N-NO3−: 13.0 mM; N-NH4+: 1.0 mM; S: 1.75 mM; P: 1.5 mM; K: 5.0 mM; Ca: 4.5 mM; Mg: 2.0 mM; Fe: 20 µM; Mn: 9 µM; Cu: 0.3 µM; Zn: 1.6 µM; B: 20 µM; Mo: 0.3 µM; pH 6.0), and the NSs with NaCl, KCl, or CaCl2 were obtained by adding 20 mM NaCl, 20 mM KCl, or 13.3 mM CaCl2 respectively, to yield isosmotic NSs with a final molar concentration of 40 mM. All NSs were prepared using deionized water. Leaves were harvested at the commercial stage of the no-salt control treatment: 25 and 29 DAS for GSB and RSB respectively, at the first harvest, and 43 and 45 DAS for GSB and RSB respectively, at the second harvest.
2.2. Morphological Measurements
At the two harvests, we measured the height of the plants, the number of leaves per plant, and the fresh weight (fw) of the leaves using a laboratory scale (ME-T Analytical Balance, Mettler Toledo, Italy). The surface of each leaf was measured with a bench-top area meter (LiCor 3100C; LI-COR Biosciences, Lincoln, NE, USA). Leaves were then stored in paper bags, dried in a forced-air oven at 70 °C for three days, and then weighted to obtain the dry weight (dw). The specific leaf area (SLA) was calculated as the ratio between the area and dw of the leaves [37] and expressed as cm2/g dw. Leaf succulence (LSU) was calculated as the ratio between the leaf area and the water content at harvest (fw − dw), expressed in mg (of H20) per cm2 [38].
2.3. Quantification of Polyphenols
At harvest, leaves were freeze-dried in an Alpha 1-4 freeze drier (Christ, Osterode, Germany), and total polyphenols were extracted from approximately 0.2 g of lyophilized leaf powder, as reported [39]. Briefly, samples were homogenized in eight mL of a methanol-water-formic acid solution (25/24/3 v/v/v), mixed with a vortex, sonicated, mixed again on a rotatory shaker, cold-centrifuged twice, and filtered through a 0.22 mm gauge cellulose-based filter. For HPLC analysis, a 20 μL aliquot of the clean supernatant was analyzed (two technical replicates) according to previously described procedures [38] using a Prodigy ODS3 250 mm × 4.6 reversed-phase C-18 (Phenomenex, CA, USA) column, with a flow rate of 1 mL/min, installed into LC-10A apparatus (Shimadzu, Kyoto, Japan) equipped with an SPD-M10A DA detector (Shimadzu) and a Series 200 Autosampler (Perkin Elmer, Waltham, MA). Five phenolic acids were identified by LC-MS and quantified on calibration curves built with chlorogenic acid and cichoric acid (with HPLC). The cyanidin-malonyl glucoside was identified by LC-MS and quantified using the oenin to build a standard curve. HPLC-grade solvents and standards were purchased from Sigma-Aldrich (Milan, Italy).
2.4. Statistical Analysis
Morphological measurements were performed on ten lettuce plants per experimental block, replicated three times (for a total of thirty plants per treatment). For the biochemical evaluation, we analyzed leaves from three plants per each experimental block. Data are reported as mean ± standard error of the mean (SEM). Mean separation among factors (i.e., Cultivar, Salt, and Harvest) and their interaction on the dependent variable was performed with a three-way analysis of variance (ANOVA), using a significance level (α) of 0.05. A user-defined contrast analysis was carried out to compare the different levels of salt treatment, namely, “no salt vs. CaCl2-KCl-NaCl (named L1), “CaCl2 vs. KCl-NaCl” (L2), “KCl vs. CaCl2-NaCl” (L3), and “NaCl vs. CaCl2-KCl” (L4). A simple effects analysis (e.g., pairwise comparisons) was also performed to look at the effect of the Salt factor at individual levels of the other independent variables (Harvest and Cultivar). Post-hoc analysis for the main effect of the categorical variables was performed with the Tukey HSD procedure for the Salt factor, or the independent two-tailed Student’s t-test for the Cultivar and the Harvest factors. All statistical tests were carried out with the IBM SPSS 26 software (Armonk, NY, USA). The principal component analysis (PCA) was performed in R as described in [40].
3. Results
3.1. Effects on the Morphological Parameters
The Cultivar (C) was the investigated experimental factor that had a significant influence on all the analyzed morphological parameters (Table 1). Specifically, post-hoc mean separation by a t-test revealed that the GSB plants were slightly taller (+4.6%) and with a higher number (+15.4%) of heavier (+17.2%) leaves, irrespective of the slightly shorter time to reach commercial maturity compared to RSB. GSB plants also had a higher succulence (+9.0%) and a lower specific leaf area (−13.4%), indicating that this variety was less efficient in building leaf surface per dry matter and that the higher mass is due mainly to higher water content.

Table 1.
Effect of the experimental factors (Cultivar: C, Harvest: H, Salt: S) on the height, number of leaves, leaf fresh weight (LFW), specific leaf area (SLA), and leaf succulence (LSU) of the two baby lettuce varieties, GSB and RSB. The significance of the factors and their interactions was evaluated with a three-way ANOVA, using the Tukey test as a post-hoc test for mean separation among conditions. Data are mean ± SEM. n.s.: not significant; *: p < 0.05; **: p < 0.01; ***: p < 0.001.
The main effect of the factor Salt was a reduction of the plant height (on average −16.0%) and of the fresh weight of the leaves (−16.2%) compared to the no-salt condition (Table 1). With the aim of determining whether the influence of the different salts is significantly different from one another, we performed a contrast analysis. The data indicated that the salt treatment (e.g., the increase of the NS molarity) statistically influences the height and the fresh weight, and that the main effect of each added salt (for example, KCl) was not different from that of the other two (for example, CaCl2-NaCl) (Supplementary Table S1). To obtain a detailed view of the effect of the salt in each experimental condition, we also built a pairwise comparison table. The data indicated that at any level of the other categorical variables (Cultivar and Harvest), the effect of each type of salt (i.e., KCl, CaCl2, and NaCl) on plant height and fresh weight was not statistically different (Supplementary Tables S2 and S3). In addition, pairwise comparisons indicated that the reduction in height was similarly present for both cultivars and for all the saline conditions at the second harvest (Supplementary Table S3). On the other hand, a significant reduction of the amount of water per dry matter (e.g., succulence) was observed for the NaCl treatment (−5.7% compared to the no-salt control) (Table 1).
The main effect of the factor Harvest was present for the number of leaves, higher at H2 (Table 1). At the second harvest, the leaves were more numerous but with a significantly lower specific leaf area, while succulence was not affected. Overall, the main effect of the factors S and H on the measured morphological parameters was different, except for fresh weight.
There were significant two-way interaction effects between H with C or S on the height of the plants and between H and C on the fresh weight and succulence of the leaves (Table 1). Interestingly, the analysis of the factors’ interaction revealed that the response of the two cultivars to the salt was substantially similar. The significant two-way interaction between S and H on plant height can be explained considering that in the no-salt condition, the GSB were taller than RSB, but plants growing in saline conditions had equal height, with a stronger reduction only for the RSB plant with KCl (Table 1). The response of the two cultivars to the harvest was markedly different. While at the first harvest the GBS plants were taller than at H2, the RSB were higher, which rendered the height of the two cultivars not significantly different at the second harvest. A difference between the two varieties was that the leaves of the RSB plants were higher at the second cut, while the weight of GSB was not different. Finally, there was not a two-way interaction that varies across levels of a third categorical variable.
3.2. Effect on Polyphenols and Anthocyanins
The quantification of main polyphenols in leaves (caffeoyltartaric acid: CTA; 5-O-caffeoylquinic acid, aka chlorogenic acid: 5-CQA; caffeoylmalic acid: CMA; di-O-caffeoyltartaric acid, aka cichoric acid: DCTA; meso-di-O-caffeoyltartaric acid: m-DCTA; 3,5-di-O-caffeoylquinic acid, aka isochlorogenic acid: 3,5-DCQA) indicated that the factor Cultivar played a significant role in determining the phytochemical profile, with all the polyphenols significantly affected (Table 2). Considering the averaged value across the levels of the other categorical variables, the RSB cultivar accumulated a 3 times higher amount of the analyzed polyphenols compared to the GSB. This was mainly due to 5-CQA and DCTA, which represented around 85% of the total sum of these phytochemicals.

Table 2.
Effect of the experimental factors (Cultivar: C, Harvest: H, Salt: S) on the polyphenols and anthocyanins of the two baby lettuce varieties, GSB and RSB. CTA: Caffeoyltartaric acid: CTA; 5-CQA: 5-O-caffeoylquinic acid, aka chlorogenic acid; CMA: caffeoylmalic acid; DCTA: di-O-caffeoyltartaric acid, aka cichoric acid; m-DCTA: meso-di-O-caffeoyltartaric acid: m-DCTA; 3,5-DCQA: 3,5-di-O-caffeoylquinic acid, aka isochlorogenic acid; CMG: cyanidin-3-(6-malonylglucoside). Data are mean ± SEM. The significance of the factors and their interactions was evaluated with a three-way ANOVA, using the Tukey test as a post-hoc test for mean separation among conditions. n.a.: not available; n.s.: not significant; *: p < 0.05; **: p < 0.01; ***: p < 0.001.
The effect of the factor Salt was similarly critical, with all compounds but 3,5-CQA significantly altered (Table 2). The post-hoc analysis indicated a significant main effect of the different salts employed over the accumulation of specific polyphenols, with either CaCl2 or KCl providing the largest increase. To test differences between groups within our data in relation to the salt in the NS (i.e., KCl, CaCl2, or NaCl), we performed a contrast analysis on the polyphenols. Interestingly, the data indicated the importance of each specific salt added to the NS, rather than an effect due to the simple increase of the salinity (Supplementary Table S4). Specifically, the main effect of the CaCl2-NS on the various polyphenols was a significant increase of the various compounds compared to the other two saline conditions, while the KCl-NS had the opposite effect. This is also the reason why the effect of the NaCl-NS was not significantly different from that of the other two NSs. The pairwise comparison between experimental treatments indicated that the CaCl-NS specifically increased CTA in the RSB leaves at the second harvest (Supplementary Table S5). For 5-CQA, the effect of the three salts was different only at the second harvest in the RSB variety (Supplementary Table S6). In particular, the KCl treatment associated with a significant reduction of 5-CQA compared to the other salts and the controls, comparable to that of the GSB variety under the same condition. At H2, leaves of the RSB plant treated with CaCl2-enriched NS yielded the highest quantity of this polyphenol, higher than the other two saline solutions. Similar behavior was present for the DCTA and for m-DCTA. The pairwise comparisons also indicated that for the RSB variety and at the second harvest, the CaCl2 provided a significantly higher amount compared to the no-salt and the KCl and NaCl nutrient solutions (Supplementary Tables S7 and S8). On the other hand, leaves of the plants treated with KCl-NS have a significantly lower amount of DCTA and m-DCTA compared to the other two saline conditions (Supplementary Tables S7 and S8). Pairwise differences of 3,5-CQA between salt treatments in the various experimental conditions were more limited, with a significant variation between CaCl2 and KCl in the RSB leaves of the second harvest (Supplementary Table S9). The analysis of the sum of polyphenols according to the salt indicated that their accumulation in RSB at H2 was significantly different in the CaCl2 and KCl compared to all the other conditions, while in the same experimental condition, the NaCl treatment was different only from the other saline treatments (Supplementary Table S10). The main effect of the harvest was significant over all the analyzed polyphenols, with an almost 3-fold increase at the second harvest (Table 2).
Nonetheless, the combined effect of factors on polyphenols’ accumulation in leaves (and their sum) was important because there were significant three-way interactions among the categorical variables for all compounds. In relative terms, the highest positive combined effect among the two-way interactions was present for Harvest and the Cultivar, with the RSB variety showing the highest increase at H2.
The anthocyanin cyanidin-3-(6-malonylglucoside) was detected only in the red-pigmented lettuce variety. While this did not allow to study the effect of the cultivar, it still permitted to highlight the different effects of the employed salts. This major anthocyanin was present at the second harvest in a higher quantity (+150%) (Table 2). Among the employed salts, the CaCl2 treatment was associated with the highest increase, while KCl induced a reduction. The S × H interaction on CMG accumulation in leaves was modest and positive (Table 2).
3.3. Multivariate Analysis of the Lettuce Response
To summarize the information content of our agronomic and phytochemical dataset, we performed a principal component analysis on all the recorded traits, except CMG, absent in the GSB variety. The first two components explained 80.6% of the total variance (Supplementary Figure S1) and were used to graphically represent the relation among samples and treatments (Figure 1).
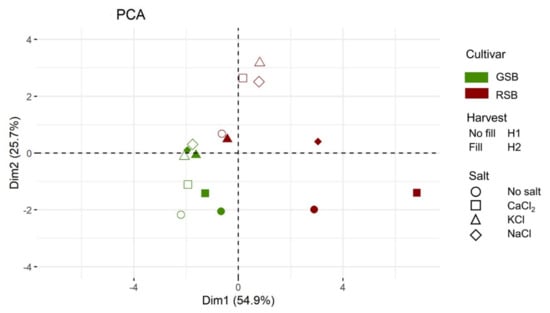
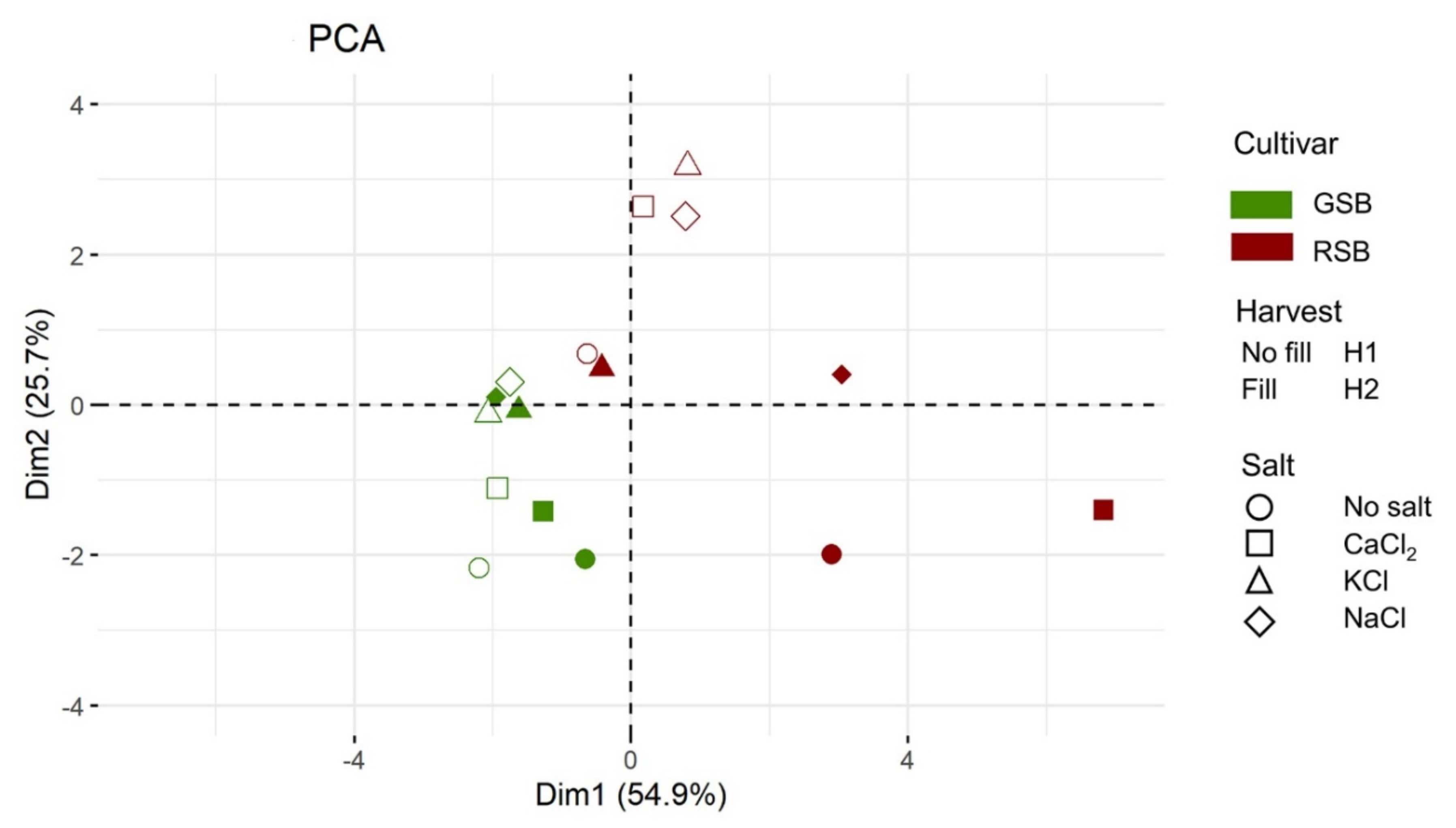
Figure 1.
Principal component analysis (PCA) of the lettuce response. Each condition is colored according to the variety (green: GSB, deep red: RSB). The shape of the symbol indicates the level of the factor Salt (circle: no salt, square: CaCl2, triangle: KCl, diamond: NaCl). The plot displays the symbol as empty (H1) or filled (H2) according to the harvest. The color and symbol legends are reported on the right side.
The plot revealed that samples were separated coherently with the genotype along the first principal component (PC). Moreover, the distribution of the RSB samples along PC1 and PC2 was much wider compared to the GSB samples, indicating a more variable response to the experimental factors. The multidimensional reduction also highlighted another difference between the cultivars. While the salt treatment mainly separated experimental conditions of the GSB along the second principal component, the factor Harvest mainly distributed samples along PC1, with those of the second harvest shifted toward less negative values compared to those of the first harvest. This resulted in a coherent clustering of the GSB samples, with, for instance, the no-salt treatment having the higher (negative) values along PC2. The response of the RSB to the experimental condition was more complex. For instance, the factor Harvest separated the RSB samples along the two main components. Moreover, at the second harvest, RSB samples are more dispersed. Overall, the multivariate analysis indicated that a large proportion of the variance can be linearly explained by two main components, with sorting of the samples strongly influenced by the genotype (e.g., cultivars were fully separated). The variation due to the saline treatments between the two cultivars was different, with a more linear and tight ordination for the GSB.
4. Discussion
By using iso-osmotic nutrient solutions, this study investigated the effect of mild stress of three salts in lettuce and its interaction with successive harvests in two differently pigmented baby leaf cultivars. It is known that controlled saline stress applied in a short growing cycle can be employed to alter the phytochemical value of the edible product of several vegetables [10]. We mainly focused on the phytochemical profile to understand if different salts can provide significantly different responses, and if these responses are also modulated by the variety and the harvest.
We did not observe significant three-way interactions on the key morphological traits under investigation, despite their plasticity [41]. Specifically, the morphological analysis indicated that the main effects of the salt treatment and the harvest were different, and there was little interaction. Conversely, a main effect on all traits was detected for the factor Cultivar. In the control condition (no-salt), the GSB variety performed better compared to the RSB, with slightly taller plants with more leaves. Nonetheless, the RSB compensated for this difference with higher efficiency in terms of leaf area built per biomass. Under non-limiting conditions, the GSB variety was able to produce denser leaves and to increase leaf mass more than leaf area, resulting in a reduction of the SLA. The main effect of the factor Cultivar was not altered by the saline treatments, with the two varieties having a similar morphological response. The response to the saline condition was, as expected, characterized by a growth reduction, and this was not influenced by the kind of salt employed [42]. These alterations are, therefore, mainly due to the higher osmolarity of the saline NS, as also reported in basil [43]. The presence of essential elements such as K and Ca in the saline NSs was not able to provide significant advantages or to mitigate the negative effects of increased salinity, at least in the typical short growing cycle of the baby lettuce under non-limiting mineral availability. The excess of NaCl can cause nutrient deficiencies or imbalances because of the antagonist competitions of the ions with similar features on their availability, transport, and partitioning in plants [44]. An increased supply of a mineral nutrient to the plants under saline conditions is not supposed to improve plant growth if nutrients are already present in adequate amounts in the growth medium [44]. This will also occur when the salt stress is severe, but we did not employ chlorine concentrations that are expected to induce toxicity symptoms [45]. For instance, in cucumber, CaCl2 salinity reduced yield at the high EC, to a level corresponding to low NaCl salinity [46]. Nonetheless, a specific main effect of the NaCl was evident for the leaf succulence only if compared to the no-salt condition, suggesting a slightly more severe effect on this parameter compared to the other two employed salts.
The analysis of polyphenols indicated that the accumulation of these compounds was highly affected by the experimental factors, with significant three-way interactions. This is not unexpected considering that we tested the interaction among genotypic (i.e., the cultivars), environmental (i.e., the type of saline stress), and technical (i.e., successive harvest) factors over a class of highly responsive chemicals. For instance, the analysis of the main effect of the Cultivar highlighted that the higher accumulation in the no-salt condition and the responsiveness to stress of the RSB variety are genetically determined. Red leaf lettuces have a more complex phytochemical profile, and, in the future, it will be possible to test if the association between leaf coloration and flexibility of the response in polyphenols’ accumulation holds true in an ampler panel of red cultivars. It is also necessary to add that the initial level of total phenolic acids and flavonoids strongly varied between the two analyzed cultivars, and this factor has been shown to be common in crop species, and possibly also related to the salt tolerance [47,48,49]. Contrarily to the analysis of the morphological traits, the accumulation of specific phenolic compounds showed differences among the employed salts. In a complex framework of interactions, the CaCl2 solution provided the most interesting results in the RSB cultivar at the second harvest. Finally, our study focused on the quantitative variation of polyphenols, and more comprehensive metabolomics studies may clarify whether the different salts stimulate the synthesis of new polyphenolics.
Considering the multiple interactions and the different nature of the variables of our dataset, we performed a PCA to increase the interpretability of the overall lettuce response and to investigate similarities between samples in the various experimental conditions. The relevance of the two main linear dimensions (explaining 80.6% of the total variance) along with a coherent separation according to at least one factor (the cultivar) suggest that, even in the presence of multiple interactions, meaningful (linear) biological information is contained in the observed responses. Moreover, the PCA highlighted the discriminatory force of the genetic factor and the different variability of the two varieties. Specifically, the response of the GSB to salt was consistently altered by the harvest, with the NaCl condition being more distant from the no-salt control. For the RSB variety, the PCA highlighted the strong interaction between factors, with samples much more spread out at the second harvest than at the first one, and with a lack of a clear clustering of the samples according to salt.
5. Conclusions
Our study revealed the complexity of the lettuce response to mild salinity and supports the notion that certain combinations of pre-harvest factors provide specific results. Notably, the effects of the genotypic factor and its interactions were large even in two relatively similar varieties that belong to the same horticultural group. All this may contribute to explain why in lettuce, and other species, apparently inconclusive results are reported in the literature, especially with respect to the phenolic content and its relation to salt stress and its levels [33,50,51]. The morphological response of baby lettuce to the various saline solutions was wide-ranging and mainly influenced by the osmolarity, with negligible ion-specific alleviating or detrimental effects. The analysis of the phytochemicals indicated the highest level of interaction among experimental factors, further showing the responsiveness of phenolic compounds to environmental factors, as well as the possible role of breeding in fixing divergent features (such as color and phytochemical composition) in differently colored lettuce varieties [52].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae7090264/s1, Figure S1: Scree plot of the PCA representing the proportion of variance accounted for by the principal components. Table S1: Contrast results of the morphological traits significantly affected by the salt (height and fresh weight of the leaves, FWL). The user-defined contrasts (L1, L2, L3, and L4) are specified in the Materials and Methods section. Table S2: Pairwise multiple comparisons table of the effect of the salt treatment on the plant height. Table S3: Pairwise multiple comparison three-way ANOVA table of the effect of the salt treatment on the fresh weight of the leaves. Table S4: Contrast results of the polyphenols significantly affected by the salt. The user-defined contrasts (L1, L2, L3, and L4) are specified in the Materials and Methods section. CE: Contrast Estimate (mg/100 g dw). Table S5: Pairwise multiple comparison table of the effect of the salt treatment on CTA. Table S6: Pairwise multiple comparison table of the effect of the salt treatment on 5-CQA. Table S7: Pairwise multiple comparison table of the effect of the salt treatment on DCTA. Table S8: Pairwise multiple comparison table of the effect of the salt treatment on m-DCTA. Table S9: Pairwise multiple comparison table of the effect of the salt treatment on m-3,5-CQA. Table S10: Pairwise multiple comparison table of the effect of the salt treatment on the sum of polyphenols.
Author Contributions
Conceptualization, G.C. and Y.R.; methodology, G.C. and Y.R.; software, G.A.S.; validation, G.C. and G.A.S.; formal analysis, G.C., P.V., and G.A.S.; investigation, G.C. and G.A.S.; resources, Y.R.; data curation, G.C., G.A.S., and Y.R.; writing—original draft preparation, G.C.; writing—review and editing, G.C., P.V., G.A.S., M.C.K., and Y.R.; visualization, G.C. and Y.R.; supervision, G.C. and Y.R.; project administration, Y.R.; funding acquisition, G.C. and Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material. The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to Francesco Napolitano, Emilio Di Stasio, and Giampaolo Raimondi for their assistance in the greenhouse management.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mou, B. Lettuce. Vegetables I; Springer: New York, NY, USA, 2008; pp. 75–116. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Tudela, J.A.; Marín, A.; Martínez-Sánchez, A.; Luna, M.C.; Gil, M.I. Preharvest and postharvest factors related to off-odours of fresh-cut iceberg lettuce. Postharvest Biol. Technol. 2013, 86, 463–471. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Timmermann, B.N.; Steelink, C.; Loewus, F.A. Phytochemical Adaptations to Stress; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 18. [Google Scholar]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food–the impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Franken, P.; Krumbein, A.; Kläring, H.-P.; Bar-Yosef, B. Nutrient Management in Soilless Culture in the Conflict of Plant, Microorganism, Consumer and Environmental Demands. In Proceedings of the International Symposium on Soilless Culture and Hydroponics 843, Lima, Peru, 25–28 August 2008; pp. 27–34. [Google Scholar]
- Tomasi, N.; Pinton, R.; Dalla Costa, L.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New ‘solutions’ for floating cultivation system of ready-to-eat salad: A review. Trends Food Sci. Technol. 2015, 46, 267–276. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Ghoname, A.; Abou-Hussein, S.; El-Tohamy, W. Eustress (positive stress) salinity as an enhancement tool for bioactive ingredients and quality characteristics of vegetables. A review. Sciences 2019, 9, 456–463. [Google Scholar]
- Zushi, K.; Matsuzoe, N. Metabolic profile of organoleptic and health-promoting qualities in two tomato cultivars subjected to salt stress and their interactions using correlation network analysis. Sci. Hortic. 2015, 184, 8–17. [Google Scholar] [CrossRef]
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.F.; Urrestarazu, M.; Álvaro, J.E. Increased electrical conductivity in nutrient solution management enhances dietary and organoleptic qualities in soilless culture tomato. HortScience 2017, 52, 868–872. [Google Scholar] [CrossRef]
- Marin, A.; Rubio, J.S.; Martinez, V.; Gil, M.I. Antioxidant compounds in green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J. Sci. Food Agric. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Giuffrida, F.; Cassaniti, C.; Malvuccio, A.; Leonardi, C. Effects of salt stress imposed during two growth phases on cauliflower production and quality. J. Sci. Food Agric. 2017, 97, 1552–1560. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]
- Hegazi, A.M.; El-Shraiy, A.M.; Ghoname, A. Alleviation of salt stress adverse effect and enhancing phenolic anti-oxidant content of eggplant by seaweed extract. Gesunde Pflanz. 2015, 67, 21–31. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, A.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Berenguer, C.; Martínez-Ballesta, M.A.D.C.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Growing hardier crops for better health: Salinity tolerance and the nutritional value of broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kogi, M.; Yanagisawa, T. Effects of salinity and nutrients in seawater on hydroponic culture of red leaf lettuce. Environ. Control Biol. 2014, 52, 189–195. [Google Scholar] [CrossRef][Green Version]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. Acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Svecova, E.; Rea, E.; Lucini, L. Effects of saline stress on mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon genotypes grown in floating system. J. Sci. Food Agric. 2013, 93, 1119–1127. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Martinez-Ballesta, M.C.; Riquelme, F.; Carvajal, M.; Garcia-Viguera, C.; Moreno, D.A. Novel varieties of broccoli for optimal bioactive components under saline stress. J. Sci. Food Agric. 2011, 91, 1638–1647. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar]
- Carillo, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Kyriacou, M.C.; Sifola, M.I.; Rouphael, Y. Physiological and nutraceutical quality of green and red pigmented lettuce in response to NaCl concentration in two successive harvests. Agronomy 2020, 10, 1358. [Google Scholar] [CrossRef]
- Carillo, P.; Soteriou, G.A.; Kyriacou, M.C.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Mola, I.D.; Mori, M.; Rouphael, Y. Regulated salinity eustress in a floating hydroponic module of sequentially harvested lettuce modulates phytochemical constitution, plant resilience, and post-harvest nutraceutical quality. Agronomy 2021, 11, 1040. [Google Scholar] [CrossRef]
- Neocleous, D.; Koukounaras, A.; Siomos, A.; Vasilakakis, M. Assessing the salinity effects on mineral composition and nutritional quality of green and red “baby” lettuce. J. Food Qual. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Gary, C.; Jones, J.; Longuenesse, J. Modelling Daily Changes in Specific Leaf Area of Tomato: The Contribution of the Leaf Assimilate Pool. In Proceedings of the International Workshop on Greenhouse Crop Models 328, Saumane, France, 25 August 1991; pp. 205–210. [Google Scholar]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Corrado, G.; De Micco, V.; Lucini, L.; Miras-Moreno, B.; Senizza, B.; Zengin, G.; El-Nakhel, C.; De Pascale, S.; Rouphael, Y. Isosmotic macrocation variation modulates mineral efficiency, morpho-physiological traits, and functional properties in hydroponically grown lettuce varieties (Lactuca sativa L.). Front. Plant Sci. 2021, 12, 1010. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, S.; Milla, R. Differential plasticity to water and nutrients between crops and their wild progenitors. Environ. Exp. Bot. 2018, 145, 54–63. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar]
- Scagel, C.F.; Bryla, D.R.; Lee, J. Salt exclusion and mycorrhizal symbiosis increase tolerance to NaCl and CaCl2 salinity in ‘Siam Queen’ basil. HortScience 2017, 52, 278–287. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Rosales, M.A.; Cubero-Font, P.; Calvo, P.; Álvarez, R.; Diaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 2019, 99, 815–831. [Google Scholar]
- Trajkova, F.; Papadantonakis, N.; Savvas, D. Comparative effects of NaCl and CaCl2 salinity on cucumber grown in a closed hydroponic system. HortScience 2006, 41, 437–441. [Google Scholar] [CrossRef]
- Hichem, H.; Mounir, D. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop. Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Ghazanfar, A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 2006, 163, 723–730. [Google Scholar] [CrossRef]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby romaine lettuce (Lactuca sativa L. Cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Huang, J.; Gruber, M.Y.; Kaddour, R.; Lachaal, M.; Ouerghi, Z.; Hannoufa, A. The impact of genotype and salinity on physiological function, secondary metabolite accumulation, and antioxidative responses in lettuce. J. Agric. Food Chem. 2010, 58, 5122–5130. [Google Scholar] [CrossRef]
- Zhang, L.; Su, W.; Tao, R.; Zhang, W.; Chen, J.; Wu, P.; Yan, C.; Jia, Y.; Larkin, R.M.; Lavelle, D. RNA sequencing provides insights into the evolution of lettuce and the regulation of flavonoid biosynthesis. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).