Advances and Challenges in RNA Interference Technology for Citrus Huanglongbing Vector Control
Abstract
:1. Introduction
2. RNAi Machinery
3. Challenges in RNAi-Mediated Silencing for Pest Control
3.1. dsRNA Uptake and Spread into the Insect Body
3.2. dsRNA Delivery
3.3. dsRNA Stability and Efficiency
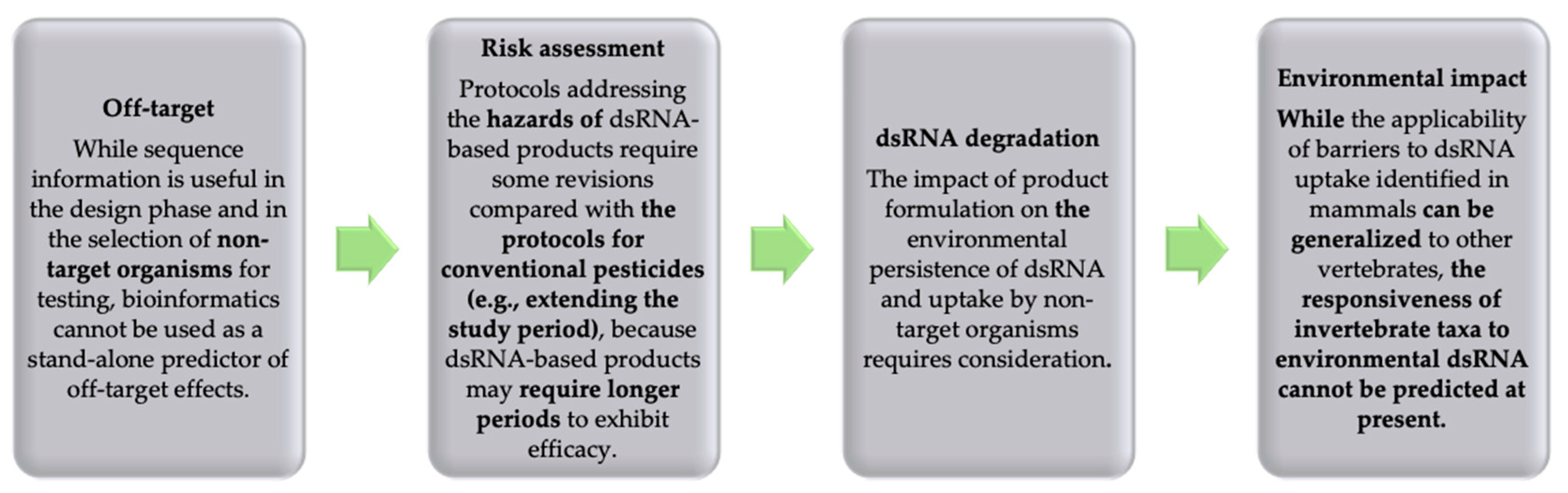
3.4. Off-Targets
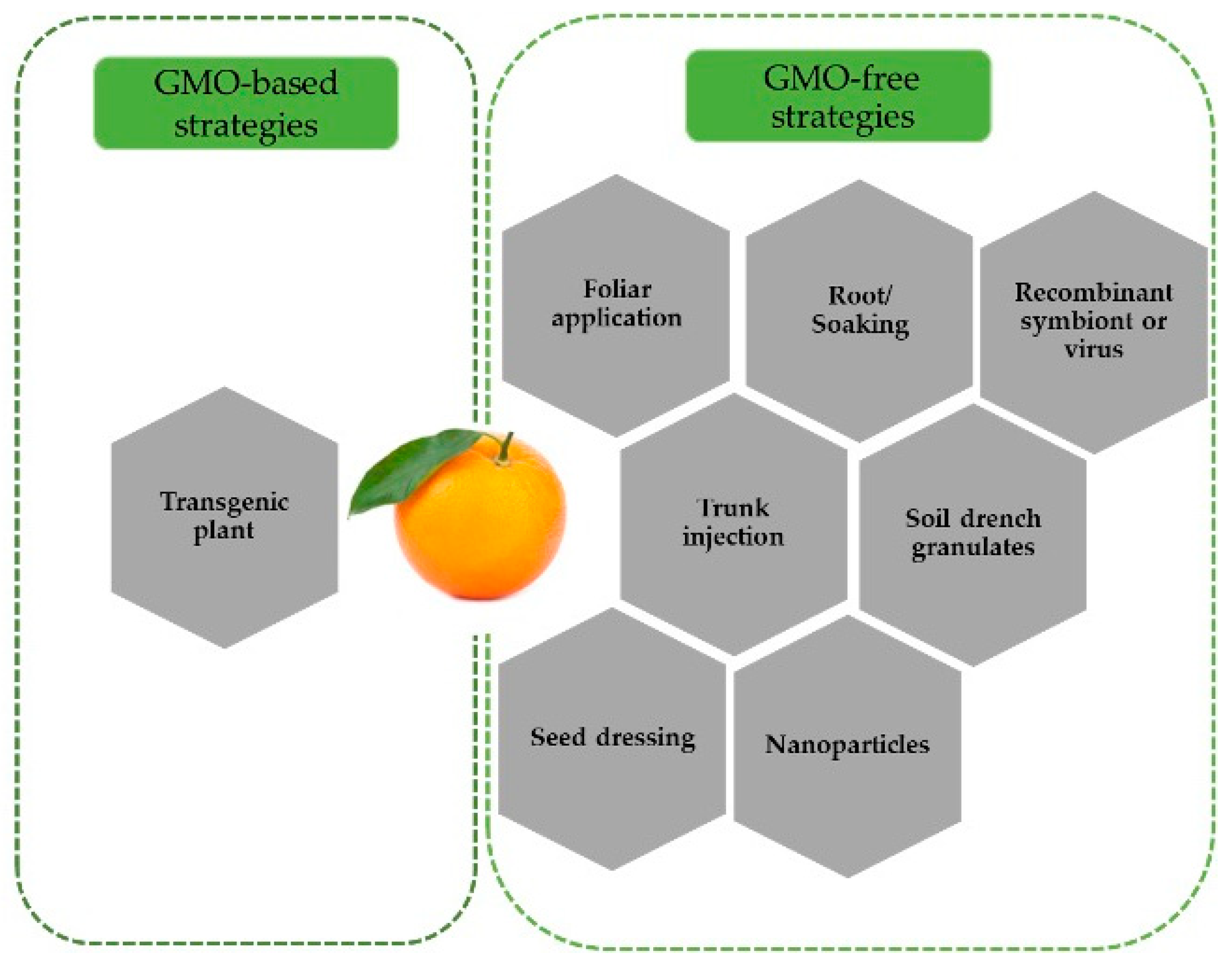
4. ACP Control Using RNAi Technology
5. Primary Considerations and Future Prospects of RNAi Strategies for ACP Control in Citrus Orchards
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rampersaud, G.C.; Valim, M.F. 100% Citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef]
- Neves, M.F.; Trombin, V.G.; Marques, V.N.; Martinez, L.F. Global orange juice market: A 16-year summary and opportunities for creating value. Trop. Plant Pathol. 2020, 45, 166–174. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Spreen, T.H.; Zansler, M.L. Economic analysis of incentives to plant citrus trees in Florida. HortTechnology 2016, 26, 720–726. [Google Scholar] [CrossRef]
- Ozkan, B.; Akcaoz, H.; Karadeniz, F. Energy requirement and economic analysis of citrus production in Turkey. Energy Convers. Manag. 2004, 45, 1821–1830. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Lopes, S.A.; de Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus Huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Zhou, C. The status of citrus Huanglongbing in China. Trop. Plant Pathol. 2020, 45, 279–284. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Zheng, Z.; Chen, J.; Deng, X. Historical perspectives, management, and current research of citrus HLB in Guangdong province of China, where the disease has been endemic for over a hundred years. Phytopathology 2018, 108, 1224–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of Huanglongbing pathogens. PLoS ONE 2014, 9, e112331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belasque, J., Jr.; Yamamoto, P.T.; de Miranda, M.P.; Bassanezi, R.B.; Ayres, A.J.; Bové, J.M. Controle do Huanglongbing no estado de São Paulo, Brasil. Citrus Res. Technol. 2010, 31, 53–64. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jaouannet, M.; Dempsey, D.A.; Imani, J.; Coustau, C.; Kogel, K.H. RNA-based technologies for insect control in plant production. Biotechnol. Adv. 2020, 39. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.C.; Hunter, W.B. RNAi feeding bioassay: Development of a non-transgenic approach to control Asian citrus psyllid and other hemipterans. Entomol. Exp. Appl. 2017, 162, 389–396. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Yu, X.; Killiny, N. RNA interference-mediated control of Asian citrus psyllid, the vector of the Huanglongbing bacterial pathogen. Trop. Plant Pathol. 2020, 45, 298–305. [Google Scholar] [CrossRef]
- Alamalakala, L.; Parimi, S.; Patel, N.; Char, B. Insect RNAi: Integrating a new tool 10 in the crop protection toolkit. In Trends in Insect Molecular Biology and Biotechnology; Kumar, D., Gong, C., Eds.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-61343-7. [Google Scholar]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A Perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Nehela, Y.; Killiny, N. Revisiting the complex pathosystem of Huanglongbing: Deciphering the role of citrus metabolites in symptom development. Metabolites 2020, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Flynt, A.S. Insecticidal RNA interference, thinking beyond long dsRNA. Pest Manag. Sci. 2021, 77, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef]
- Sijen, T.; Fleenor, J.; Simmer, F.; Thijssen, K.L.; Parrish, S.; Timmons, L.; Plasterk, R.H.; Fire, A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 2001, 107, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Taning, C.N.T.; Andrade, E.C.; Hunter, W.B.; Christiaens, O.; Smagghe, G. Asian citrus psyllid RNAi pathway-RNAi evidence. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivashuta, S.; Zhang, Y.; Wiggins, B.E.; Ramaseshadri, P.; Segers, G.C.; Johnson, S.; Meyer, S.E.; Kerstetter, R.A.; McNulty, B.C.; Bolognesi, R.; et al. Environmental RNAi in herbivorous insects. RNA 2015, 21, 840–850. [Google Scholar] [CrossRef] [Green Version]
- Winston, W.M.; Sutherlin, M.; Wright, A.J.; Feinberg, E.H.; Hunter, C.P. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc. Natl. Acad. Sci. USA 2007, 104, 10565–10570. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, E.H.; Hunter, C.P. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003, 301, 1545–1547. [Google Scholar] [CrossRef] [Green Version]
- Shih, J.D.; Hunter, C.P. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA 2011, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.M.W.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Winston, W.M.; Molodowitch, C.; Hunter, C.P. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 2002, 295, 2456–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Han, Z. Cloning and phylogenetic analysis of SID-1-like genes from aphids. J. Insect Sci. 2008, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; Gao, X.; Xu, J.; Liang, X.; Li, Q.; Yao, J.; Zhu, K.Y. Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem. Mol. Biol. 2015, 60, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.G.; Robinson, K.E.; Asgari, S.; Mitter, N. Current scenario of RNAi-based hemipteran control. Pest Manag. Sci. 2021, 77, 2188–2196. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- El-Shesheny, I.; Hijaz, F.; El-Hawary, I.; Mesbah, I.; Killiny, N. Impact of different temperatures on survival and energy metabolism in the Asian citrus psyllid, Diaphorina citri Kuwayama. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 192, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ortega, Y.; Killiny, N. Silencing of sucrose hydrolase causes nymph mortality and disturbs adult osmotic homeostasis in Diaphorina citri (Hemiptera: Liviidae). Insect Biochem. Mol. Biol. 2018, 101, 131–143. [Google Scholar] [CrossRef]
- Killiny, N.; Kishk, A. Delivery of dsRNA through topical feeding for RNA interference in the citrus sap piercing-sucking hemipteran, Diaphorina citri. Arch. Insect Biochem. Physiol. 2017, 95, 1–13. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Yao, J.; Luttrell, R. Identification of genes potentially responsible for extra-oral digestion and overcoming plant defense from salivary glands of the tarnished plant bug (Hemiptera: Miridae) using cDNA sequencing. J. Insect Sci. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Gowda, S.; Killiny, N. Double-stranded RNA delivery through soaking mediates silencing of the muscle protein 20 and increases mortality to the Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2017, 73, 1846–1853. [Google Scholar] [CrossRef]
- Yu, X.; Killiny, N. Effect of silencing a boule homologue on the survival and reproduction of Asian citrus psyllid Diaphorina citri. Physiol. Entomol. 2018, 43, 268–275. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Z.; Zhang, C.; Liu, X.; Wang, J.; Xin, T.; Xia, B. Knockdown of the trehalose-6-phosphate synthase gene using rna interference inhibits synthesis of trehalose and increases lethality rate in Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Insects 2020, 11, 605. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, H.B.; Fan, J.Y.; Liu, T.Y.; Meng, L.W.; Liu, Y.; Yu, H.Z.; Dou, W.; Wang, J.J. An odorant-binding protein of Asian citrus psyllid, Diaphorina citri, participates in the response of host plant volatiles. Pest Manag. Sci. 2021. [Google Scholar] [CrossRef]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double-stranded RNA oral delivery methods to induce RNA interference in phloem and plant-sap-feeding hemipteran insects. J. Vis. Exp. 2018, 135, 57390. [Google Scholar] [CrossRef] [Green Version]
- Andrade, E.D.; Hunter, W.B. RNA Interference—Natural Gene-Based Technology for Highly Specific Pest Control (HiSPeC). In RNA Interference; Abdurakhmonov, I.Y., Ed.; IntechOpen: Rijeka, Croatia, 2016; pp. 391–409. ISBN 978-953-51-2272-2. [Google Scholar]
- Arnosti, A.; Delalibera Jr, I.; Conceschi, M.R.; Travaglini, R.V.; Camargo-Mathias, M.I. Morphological mapping of the integument of adult females of Diaphorina citri Kuwayama, targeting the development of control strategies. Int. J. Adv. Agric. Res. 2016, 4, 57–64. [Google Scholar] [CrossRef]
- Silver, K.; Cooper, A.M.W.; Zhu, K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021. [Google Scholar] [CrossRef]
- Christiaens, O.; Swevers, L.; Smagghe, G. DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014, 53, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Liu, Z.C.; Huang, S.L.; Chen, Z.Q.; Sun, Y.W.; Duan, P.F.; Ma, Y.Z.; Xia, L.Q. RNAi-mediated plant protection against Aphids. Pest Manag. Sci. 2016, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, R.; Guo, H.; Miao, X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 2015, 38, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Khajuria, C.; Rangasamy, M.; Gandra, P.; Fitter, M.; Geng, C.; Woosely, A.; Hasler, J.; Schulenberg, G.; Worden, S.; et al. Long dsRNA but not siRNA initiates RNAi in western corn rootworm larvae and adults. J. Appl. Entomol. 2015, 139, 432–445. [Google Scholar] [CrossRef]
- El-Shesheny, I.; Hajeri, S.; El-Hawary, I.; Gowda, S.; Killiny, N. Silencing abnormal wing disc gene of the Asian citrus psyllid, Diaphorina citri disrupts adult wing development and increases nymph mortality. PLoS ONE 2013, 8, e65392. [Google Scholar] [CrossRef] [Green Version]
- Killiny, N.; Hajeri, S.; Tiwari, S.; Gowda, S.; Stelinski, L.L. Double-stranded RNA uptake through topical application, mediates silencing of five CYP4 genes and suppresses insecticide resistance in Diaphorina citri. PLoS ONE 2014, 9, e110536. [Google Scholar] [CrossRef] [Green Version]
- Galdeano, D.M.; Breton, M.C.; Lopes, J.R.S.; Falk, B.W.; Machado, M.A. Oral delivery of double-stranded RNAs induces mortality in nymphs and adults of the Asian citrus psyllid, Diaphorina citri. PLoS ONE 2017, 12, e0171847. [Google Scholar] [CrossRef] [PubMed]
- Kishk, A.; Hijaz, F.; Anber, H.A.I.; AbdEl-Raof, T.K.; El-Sherbeni, A.E.H.D.; Hamed, S.; Killiny, N. RNA Interference of Acetylcholinesterase in the Asian Citrus Psyllid, Diaphorina citri, Increases its Susceptibility to Carbamate and Organophosphate Insecticides; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 143, ISBN 1863956883. [Google Scholar]
- Angelotti-Mendonça, J.; Bassan, M.M.; Marques, J.P.R.; Yamamoto, P.T.; Figueira, A.; Piedade, S.M.D.S.; Mourão Filho, F.A.A. Knockdown of calreticulin, laccase, and snf7 genes through RNAi is not effective to control the Asian citrus psyllid (Hemiptera: Livideae). J. Econ. Entomol. 2020, 113, 2931–2940. [Google Scholar] [CrossRef]
- Hunter, W.B.; Sinisterra-Hunter, X.H. Emerging RNA Suppression Technologies to Protect Citrus Trees from Citrus Greening Disease Bacteria, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 55, ISBN 9780128150795. [Google Scholar]
- Bennett, M.; Deikman, J.; Hendrix, B.; Iandolino, A. Barriers to efficient foliar uptake of dsRNA and molecular barriers to dsRNA activity in plant cells. Front. Plant Sci. 2020, 11, 816. [Google Scholar] [CrossRef]
- Dias, N.P.; Cagliari, D.; dos Santos, E.A.; Smagghe, G.; Jurat-Fuentes, J.L.; Mishra, S.; Nava, D.E.; Zotti, M.J. Insecticidal gene silencing by RNAi in the neotropical region. Neotrop. Entomol. 2020, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ren, B.Y.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pugsley, C.E.; Isaac, R.E.; Warren, N.J.; Cayre, O.J. Recent advances in engineered nanoparticles for RNAi-mediated crop protection against insect pests. Front. Agron. 2021, 3, 9. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Dziewięcka, M.; Pawlyta, M.; Majchrzycki, Ł.; Balin, K.; Barteczko, S.; Czerkawska, M.; Augustyniak, M. The structure—properties—cytotoxicity interplay: A crucial pathway to determining graphene oxide biocompatibility. Int. J. Mol. Sci. 2021, 22, 5401. [Google Scholar] [CrossRef]
- Laisney, J.; Loczenski Rose, V.; Watters, K.; Donohue, K.V.; Unrine, J.M. Delivery of short hairpin RNA in the neotropical brown stink bug, Euschistus heros, using a composite nanomaterial. Pestic. Biochem. Physiol. 2021, 177, 104906. [Google Scholar] [CrossRef]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef]
- Neumeier, J.; Meister, G. siRNA Specificity: RNAi mechanisms and strategies to reduce off-target effects. Front. Plant Sci. 2021, 11, 2196. [Google Scholar] [CrossRef]
- Hannus, M.; Beitzinger, M.; Engelmann, J.C.; Weickert, M.T.; Spang, R.; Hannus, S.; Meister, G. SiPools: Highly complex but accurately defined siRNA pools eliminate off-target effects. Nucleic Acids Res. 2014, 42, 8049–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. DsRNA uptake in plant pests and pathogens: Insights into RNAi-based insect and fungal control technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vélez, A.M.; Fishilevich, E. The mysteries of insect RNAi: A focus on dsRNA uptake and transport. Pestic. Biochem. Physiol. 2018, 151, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Pampolini, F.; Rieske, L.K. Emerald Ash borer specific gene silencing has no effect on non-target organisms. Front. Agron. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Lück, S.; Kreszies, T.; Strickert, M.; Schweizer, P.; Kuhlmann, M.; Douchkov, D. siRNA-Finder (si-Fi) Software for RNAi-target design and off-target prediction. Front. Plant Sci. 2019, 10, 1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelsohn, M.L.; Gathmann, A.; Kardassi, D.; Sachana, M.; Hopwood, E.M.; Dietz-Pfeilstetter, A.; Michelsen-Correa, S.; Fletcher, S.J.; Székács, A. Summary of discussions from the 2019 OECD conference on RNAi based pesticides. Front. Plant Sci. 2020, 11, 740. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Petrick, J.S. Safety considerations for humans and other vertebrates regarding agricultural uses of externally applied RNA molecules. Front. Plant Sci. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzetti, B.; Smagghe, G.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Kostov, K.; Sabbadini, S.; Opsahl-Sorteberg, H.G.; Ventura, V.; et al. RNAi: What is its position in agriculture? J. Pest Sci. 2020, 93, 1125–1130. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Koci, J.; Ramaseshadri, P.; Bolognesi, R.; Segers, G.; Flannagan, R.; Park, Y. Ultrastructural changes caused by Snf7 RNAi in larval enterocytes of western corn rootworm (Diabrotica virgifera virgifera Le Conte). PLoS ONE 2014, 9, e83985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNAi-mediated mortality in southern green stinkbug Nezara viridula by oral delivery of dsRNA. Pest Manag. Sci. 2021, 77, 77–84. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Smagghe, G.; Sharma, R.; Oliveira, E.E.; Christiaens, O. Liposome encapsulation and EDTA formulation of dsRNA targeting essential genes increase oral RNAi-caused mortality in the Neotropical stink bug Euschistus heros. Pest Manag. Sci. 2019, 75, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Abdellatef, E. Tuning beforehand: A foresight on RNA interference (RNAi) and in vitro-derived dsRNAs to enhance crop resilience to biotic and abiotic stresses. Mol. Sci. 2021, 22, 7687. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus Huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Guo, S.H.; Liu, Y.M.; Wang, Z.Y.; Wang, F.F.; Mao, Y.K.; Hu, Y.W.; Han, P.; Cuthbertson, A.G.S.; Qiu, B.L.; Sang, W. Transcriptome analysis reveals TOR signalling-mediated plant flush shoots governing Diaphorina citri Kuwayama oviposition. Insect. Mol. Biol. 2021, 30, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, D.; Avila dos Santos, E.; Dias, N.; Smagghe, G.; Zotti, M. Nontransformative strategies for RNAi in crop protection. Modul. Gene Expr.-Abridging RNAi Cris. Technol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef] [PubMed]
- Campos, H. The Innovation Revolution in Agriculture; Springer: Cham, Switzerland, 2021; ISBN 9783030509903. [Google Scholar]
| Target Gene | Gene Target Function | dsRNA Delivery | Developmental Stage | Result | Reference |
| Abnormal wings disc (awd) | Essential for adult wing formation | Topical application | Fifth instar nymphs | Increased nymph mortality and abnormal wing development in adults | [53] |
| Cytochrome P450 gene family 4 (CYP4) | Detoxifying enzyme related to insecticide resistance | Topical application | Adults | Increased insecticide susceptibility | [54] |
| Arginine kinase | Energy mobilization | In plant system | Adults | Increased adult mortality | [26] |
| Superoxide dismutase (SOD) | Antioxidant defense | In plant system | Adults | Increases adult mortality | |
| Pterin-4-alpha-carbinolamine dehydratase (PCDB1) | Cell metabolism | In plant system | Adults | None | |
| Tomosyn | Nervous system | In plant system | Adults | None | |
| Vitellogenin | Reproduction | In plant system | Adults | None | |
| Cathepsin D | Metamorphic events | Ingestion through artificial diet and in plant system | Adults and fifth instar nymphs | Increased insect mortality | [55] |
| Chitin synthase | Chitin synthesis | Ingestion through artificial diet and in plant system | Adults and fifth instar nymphs | Increased insect mortality | |
| Inhibitor of apoptosis | Regulation of the apoptotic machinery | Ingestion through artificial diet and in plant system | Adults and fifth instar nymphs | Increased insect mortality | |
| Esterase FE4-like (EstFE4); acetylcholinesterase (AChe) | Detoxifying enzyme related to insecticide resistance | Topical feeding | Fourth and fifth instar nymphs | Increased mortality of nymphs and, consequently, of emerged adults | [56] |
| Muscle protein 20 (DcMP20) | Encodes a cytoskeletal protein | Soaking | Third and fourth instar nymphs | Increased nymph mortality | [41] |
| Abnormal wings disc (awd) | Essential for adult wing formation | Topical feeding | Adults and fifth instar nymphs | Increased nymph mortality and deformed adults | [39] |
| Boule | Fertility | Feeding in artificial diet | Adults | Increased adult mortality, but no effect on number of eggs | [42] |
| Sucrose hydrolase (DcSuh) | Enhanced absorption of sucrose from the midgut | Topical application | Fifth instar nymphs | Increased nymph mortality and shortened insect lifecycle duration | [38] |
| Laccase-1-S | Detoxification of secondary plant compounds in insects | In plant system | Adults | None | [57] |
| DcSnf7 | Transport of proteins for degradation via the endosomal autophagic pathway | In plant system | Adults | None | |
| Calreticulin | Calcium ion chelation, which helps maintain phloem circulation | In plant system | Adults | None | |
| Trehalose-6-phosphate synthase | Energy synthesis; metamorphosis | Feeding in artificial diet | Fifth instar nymphs | Increased nymph mortality and deformed adults | [43] |
| Odorant-binding 7 | Host plant volatile compound recognition | Ingestion through artificial diet | Adults | Suppressed electrophysiological responses of antennae and disrupted insect behavioral responses | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, V.V.; Angelotti-Mendonça, J.; Roberto, S.R. Advances and Challenges in RNA Interference Technology for Citrus Huanglongbing Vector Control. Horticulturae 2021, 7, 277. https://doi.org/10.3390/horticulturae7090277
Marques VV, Angelotti-Mendonça J, Roberto SR. Advances and Challenges in RNA Interference Technology for Citrus Huanglongbing Vector Control. Horticulturae. 2021; 7(9):277. https://doi.org/10.3390/horticulturae7090277
Chicago/Turabian StyleMarques, Viviani Vieira, Jéssika Angelotti-Mendonça, and Sergio Ruffo Roberto. 2021. "Advances and Challenges in RNA Interference Technology for Citrus Huanglongbing Vector Control" Horticulturae 7, no. 9: 277. https://doi.org/10.3390/horticulturae7090277
APA StyleMarques, V. V., Angelotti-Mendonça, J., & Roberto, S. R. (2021). Advances and Challenges in RNA Interference Technology for Citrus Huanglongbing Vector Control. Horticulturae, 7(9), 277. https://doi.org/10.3390/horticulturae7090277








