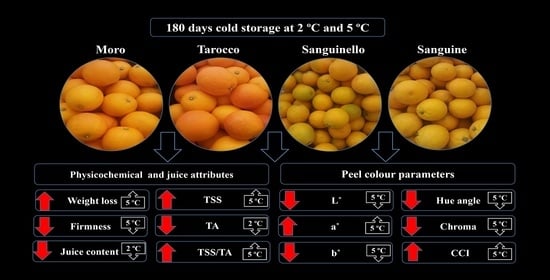
Physicochemical Changes, Peel Colour, and Juice Attributes of Blood Orange Cultivars Stored at Different Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Storage Conditions
2.2. Weight Loss
2.3. Firmness
2.4. Chemical Attributes of Juice
2.5. Juice Content
2.6. Peel Colour
2.7. Statistical Analysis
3. Results
3.1. Weight Loss
3.2. Firmness
3.3. Chemical Attributes of Juice
3.4. Juice Content
3.5. Peel Colour
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons Control Fruit-Specific, Cold-Dependent Accumulation of Anthocyanins in Blood Oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef] [Green Version]
- Piero, A.R.L. The State of the Art in Biosynthesis of Anthocyanins and Its Regulation in Pigmented Sweet Oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bellomo, S.E.; Intelisano, S. Storage Temperature Effects on Blood Orange Fruit Quality. J. Agric. Food Chem. 2001, 49, 3230–3235. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Galvano, F.; Mistretta, A.; Marventano, S.; Nolfo, F.; Calabrese, G.; Buscemi, S.; Drago, F.; Veronesi, U.; Scuderi, A.G. Red Orange: Experimental Models and Epidemiological Evidence of Its Benefits on Human Health. Oxidative Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ -aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D. Changes in Bioactive Compounds, Antioxidant Activity, and Nutritional Quality of Blood Orange Cultivars at Different Storage Temperatures. Antioxidants 2020, 9, 1016. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, C.; Xu, S.; Chen, Y.; Wang, Y.; Li, X.; Sun, C. The effects of transportation temperature on the decay rate and quality of postharvest Ponkan (Citrus reticulata Blanco) fruit in different storage periods. Sci. Hortic. 2019, 247, 42–48. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Effect of Various Postharvest Treatment on Aroma Volatile Compounds of Blood Orange Fruit Exposed to Chilling Temperature After Long-Term Storage. Food Bioprocess Technol. 2020, 13, 2054–2064. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Valero, D. Susceptibility of Blood Orange Cultivars to Chilling Injury Based on Antioxidant System and Physiological and Biochemical Responses at Different Storage Temperatures. Foods 2020, 9, 1609. [Google Scholar] [CrossRef]
- Habibi, F.; García-Pastor, M.E.; Guillén, F.; Serrano, M.; Valero, D. Fatty acid composition in relation to chilling susceptibility of blood orange cultivars at different storage temperatures. Plant Physiol. Biochem. 2021, 166, 770–776. [Google Scholar] [CrossRef]
- Strano, M.C.; Di Silvestro, S.; Allegra, M.; Russo, G.; Caruso, M. Effect of cold storage on the postharvest quality of different Tarocco sweet orange clonal selections. Sci. Hortic. 2021, 285, 110167. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A. Vacuum infiltration of putrescine enhances bioactive compounds and maintains quality of blood orange during cold storage. Food Chem. 2017, 227, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moscoso-Ramírez, P.A.; Palou, L. Effect of ethylene degreening on the development of postharvest penicillium molds and fruit quality of early season citrus fruit. Postharvest Biol. Technol. 2014, 91, 1–8. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Sun, C.-D.; Zhang, L.-L.; Dai, X.; Xu, C.-J.; Chen, K.-S. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hortic. 2010, 126, 229–235. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sun, X.-H.; Xiong, J.-J.; Zhu, A.-D.; Zhang, L.; Ma, Q.-L.; Xu, J.; Cheng, Y.-J.; Deng, X.-X. Sugars and organic acids changes in pericarp and endocarp tissues of pumelo fruit during postharvest storage. Sci. Hortic. 2012, 142, 112–117. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Asencio, A.D.; Serrano, M.; Martínez, S.G.; Pretel, M.T. Organic acids, sugars, antioxidant activity, sensorial and other fruit characteristics of nine traditional Spanish Citrus fruits. Eur. Food Res. Technol. 2018, 244, 1497–1508. [Google Scholar] [CrossRef]
- Habibi, F.; Serrano, M.; Zacarías, L.; Valero, D.; Guillén, F. Postharvest Application of 24-Epibrassinolide Reduces Chilling Injury Symptoms and Enhances Bioactive Compounds Content and Antioxidant Activity of Blood Orange Fruit. Front. Plant Sci. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Attanayake, S.; Walker, S.; Gunson, A.; Boldingh, H.; MacRae, E. Acidity and taste in kiwifruit. Postharvest Biol. Technol. 2004, 32, 159–168. [Google Scholar] [CrossRef]
- Cao, J.; Kang, C.; Chen, Y.; Karim, N.; Wang, Y.; Sun, C. Physiochemical changes in Citrus reticulata cv. Shatangju fruit during vesicle collapse. Postharvest Biol. Technol. 2020, 165, 111180. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquezar, B.; Alos, E.; Lado, J.; Zacarias, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Carmona, L.; Zacarías, L.; Rodrigo, M.J. Stimulation of coloration and carotenoid biosynthesis during postharvest storage of ‘Navelina’ orange fruit at 12 °C. Postharvest Biol. Technol. 2012, 74, 108–117. [Google Scholar] [CrossRef]
- Ramezanian, A.; Dadgar, R.; Habibi, F. Postharvest Attributes of “Washington Navel” Orange as Affected by Preharvest Foliar Application of Calcium Chloride, Potassium Chloride, and Salicylic Acid. Int. J. Fruit Sci. 2017, 18, 68–84. [Google Scholar] [CrossRef]
- Van Wyk, A.A.; Huysamer, M.; Barry, G.H. Extended low-temperature shipping adversely affects rind colour of ‘Palmer Navel’ sweet orange [Citrus sinensis (L.) Osb.] due to carotenoid degradation but can partially be mitigated by optimising post-shipping holding temperature. Postharvest Biol. Technol. 2009, 53, 109–116. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y.; Kato, M.; Nakajima, N.; Hasegawa, Y. Effect of Postharvest Temperature and Ethylene on Carotenoid Accumulation in the Flavedo and Juice Sacs of Satsuma Mandarin (Citrus unshiu Marc.) Fruit. J. Agric. Food Chem. 2009, 57, 4724–4732. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, F.; Guillén, F.; Serrano, M.; Valero, D. Physicochemical Changes, Peel Colour, and Juice Attributes of Blood Orange Cultivars Stored at Different Temperatures. Horticulturae 2021, 7, 320. https://doi.org/10.3390/horticulturae7090320
Habibi F, Guillén F, Serrano M, Valero D. Physicochemical Changes, Peel Colour, and Juice Attributes of Blood Orange Cultivars Stored at Different Temperatures. Horticulturae. 2021; 7(9):320. https://doi.org/10.3390/horticulturae7090320
Chicago/Turabian StyleHabibi, Fariborz, Fabián Guillén, María Serrano, and Daniel Valero. 2021. "Physicochemical Changes, Peel Colour, and Juice Attributes of Blood Orange Cultivars Stored at Different Temperatures" Horticulturae 7, no. 9: 320. https://doi.org/10.3390/horticulturae7090320