Mitigation of Commercial Food Waste-Related Salinity Stress Using Halotolerant Rhizobacteria in Chinese Cabbage Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Isolation of Rhizospheric Bacteria
2.2. Screening Rhizobacterial Isolates for Plant Growth Promotion
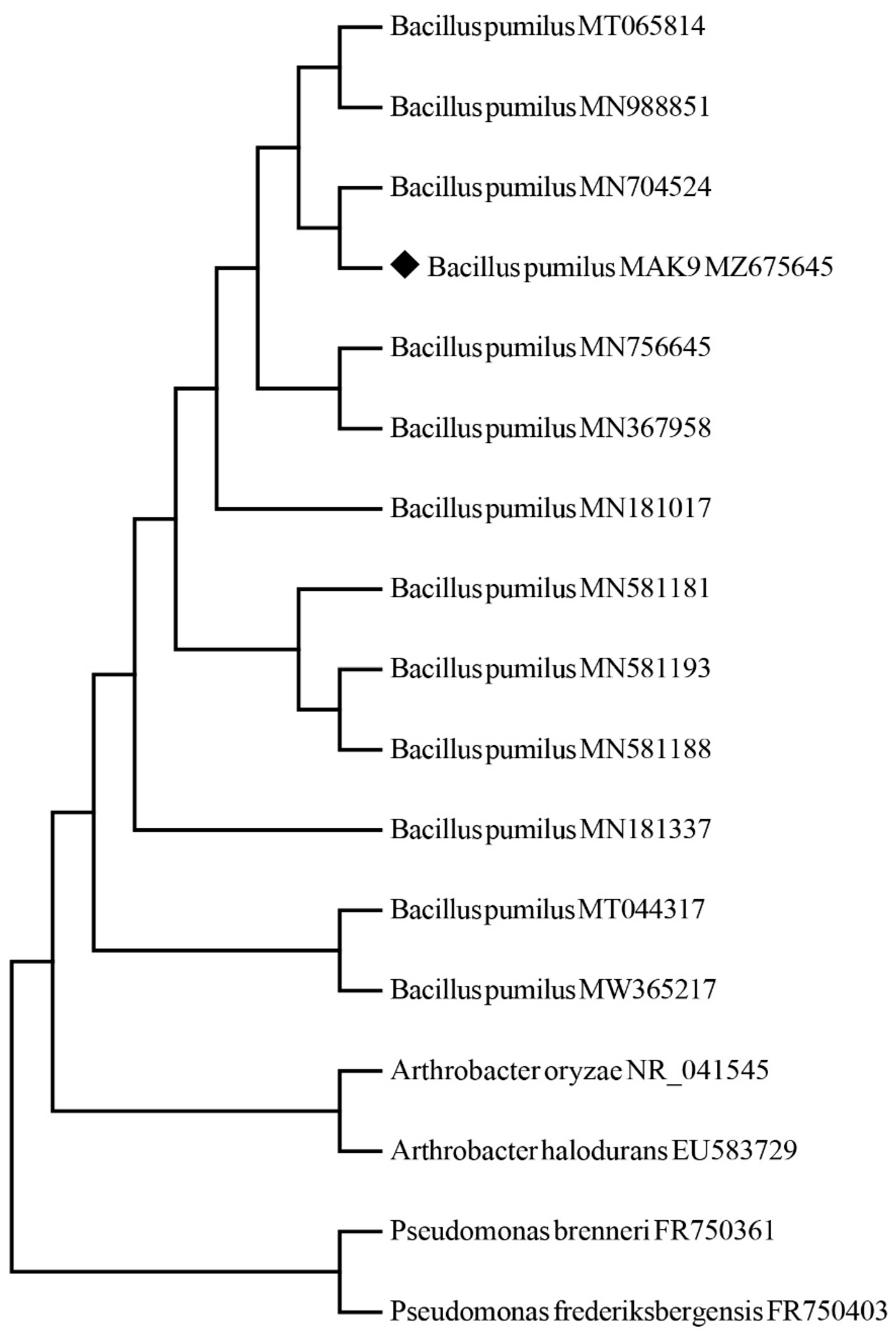
2.3. Screening of MAK9 for Halotolerance and Bacterial Identification
2.4. Screening of MAK9 Culture Filtrate for Organic Acid and IAA Production
2.5. Effects of MAK9 on the Growth Attributes of Chinese Cabbage Plants
2.6. Estimation of Enzymatic Activities
2.7. Quantification of Endogenous ABA and SA
2.8. Inductively Coupled Plasma (ICP) Analysis of Ion Uptake
3. Analysis of Sugars
4. Statistical Analysis
5. Results
5.1. Isolation, Screening, and Identification
5.2. In Vitro IAA and Organic Acid Production of Isolate MAK9
5.3. Bacterial Isolates Regulate Chinese Cabbage Growth under Food Waste
5.4. Effect of Food Waste on ABA and SA Content
5.5. Quantification of Antioxidants in Chinese Cabbage under Food Waste Treatments
5.6. ICP Analysis of Na, K, P, and Ca Content
5.7. Sugar Content Analysis
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bratovcic, A.; Zohorović, M.; Odobasic, A.; Šestan, I. Efficiency of food waste as an organic fertilizer. Int. J. Eng. Sci. Res. Technol. 2018, 7, 527–530. [Google Scholar] [CrossRef]
- FAO. Global food losses and food waste–Extent, causes and prevention. In SAVE FOOD: An Initiative on Food Loss and Waste Reduction; FAO: Rome, Italy, 2011. [Google Scholar]
- Schanes, K.; Dobernig, K.; Gözet, B. Food waste matters—A systematic review of household food waste practices and their policy implications. J. Clean. Prod. 2018, 182, 978–991. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. BioMed Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- FAO. Towards the Future We Want: End Hunger and Make the Transition to Sustainable Agricultural and Food Systems; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; pp. 1–42. [Google Scholar]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Analysing global food waste problem: Pinpointing the facts and estimating the energy content. Cent. Eur. J. Eng. 2013, 3, 157–164. [Google Scholar] [CrossRef]
- Kawashima, T. The use of food waste as a protein source for animal feed—Current status and technological development in Japan. In Proceedings of the FAO Animal Production and Health Proceedings (FAO), Protein Sources for the Animal Feed Industry, Expert Consultation and Workshop, Bangkok, Thailand, 29 April–3 May 2002; pp. 303–311. [Google Scholar]
- Kim, J.D.; Park, J.S.; In, B.H.; Kim, D.; Namkoong, W. Evaluation of pilot-scale in-vessel composting for food waste treatment. J. Hazard. Mater. 2008, 154, 272–277. [Google Scholar] [CrossRef]
- Hamid, H.A.; Qi, L.P.; Harun, H.; Sunar, N.M.; Ahmad, F.H.; Muhamad, M.S. Development of Organic Fertilizer from Food Waste by Composting in UTHM Campus Pagoh. J. Des. Sustain. Environ. 2019, 1, 1–6. [Google Scholar]
- Tiwari, A.; Khawas, R. Food Waste and Agro By-Products: A Step towards Food Sustainability; IntechOpen: London, UK, 2021. [Google Scholar]
- Hossain, M.Z.; Von Fragstein, P.; Von Niemsdorff, P.; Heß, J. Effect of different organic wastes on soil properties and plant growth and yield: A review. Sci. Agric. Bohem. 2017, 48, 224–237. [Google Scholar]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Mahmood, A.; Iguchi, R.; Kataoka, R. Multifunctional food waste fertilizer having the capability of Fusarium-growth inhibition and phosphate solubility: A new horizon of food waste recycle using microorganisms. Waste Manag. 2019, 94, 77–84. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shaffique, S.; Kim, L.-R.; Kwon, E.-H.; Kim, S.-H.; Lee, Y.-H.; Kalsoom, K.; Aaqil Khan, M.; Lee, I.-J. Effects of Organic Fertilizer Mixed with Food Waste Dry Powder on the Growth of Chinese Cabbage Seedlings. Environments 2021, 8, 86. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Trends and Challenges; Annual Report; FAO: Rome, Italy, 2017; Volume 296. [Google Scholar]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, S.J.; Hwang, H.Y.; Kim, M.S.; Jung, H.i.; Luyima, D.; Hong, S.Y.; Oh, T.K.; Kim, S.H. Effects of food waste compost on the shift of microbial community in water saturated and unsaturated soil condition. Appl. Biol. Chem. 2019, 62, 36. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kang, Y.-G.; Luyima, D.; Park, S.-J.; Oh, T.-K.; Lee, C.H. Characteristics of food waste: Water and salinity contents. Korean J. Agric. Sci. 2020, 47, 375–380. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Tartoura, K.A.H.; Youssef, S.A.; Tartoura, E.-S.A.A. Compost alleviates the negative effects of salinity via up-regulation of antioxidants in Solanum lycopersicum L. plants. Plant Growth Regul. 2014, 74, 299–310. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.A.; Lee, K.E.; Kang, S.M.; Dhungana, S.K.; Bhusal, N.; Lee, I.J. The Halotolerant Rhizobacterium-Pseudomonas koreensis MU2 Enhances Inorganic Silicon and Phosphorus Use Efficiency and Augments Salt Stress Tolerance in Soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Lubna; Asaf, S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; Lee, I.-J.; Hussain, A. Salt tolerance of Glycine max L. induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol. Biochem. 2018, 128, 13–23. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [Green Version]
- Kubi, H.A.A.; Khan, M.A.; Adhikari, A.; Imran, M.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Silicon and Plant Growth-Promoting Rhizobacteria Pseudomonas psychrotolerans CS51 Mitigates Salt Stress in Zea mays L. Agriculture 2021, 11, 272. [Google Scholar] [CrossRef]
- Chadha, N.; Prasad, R.; Varma, A. Plant Promoting Activities of Fungal Endophytes Associated with Tomato Roots from Central Himalaya, India and Their Interaction with Piriformospora Indica. Int. J. Pharma Bio Sci. 2015, 6, 333–343. [Google Scholar]
- Yu-Na, K.; Muhammad Aaqil, K.; Sang-Mo, K.; Muhammad, H.; In-Jung, L. Enhancement of Drought-Stress Tolerance of Brassica oleracea var. italica L. by Newly Isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.-W.; Kang, S.-M.; Lee, I.-J. Rhizospheric Bacillus spp. Rescues Plant Growth Under Salinity Stress via Regulating Gene Expression, Endogenous Hormones, and Antioxidant System of Oryza sativa L. Front. Plant Sci. 2021, 12, 1145. [Google Scholar] [CrossRef]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.-W.; Khan, A.L.; Lee, I.-J. Melatonin Ameliorates Thermotolerance in Soybean Seedling through Balancing Redox Homeostasis and Modulating Antioxidant Defense, Phytohormones and Polyamines Biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, Y.; Mun, B.-G.; Khan, A.L.; Waqas, M.; Kim, H.-H.; Shahzad, R.; Imran, M.; Yun, B.-W.; Lee, I.-J. Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS ONE 2018, 13, e0192650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.-M.; Kim, K.-M.; Jan, R.; Lee, I.-J. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Asard, H.; Pinto, A.; Soares, C.; Branco-Neves, S.; Braga, T.; Azenha, M.; Selim, S.; Al Jaouni, S.; et al. Metalaxyl Effects on Antioxidant Defenses in Leaves and Roots of Solanum nigrum L. Front. Plant Sci. 2017, 8, 1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.L.; Gilani, S.A.; Waqas, M.; Al-Hosni, K.; Al-Khiziri, S.; Kim, Y.-H.; Ali, L.; Kang, S.-M.; Asaf, S.; Shahzad, R.; et al. Endophytes from medicinal plants and their potential for producing indole acetic acid, improving seed germination and mitigating oxidative stress. J. Zhejiang Univ. Sci. B 2017, 18, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 2020, 15, e0232228. [Google Scholar] [CrossRef]
- Juteau, P. Review of the use of aerobic thermophilic bioprocesses for the treatment of swine waste. Livest. Sci. 2006, 102, 187–196. [Google Scholar] [CrossRef]
- Hamayun, M.; Afzal Khan, S.; Khan, A.; Shinwari, Z.; Hussain, J.; Sohn, E.-Y.; Kang, S.-M.; Kim, Y.-H.; Khan, M.; Lee, I.-J. Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. Abstr. Pap. 2010, 42, 3103–3112. [Google Scholar]
- Hamayun, M.; Sohn, E.-Y.; Afzal Khan, S.; Shinwari, Z.; Khan, A.; Lee, I.-J. Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Abstr. Pap. 2010, 42, 1713–1722. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shin, J.H.; Ahmad, B.; Shin, D.H.; Lee, I.J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010, 58, 7226–7232. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins Producing Endophytic Fungus Porostereum spadiceum AGH786 Rescues Growth of Salt Affected Soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.L.; Waqas, M.; Hamayun, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Co-synergism of endophyte Penicillium resedanum LK6 with salicylic acid helped Capsicum annuumin biomass recovery and osmotic stress mitigation. BMC Microbiol. 2013, 13, 51. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-M.; Khan, A.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant Growth Promoting Rhizobacterias reduces adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Berg, G.; Lindström, K.; Räsänen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Yoo, S.-J.; Weon, H.-Y.; Song, J.; Sang, M.K. Induced tolerance to salinity stress by halotolerant bacteria Bacillus aryabhattai H19-1 and B. mesonae H20-5 in tomato plants. J. Microbiol. Biotechnol. 2019, 29, 1124–1136. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; jong Yim, W.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef]
- Hidri, R.; Barea, J.M.; Mahmoud, O.M.-B.; Abdelly, C.; Azcón, R. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na(+) and K(+) Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Aldubise, A.; Egamberdieva, D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 2015, 10, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Shilev, S. Plant-Growth-Promoting Bacteria Mitigating Soil Salinity Stress in Plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 2016, 22, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lin, Q.; Feng, Y.; Fan, X.; Zou, F.; Yuan, D.-Y.; Zeng, X.; Cao, H. Transcriptomic Identification and Expression of Starch and Sucrose Metabolism Genes in the Seeds of Chinese Chestnut (Castanea mollissima). J. Agric. Food Chem. 2015, 63, 929–942. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Liu, W. Sugar accumulation and characterization of metabolizing enzyme genes in leafy head of Chinese cabbage (Brassica campestris L. ssp. pekinensis). Hortic. Environ. Biotechnol. 2021, 62, 17–29. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; Lee, S.-M.; Park, Y.-G.; Kim, A.-Y.; Seo, C.-W.; Lee, I.-J. Enterobacter sp. SE992-induced regulation of amino acids, sugars, and hormones in cucumber plants improves salt tolerance. Acta Physiol. Plant. 2015, 37, 149. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Song, L.; Li, K.; Zhang, X.; Liu, S.; Qin, Y.; Li, P. Polysaccharides from Grateloupia filicina enhance tolerance of rice seeds (Oryza sativa L.) under salt stress. Int. J. Biol. Macromol. 2019, 124, 1197–1204. [Google Scholar] [CrossRef]
- Chinsamy, M.; Kulkarni, M.G.; Van Staden, J. Garden-waste-vermicompost leachate alleviates salinity stress in tomato seedlings by mobilizing salt tolerance mechanisms. Plant Growth Regul. 2013, 71, 41–47. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, Y.; Guo, L.; Li, C. Root colonization of encapsulated Klebsiella oxytoca Rs-5 on cotton plants and its promoting growth performance under salinity stress. Eur. J. Soil Biol. 2014, 60, 81–87. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of Detrimental Effects of Salt Stress on Date Palm (Phoenix dactylifera L.) by the Application of Arbuscular Mycorrhizal Fungi and/or Compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Zahra, S.; Amin, B.; Ali, V.S.M.; Ali, Y.; Mehdi, Y. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl). J. Biophys. Struct. Biol. 2011, 2, 35–41. [Google Scholar]
- Ahanger, M.A.; Agarwal, R. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; IntechOpen: London, UK, 2016; pp. 463–480. [Google Scholar]
- Laouane, R.B.; Meddich, A.; Bechtaoui, N.; Oufdou, K.; Wahbi, S. Effects of arbuscular mycorrhizal fungi and rhizobia symbiosis on the tolerance of Medicago sativa to salt stress. Gesunde Pflanz. 2019, 71, 135–146. [Google Scholar] [CrossRef]





| RL (cm) | SL (cm) | FW (g/Three Plants) | DW (g/Three Plants) | CC (SPAD) | |
|---|---|---|---|---|---|
| Control | 12.0 ± 1.00 B | 17.20 ± 1.00 B | 2.66 ± 0.11 B | 0.22 ± 0.005 B | 27.10 ± 1.13 B |
| MAK9 | 15.33 ± 0.57 A | 19.33 ±0.57 A | 3.22 ± 0.10 A | 0.29 ± 0.005 A | 30.10 ± 2.54 A |
| Food waste (8%) | 5.50 ± 0.50 D | 9.16 ± 0.76 D | 1.83 ± 0.05 C | 0.14 ± 0.005 D | 21.60 ± 1.69 D |
| MAK9 + food waste (8%) | 7.80 ± 0.76 C | 12.16 ± 0.76 C | 2.16 ± 0.05 B | 0.19 ± 0.005 C | 24.50 ± 0.70 C |
| Glucose | Fructose | Sucrose | |
|---|---|---|---|
| Control | 8.80 ± 0.20 A | 5.70 ± 0.30 A | 1.21 ± 0.10 A |
| MAK9 | 8.89 ± 0.20 A | 5.89 ± 0.40 A | 1.32 ± 0.10 A |
| Food waste (8%) | 6.45 ± 0.22 C | 4.51 ± 0.27 B | 1.01 ± 0.10 B |
| MAK9 + food waste (8%) | 7.55 ± 0.25 B | 5.02 ± 0.20 B | 1.16 ± 0.03 AB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Kalsoom; Imran, M.; Lubna; Shaffique, S.; Kwon, E.-H.; Kang, S.-M.; Kim, S.-H.; Hamayun, M.; Lee, I.-J. Mitigation of Commercial Food Waste-Related Salinity Stress Using Halotolerant Rhizobacteria in Chinese Cabbage Plants. Horticulturae 2022, 8, 49. https://doi.org/10.3390/horticulturae8010049
Khan MA, Kalsoom, Imran M, Lubna, Shaffique S, Kwon E-H, Kang S-M, Kim S-H, Hamayun M, Lee I-J. Mitigation of Commercial Food Waste-Related Salinity Stress Using Halotolerant Rhizobacteria in Chinese Cabbage Plants. Horticulturae. 2022; 8(1):49. https://doi.org/10.3390/horticulturae8010049
Chicago/Turabian StyleKhan, Muhammad Aaqil, Kalsoom, Muhammad Imran, Lubna, Shifa Shaffique, Eun-Hae Kwon, Sang-Mo Kang, Seong-Heon Kim, Muhammad Hamayun, and In-Jung Lee. 2022. "Mitigation of Commercial Food Waste-Related Salinity Stress Using Halotolerant Rhizobacteria in Chinese Cabbage Plants" Horticulturae 8, no. 1: 49. https://doi.org/10.3390/horticulturae8010049
APA StyleKhan, M. A., Kalsoom, Imran, M., Lubna, Shaffique, S., Kwon, E.-H., Kang, S.-M., Kim, S.-H., Hamayun, M., & Lee, I.-J. (2022). Mitigation of Commercial Food Waste-Related Salinity Stress Using Halotolerant Rhizobacteria in Chinese Cabbage Plants. Horticulturae, 8(1), 49. https://doi.org/10.3390/horticulturae8010049







