Cluster-Zone Leaf Removal and GA3 Application at Early Flowering Reduce Bunch Compactness and Yield per Vine in Vitis vinifera cv. Pinot Gris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growing Conditions and Environmental Data
2.2. Experimental Design
2.3. Phenotypic and Yield Assessments
2.4. Total Soluble Solids and Yield
2.5. Bunch Compactness
2.6. Bunch Rot Incidence
2.7. Statistical Analysis
3. Results
3.1. Environmental Conditions
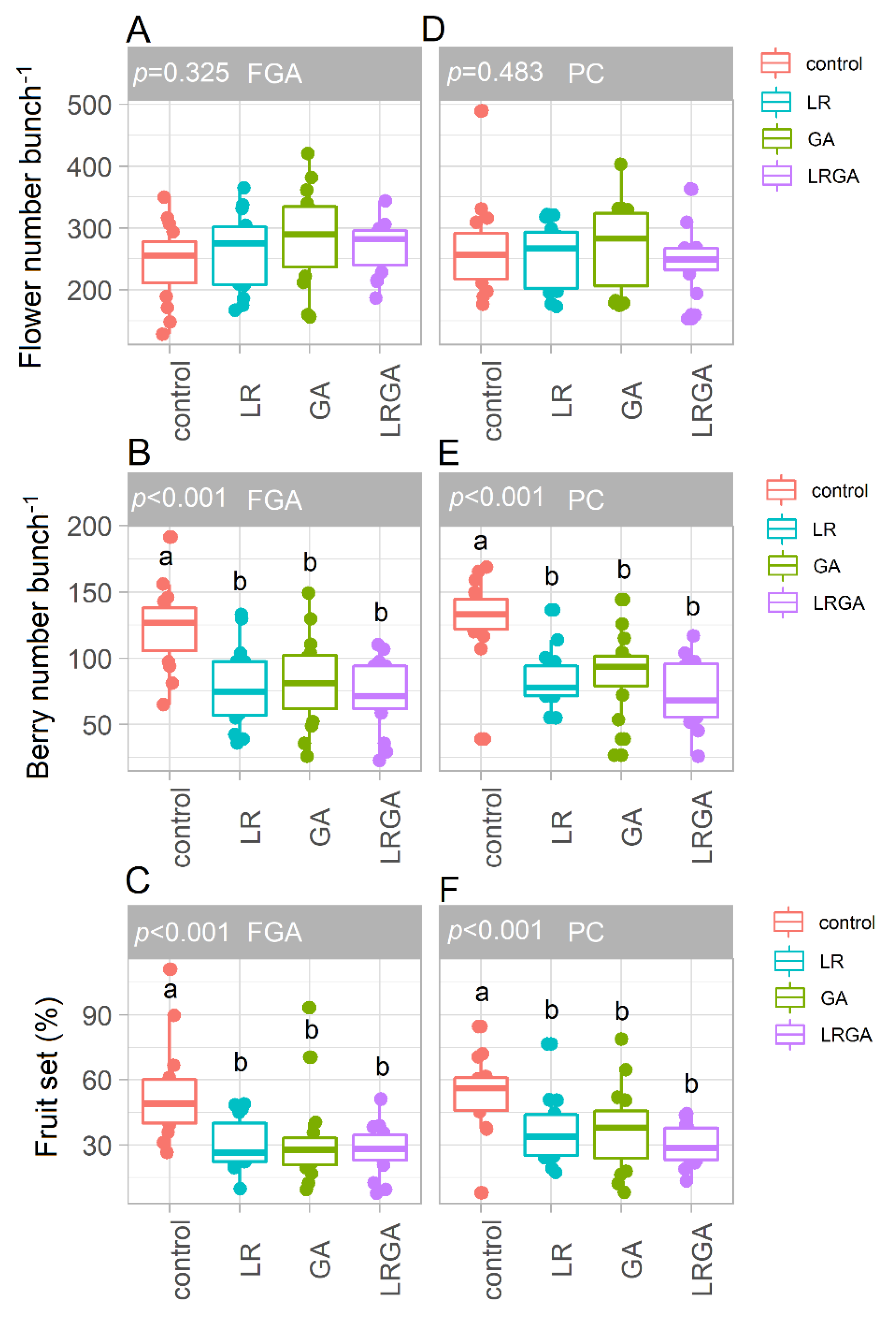
3.2. Leaf Area, Flower Number and Fruit Set
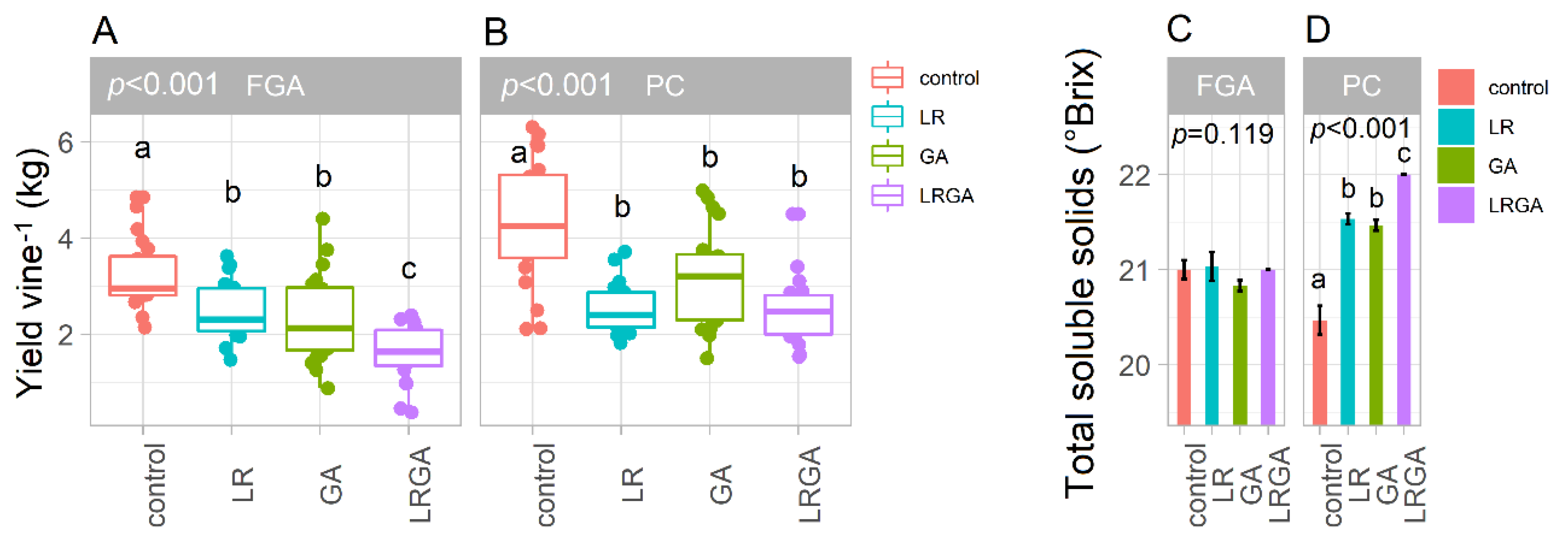
3.3. Yield per Vine and Total Soluble Carbohydrates
3.4. Bunch Compactness: LDI, TDI, SDI and IDI Indices
4. Discussion
4.1. LR and GA Reduce Fruit Set, Berry Number and Yield per Vine in Both Training Systems
4.2. LR and GA Reduce Bunch Compactness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smart, R.; Robinson, J.B.; Due, G.R.; Brien, C.J. Canopy microclimate modification for the cultivar Shiraz. I. Definition of canopy microclimate. Vitis 1985, 24, 17–31. [Google Scholar]
- Zoecklein, B.W.; Wolf, T.K.; Duncan, N.W.; Judge, J.M.; Cook, M.K. Effects of fruit zone leaf removal on yield, fruit composition, and fruit rot incidence of Chardonnay and White Riesling (Vitis vinifera L.) grapes. Am. J. Enol. Vitic. 1992, 43, 139–148. [Google Scholar]
- Filippetti, I.; Allegro, G.; Valentini, G.; Pastore, C.; Colucci, E.; Intrieri, C. Influence of vigour on vine performance and berry composition of cv. Sangiovese (Vitis vinifera L.). J. Int. Sci. Vigne Vin 2013, 47, 21–33. [Google Scholar] [CrossRef] [Green Version]
- English, J.T.; Marois, J.J.; Guble, W.D. Microclimate of grapevine canopies associated with leaf removal and control of Botrytis bunch rot. Phytopathology 1989, 79, 395–401. [Google Scholar] [CrossRef]
- Sauvage, S.; Sall, M.A. Botrytis bunch rot of grapes: Influence of trellis type and canopy microclimate. Phytopathology 1983, 50, 450–454. [Google Scholar] [CrossRef]
- Sauvage, S.; Sall, M.A. Botrytis bunch rot of grapes: Influence of selected cultural practices on infection under California condition. Plant Dis. 1984, 67, 771–774. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2010, 9, 357–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vail, M.E.; Marois, J.J. Grape cluster architecture and the susceptibility of berries to Botrytis cinerea. Phytopathology 1991, 81, 188–191. [Google Scholar] [CrossRef]
- Tello, J.; Marcos, J.I. Evaluation of indexes for the quantitative and objective estimation of grapevine bunch compactness. Vitis-J. Grapevine Res. 2014, 53, 9–16. [Google Scholar]
- Hed, B.; Ngugi, H.K.; Travis, J.W. Relationship between cluster compactness and bunch rot in Vignoles grapes. Plant Dis. 2009, 93, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Poni, S.; Casalini, L.; Bernizzoni, F.; Civardi, S.; Intrieri, C. Effects of early defoliation on shoot photosynthesis, yield components, and grape composition. Am. J. Enol. Vitic. 2006, 57, 397–407. [Google Scholar]
- Tardaguila, J.; Martinez de Toda, F.; Poni, S.; Diago, M.P. Impact of early leaf removal on yield and fruit and wine composition of Vitis vinifera L. Graciano and Carignan. Am. J. Enol. Vitic. 2010, 61, 372–381. [Google Scholar]
- Gatti, M.; Bernizzoni, F.; Civardi, S.; Poni, S. Effects of cluster thinning and pre-flowering leaf removal on growth and grape composition in cv. Sangiovese. Am. J. Enol. Vitic. 2012, 63, 325–332. [Google Scholar] [CrossRef]
- Risco, D.; Pérez, D.; Yeves, A.; Castel, J.R.; Intrigliolo, D.S. Early defoliation in a temperate warm and semi-arid Tempranillo vineyard: Vine performance and grape composition. Aust. J. Grape Wine Res. 2013, 20, 111–122. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Vaillant-Gaveau, N.; Wojnarowiez, G.; Petit, A.N.; Jacquens, L.; Panigai, L.; Clement, C.; Fontaine, F. Relationships between carbohydrates and reproductive development in Chardonnay grapevine: Impact of defoliation and fruit removal treatments during four successive growing seasons. OEN One 2014, 48, 219–229. [Google Scholar] [CrossRef]
- Hunter, J.J.; Ruffner, H.P.; Volschenk, C.G.; Le Roux, D.J. Partial defoliation of Vitis vinifera L. cv. Cabernet Sauvignon/99 Richter: Effect on root growth, canopy efficiency; grape composition, and wine quality. Am. J. Enol. Vitic. 1995, 46, 306–314. [Google Scholar]
- Poni, S.; Bernizzoni, F.; Civardi, S. The effect of early leaf removal on whole- canopy gas exchange and vine performance of Vitis vinifera L. Sangiovese. Vitis 2008, 47, 1–6. [Google Scholar]
- Palliotti, A.; Gatti, M.; Poni, S. Early leaf removal to improve vineyard efficiency: Gas exchange, source-to-sink balance, and reserve storage responses. Am. J. Enol. Vitic. 2011, 62, 219–228. [Google Scholar] [CrossRef]
- Medrano, H.; Bota, J.; Cifre, J.; Flexas, J.; Ribas-Carbó, M.; Gulías, J. Eficiencia en el uso del agua por las plantas. Investig. Geográficas 2007, 43, 63–84. [Google Scholar] [CrossRef] [Green Version]
- Siegfried, W. Gibberellin trials 2007 in wine production. Schweiz. Z. Obs.-Weinbau 2007, 144, 4–7. [Google Scholar]
- Petgen, M. Gibberellin use for quality control. Schweiz. Z. Obs.-Weinbau 2005, 141, 6–9. [Google Scholar]
- Spring, J.L.; Viret, O. Influence of thinning methods on yield, bunch morphology, grey and sour rot, and wine quality of Pinot noir. Rev. Suisse Vitic. Arboric. Hortic. 2009, 41, 95–101. [Google Scholar]
- Hed, B.; Ngugi, H.K.; Travis, J.W. Use of gibberellic acid for management of bunch rot on Chardonnay and Vignoles grape. Plant Dis. 2011, 95, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, S.; Leonardelli, L.; Malossini, U.; Stefanini, M.; Velasco, R.; Moser, C. Pinot blanc and Pinot gris arose as independent somatic mutations of Pinot noir. J. Exp. Bot. 2012, 63, 6359–6369. [Google Scholar] [CrossRef] [Green Version]
- Kocsis, M.; Csikász-Krizsics, A.; Szata, B.E.; Kovács, S.; Nagy, A.; Mátai, A.; Jakab, G. Regulation of cluster compactness and resistance to Botrytis cinerea with β-aminobutyric acid treatment in field-grown grapevine. Vitis 2018, 57, 35–40. [Google Scholar]
- Ferree, D.C.; Ellis, M.A.; McArtney, S.J.; Brown, M.V.; Scurlock, D.M. Comparison of fungicide, leaf removal and gibberellic acid on development of grape bunchs and botrytis bunch rot of ‘Vignoles’ and ‘Pinot Gris’. Small Fruits Rev. 2003, 2, 3–18. [Google Scholar] [CrossRef]
- Lemut, M.S.; Sivilotti, P.; Butinar, L.; Vrhovšek, U. Controlling microbial infection by managing grapevine canopy. In Proceedings. 46th Croatian and 6th International Symposium on Agriculture. Opatija. Croat. 2011, 984, 987. [Google Scholar]
- Sabbatini, P.; Howell, G.S. Effects of early defoliation on yield, fruit composition, and harvest season cluster rot complex of grapevines. HortScience 2010, 45, 1804–1808. [Google Scholar] [CrossRef]
- Ipach, R.; Huber, B.; Hofmann, H.; Baus, O. Richtlinie zur Prüfung von Wachstumsregulatoren zur Auflockerung der Traubenstruktur und zur Vermeidung von Fäulnis an Trauben. Outline for an EPPO-Guideline; DLR Rheinpfalz: Neustadt an der Weinstraße, Germany, 2005. [Google Scholar]
- Tello, J.; Ibáñez Marcos, J. What do we know about grapevine bunch compactness? A state-of-the-art review. Aust. J. Grape Wine Res. 2018, 24, 6–23. [Google Scholar] [CrossRef]
- Molitor, D.; Baron, N.; Sauerwein, T.; André, C.M.; Kicherer, A.; Döring, J.; Stoll, M.; Beyer, M.; Hoffmann, L.; Evers, D. Postponing first shoot topping reduces grape cluster compactness and delays bunch rot epidemic. Am. J. Enol. Vitic. 2015, 66, 164–176. [Google Scholar] [CrossRef]
- Smart, R.E.; Sinclair, T.R. Solar heating of grape berries and other spherical fruits. Agric. Meteorol. 1976, 17, 241–259. [Google Scholar] [CrossRef]
- Tello, J.; Aguirrezábal, R.; Hernáiz, S.; Larreina, B.; Montemayor, M.; Vaquero, E.; Ibáñez, J. Multicultivar study of grapevine bunch compactness. Aust. J. Grape Wine Res. 2015, 21, 277–289. [Google Scholar] [CrossRef]
- Zdunić, G.; Mucalo, A.; Budić-Leto, I.; Humar, I.; Pejić, I.; Maletić, E. Cluster architecture of old, neglected Croatian grapevine varieties (Vitis vinifera L.). VITIS-J. Grapevine Res. 2015, 54, 177–180. [Google Scholar]
- Ferreira, J.H.S.; Marais, P.G. Effect of rootstock cultivar, pruning method and crop load on Botrytis cinerea rot of Vitis vinifera cv. Chenin blanc grapes. South Afr. J. Enol. Vitic. 1987, 8, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine bud fertility and number of berries per bunch are determined by water and nitrogen stress around flowering in the previous year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Acimovic, D.; Tozzini, L.; Green, A.; Sivilotti, P.; Sabbatini, P. Identification of a defoliation severity threshold for changing fruitset, bunch morphology and fruit composition in Pinot Noir. Aust. J. Grape Wine Res. 2016, 22, 399–408. [Google Scholar] [CrossRef]
- Hed, B.; Ngugi, H.K.; Travis, J.W. Short-and long-term effects of leaf removal and gibberellin on Chardonnay grapes in the Lake Erie region of Pennsylvania. Am. J. Enol. Vitic. 2015, 66, 22–29. [Google Scholar] [CrossRef]
- Molitor, D.; Behr, M.; Fischer, S.; Hoffmann, L.; Evers, D. Timing of cluster-zone leaf removal and its impact on canopy morphology, cluster structure and bunch rot susceptibility of grapes. OENO One 2011, 45, 149–159. [Google Scholar] [CrossRef]
- Basile, B.; Caccavello, G.; Giaccone, M.; Forlani, M. Effects of early shading and defoliation on bunch compactness, yield components, and berry composition of Aglianico grapevines under warm climate conditions. Am. J. Enol. Vitic. 2015, 66, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Hed, B.; Centinari, M. Gibberellin Application Improved Bunch Rot Control of Vignoles Grape, but Response to Mechanical Defoliation Varied Between Training Systems. Plant Dis. 2021, 105, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lemut, M.S.; Sivilotti, P.; Butinar, L.; Laganis, J.; Vrhovsek, U. Pre-flowering leaf removal alters grape microbial population and offers good potential for a more sustainable and cost-effective management of a Pinot noir vineyard. Aust. J. Grape Wine Res. 2015, 21, 439–450. [Google Scholar] [CrossRef]
- Sivilotti, P.; Herrera, J.C.; Lisjak, K.; Baša Česnik, H.; Sabbatini, P.; Peterlunger, E.; Castellarin, S.D. Impact of leaf removal, applied before and after flowering, on anthocyanin, tannin, and methoxypyrazine concentrations in ‘Merlot’(Vitis vinifera L.) grapes and wines. J. Agric. Food Chem. 2016, 64, 4487–4496. [Google Scholar] [CrossRef] [Green Version]
- Evers, D.; Molitor, D.; Rothmeier, M.; Behr, M.; Fischer, S.; Hoffmann, L. Efficiency of different strategies for the control of grey mold on grapes including gibberellic acid (Gibb3), leaf removal and/or botrycide treatments. OENO One 2010, 44, 151–159. [Google Scholar] [CrossRef]
- Dokoozlian, N.K.; Peacock, W.L. Gibberellic acid applied at bloom reduces fruit set and improves size of ‘Crimson Seedless’ table grapes. HortScience 2001, 36, 706–709. [Google Scholar] [CrossRef] [Green Version]



| Vineyard | Training System | Treatment | Developmental Stage |
|---|---|---|---|
| Puncli (PC) | Pergola | Control (C) | |
| Puncli (PC) | Pergola | Leaf removal (LR) | BBCH 62 |
| Puncli (PC) | Pergola | GA3 (GA) | BBCH 65 |
| Puncli (PC) | Pergola | Leaf removal + GA3 (LRGA) | BBCH 62 and BBCH 65 |
| Fra gli Adigi (FGA) | Guyot | Control (C) | |
| Fra gli Adigi (FGA) | Guyot | Leaf removal (LR) | BBCH 62 |
| Fra gli Adigi (FGA) | Guyot | GA3 (GA) | BBCH 65 |
| Fra gli Adigi (FGA) | Guyot | Leaf removal + GA3 (LRGA) | BBCH 62 and BBCH 65 |
| FGA | PC | |||
|---|---|---|---|---|
| Before LR | After LR | Before LR | After LR | |
| LA per shoot (cm2) | 656.2 | 336.1 | 657.4 | 522.3 |
| LA per vine (cm2) | 8530.6 | 4370.2 | 9203.6 | 7312.5 |
| LA removed per shoot (cm2) | n.a. | 320.0 | n.a. | 135.0 |
| Defoliation (%) | n.a. | 49 | n.a. | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegher, M.; Faralli, M.; Bertamini, M. Cluster-Zone Leaf Removal and GA3 Application at Early Flowering Reduce Bunch Compactness and Yield per Vine in Vitis vinifera cv. Pinot Gris. Horticulturae 2022, 8, 81. https://doi.org/10.3390/horticulturae8010081
Wegher M, Faralli M, Bertamini M. Cluster-Zone Leaf Removal and GA3 Application at Early Flowering Reduce Bunch Compactness and Yield per Vine in Vitis vinifera cv. Pinot Gris. Horticulturae. 2022; 8(1):81. https://doi.org/10.3390/horticulturae8010081
Chicago/Turabian StyleWegher, Mario, Michele Faralli, and Massimo Bertamini. 2022. "Cluster-Zone Leaf Removal and GA3 Application at Early Flowering Reduce Bunch Compactness and Yield per Vine in Vitis vinifera cv. Pinot Gris" Horticulturae 8, no. 1: 81. https://doi.org/10.3390/horticulturae8010081
APA StyleWegher, M., Faralli, M., & Bertamini, M. (2022). Cluster-Zone Leaf Removal and GA3 Application at Early Flowering Reduce Bunch Compactness and Yield per Vine in Vitis vinifera cv. Pinot Gris. Horticulturae, 8(1), 81. https://doi.org/10.3390/horticulturae8010081








