Effect of the Method of Microspore Isolation on the Efficiency of Isolated Microspore Culture In Vitro for Brassicaceae Family
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Growing Donor Plants
2.3. Isolated Microspore Culture (IMC)
2.3.1. Cytological Analysis of the Microspores in Buds of Different Sizes
2.3.2. Surface Sterilization of Buds
2.3.3. Microspore Isolation Stage
2.3.4. Filtration and Washing of the Microspore Suspension
2.4. Data Visualisation
2.5. Experiment Series
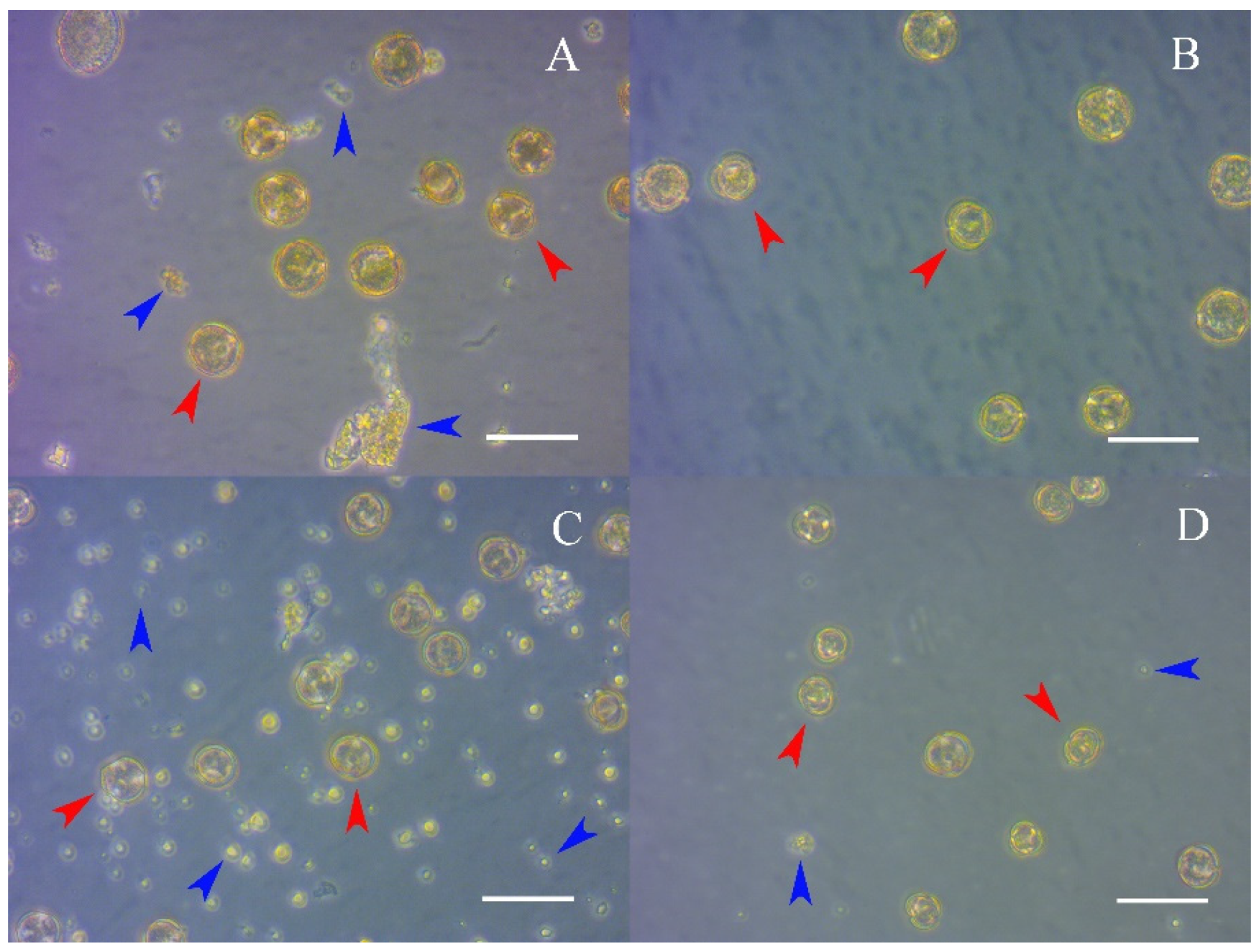
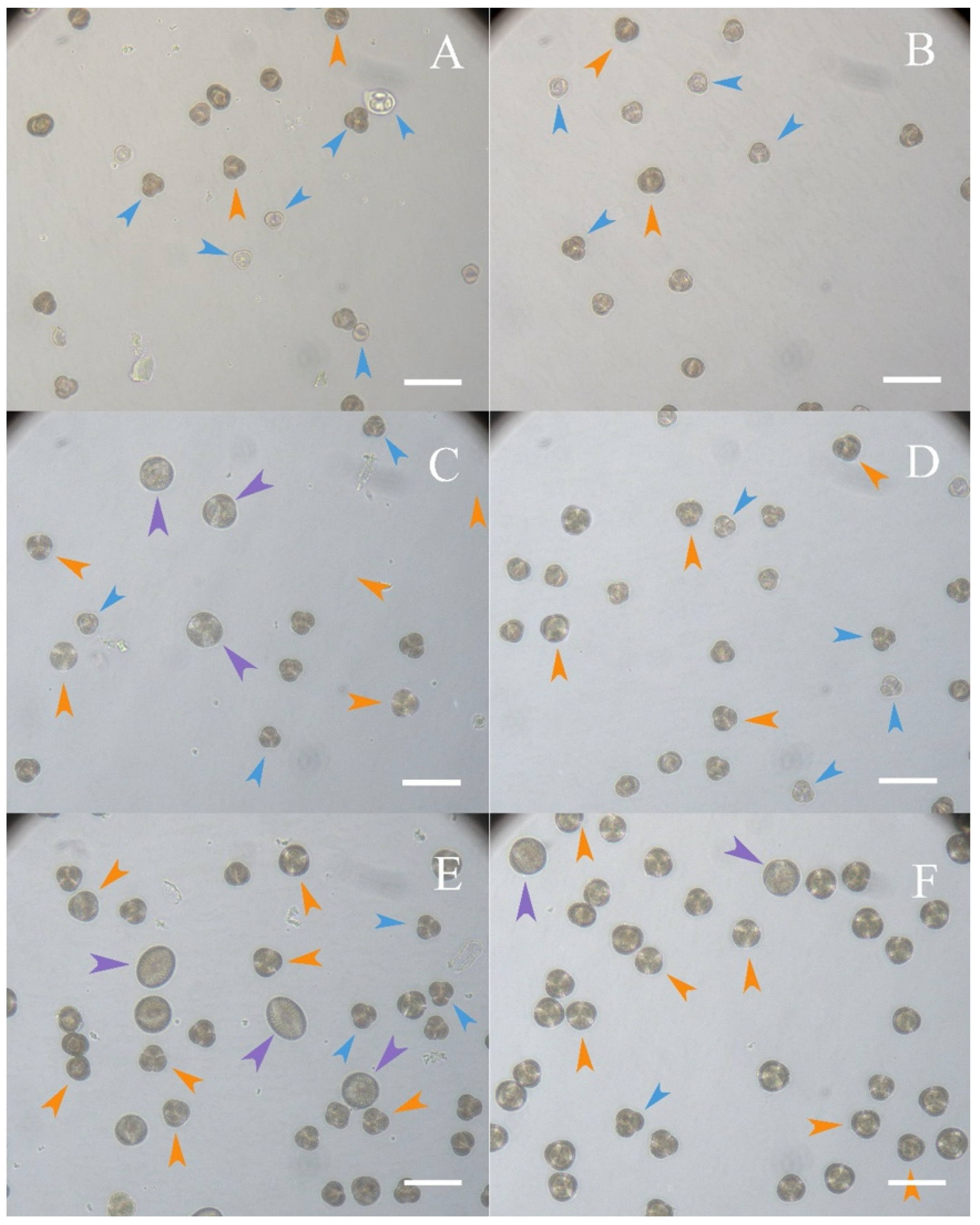
2.5.1. Assessment of the Qualitative Composition of the Microspore Population and the Foreign Matter Content in the Preparation According to the Three Methods of Microspore Isolation
2.5.2. Evaluation of the Effectiveness of the IMC Technology Depending on the Two Methods of Microspore Isolation
2.6. Statistical Analysis
3. Results
3.1. Assessment of the Qualitative Composition of the Microspore Population and the Foreign Matter Content in the Preparation, Depending on the Three Methods of Microspore Isolation
3.1.1. Assessing the Debris of the Preparation with Impurities
3.1.2. Assessment of the Qualitative Composition of the Microspore Population in the Preparation
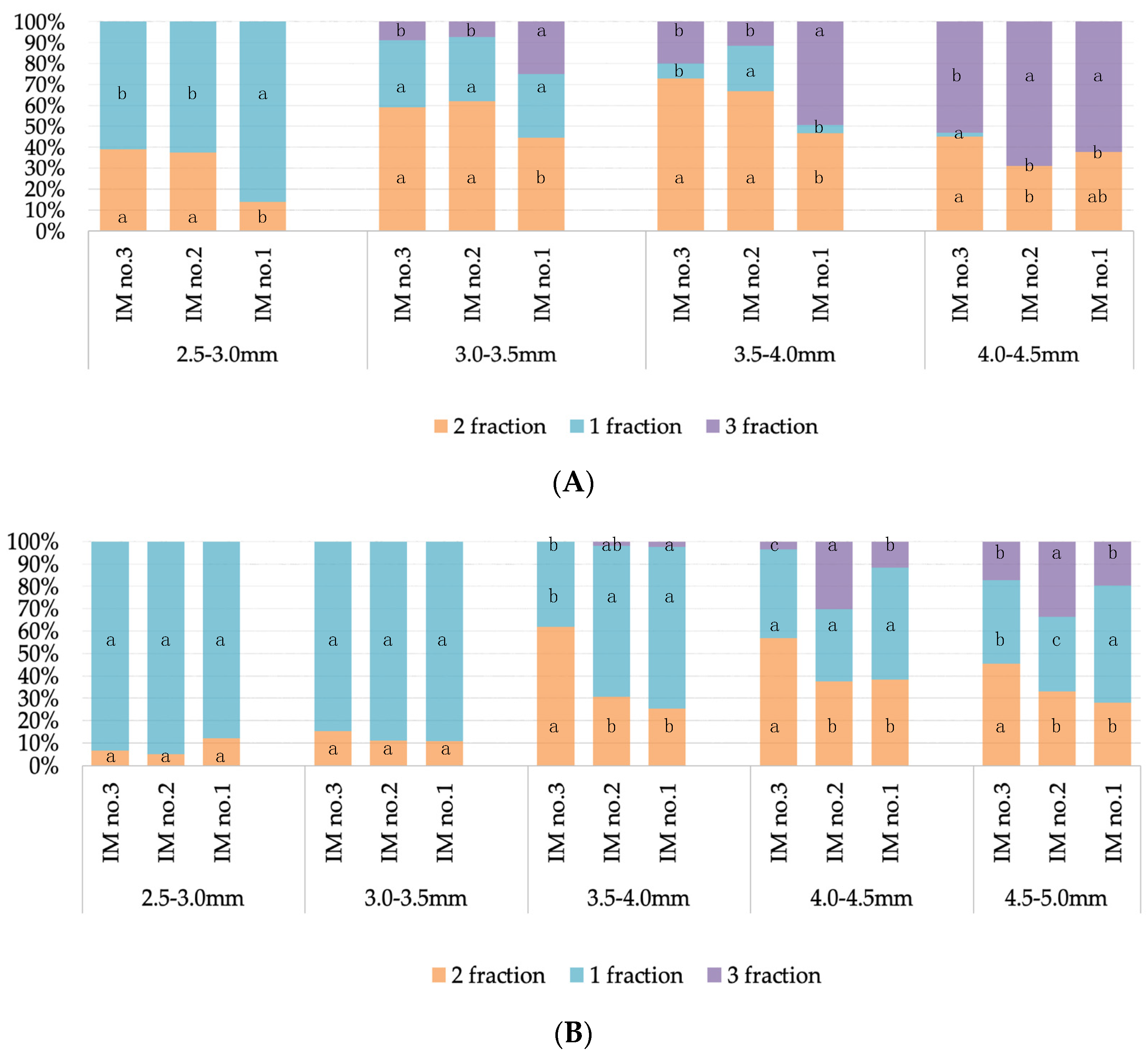
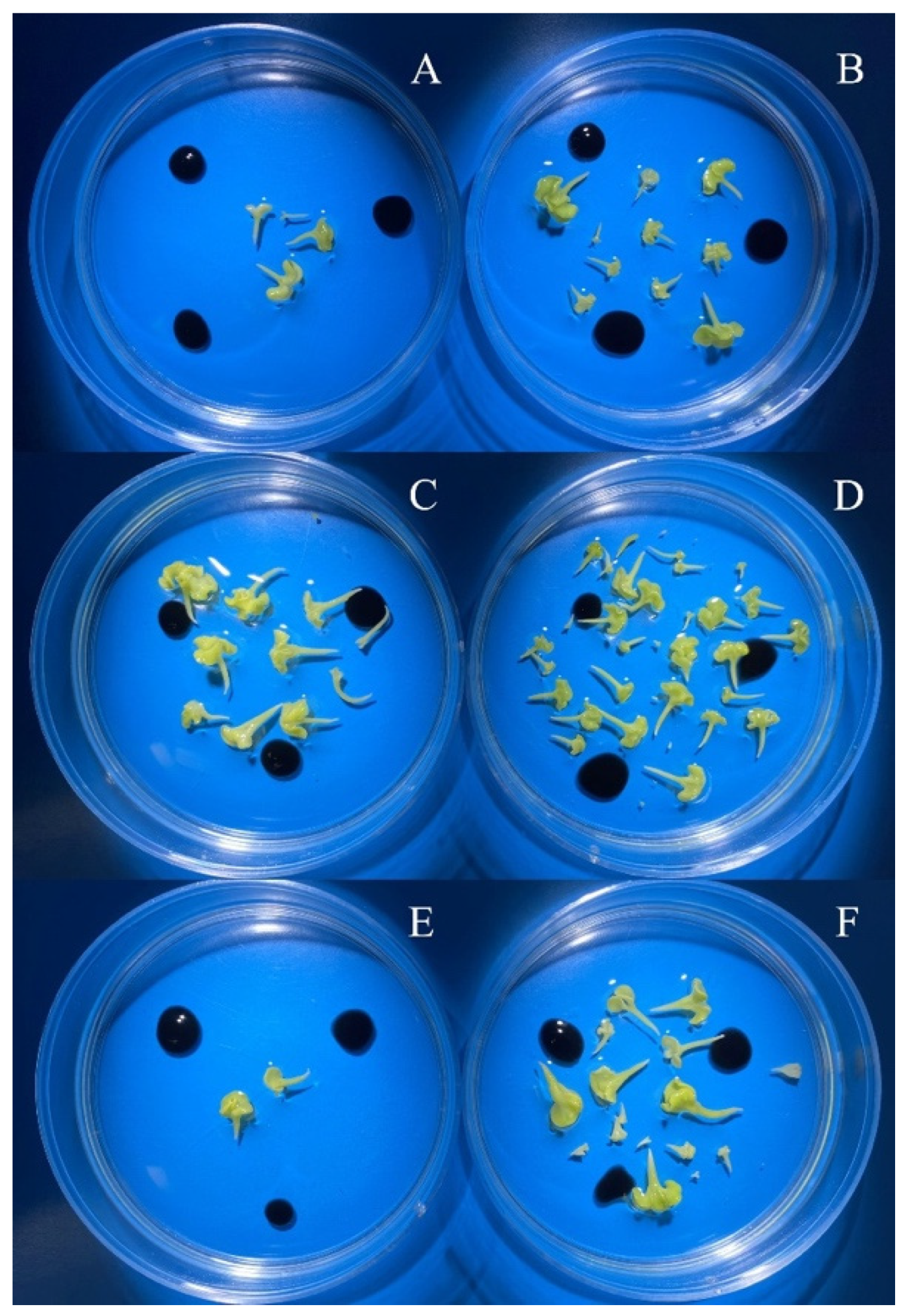
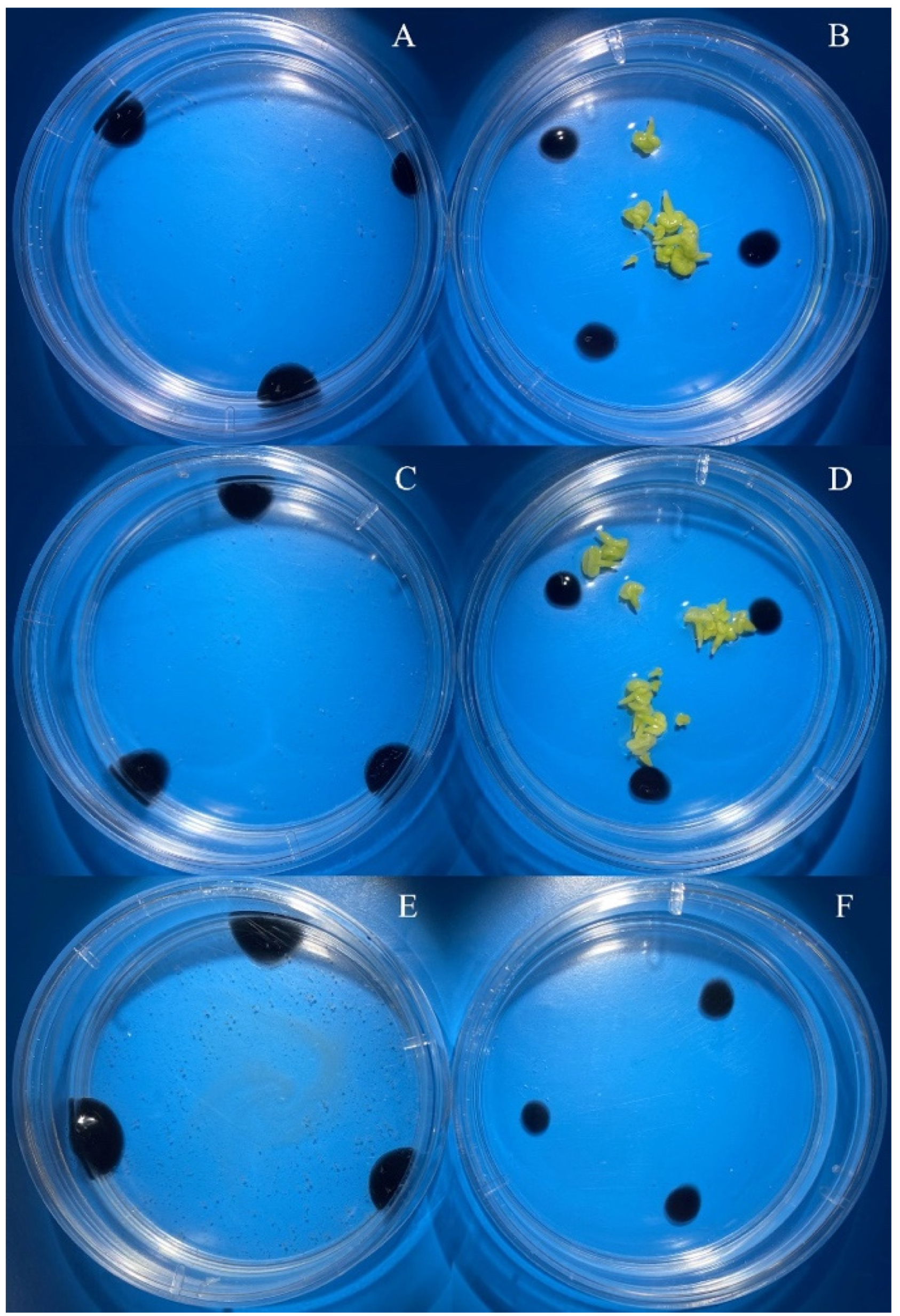
3.2. Evaluation of the Effectiveness of IMC Technology Depending on the Two Methods of Microspore Isolation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Henderson, C.A.P.; Pauls, K.P. The use of haploidy to develop plants that express several recessive traits using light-seeded canola (Brassica napus) as an example. Theor. Appl. Genet. 1992, 83, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Bansal’, V.K.; Thiagarajah’, M.R.; Stringam’, G.R.; Hardin’, R.T. Haploid plantlet screening in the development of blackleg resistant DH lines of Brassica napus. Plant Breed. 1998, 117, 103–106. [Google Scholar] [CrossRef]
- Ferrie, A.M.R.; Caswell, K.L. Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell Tissue Organ Cult. 2011, 104, 301–309. [Google Scholar] [CrossRef]
- Behla, R.; Hirani, A.H.; Zelmer, C.D.; Yu, F.; Fernando, W.G.D.; McVetty, P.; Li, G. Identification of common QTL for resistance to Sclerotinia sclerotiorum in three doubled haploid populations of Brassica napus (L.). Euphytica 2017, 213, 260. [Google Scholar] [CrossRef]
- Aslam, F.N.; Macdonald, M.V.; Loudon, P.; Ingram, D.S. Rapid-cycling Brassica Species: Inbreeding and selection of B. campestris for anther culture ability. Ann. Bot. 1990, 65, 557–566. [Google Scholar] [CrossRef]
- Dunwell, J.M. Haploids in flowering plants: Origins and exploitation. Plant Biotechnol. J. 2010, 8, 377–424. [Google Scholar] [CrossRef] [PubMed]
- Lichter, R. Induction of Haploid Plants from Isolated Pollen of Brassica napus. Z. Pflanzenphysiol. 1982, 105, 427–434. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Gao, Y.H.; Zhao, T.; Ren, G.Q.; Liu, Y.L.; Guan, B.; Jin, R.X.; Gao, F.; Zhang, Y.L.; Tan, X.F.; et al. Influencing factors and physiochemical changes of embryogenesis through in vitro isolated microspore culture in Brassica species. Biologia 2021, 76, 2629–2654. [Google Scholar] [CrossRef]
- Kott, L.S.; Polsoni, L.; Ellis, B.; Beversdorf, W.D. Autotoxicity in isolated microspore cultures of Brassica napus. Can. J. Bot. 1988, 66, 1665–1670. [Google Scholar] [CrossRef]
- Kott, L.S.; Polsoni, L.; Beversdorf, W.D. Cytological aspects of isolated microspore culture of Brassica napus. Can. J. Bot. 1988, 66, 1658–1664. [Google Scholar] [CrossRef]
- Kozar, E.V.; Domblides, E.A.; Soldatenko, A.V. Factors affecting DH plants in vitro production from microspores of European radish. Vavilovskii Zhurnal Genet. Sel. 2020, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Dey, S.S.; Parkash, C.; Sharma, K.; Sood, S.; Kumar, R. Modification of important factors for efficient microspore embryogenesis and doubled haploid production in field grown white cabbage (Brassica oleracea var. Capitata L.) genotypes in India. Sci. Hortic. 2018, 233, 178–187. [Google Scholar] [CrossRef]
- Kozar, E.; Domblides, E. Protocol of European Radish (Raphanus sativus L.) Microspore Culture for Doubled Haploid Plant Production. In When the Menorah Fades; Academic Studies Press: Boston, MA, USA, 2021; Volume 2288, pp. 217–232. ISBN 9781071613351. [Google Scholar]
- Takahata, Y.; Komatsu, H.; Kaizuma, N. Microspore culture of radish (Raphanus sativus L.): Influence of genotype and culture conditions on embryogenesis. Plant Cell Rep. 1996, 16, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, P.; Dirpaul, J.; Polowick, P.; Ferrie, A.M.R. A high throughput Brassica napus microspore culture system: Influence of percoll gradient separation and bud selection on embryogenesis. Plant Cell Tissue Organ Cult. 2011, 106, 359–362. [Google Scholar] [CrossRef]
- Dupl’Áková, N.; Dobrev, P.I.; Renák, D.; Honys, D. Rapid separation of Arabidopsis male gametophyte developmental stages using a Percoll gradient. Nat. Protoc. 2016, 11, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Corral-Martínez, P.; Camacho-Fernández, C.; Mir, R.; Seguí-Simarro, J.M. Doubled Haploid Production in High- and Low-Response Genotypes of Rapeseed (Brassica napus) Through Isolated Microspore Culture. Methods Mol. Biol. 2021, 2289, 129–144. [Google Scholar]
- Chun, C.; Park, H.; Na, H. Microspore-derived embryo formation in radish (Raphanus sativus L.) according to nutritional and environmental conditions. Hortic. Environ. Biotechnol. 2011, 52, 530–535. [Google Scholar] [CrossRef]
- Smýkalová, I.; Větrovcová, M.; Klíma, M.; Macháčková, I.; Griga, M. Efficiency of Microspore Culture for Doubled Haploid Production in the Breeding Project “Czech Winter Rape”. Czech J. Genet. Plant Breed. 2018, 42, 58–71. [Google Scholar] [CrossRef]
- Babbar, S.B.; Agarwal, P.K.; Sahay, S.; Bhojwani, S.S. Isolated microspore culture of Brassica: An experimental tool for developmental studies and crop improvement. Indian J. Biotechnol. 2004, 3, 185–202. [Google Scholar]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Biotech. Histochem. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Domblides, E.; Shmykova, N.; Shumilina, D.; Zayachkovskaya, T.; Mineykina, A.; Kozar, E.; Ahramenko, V.; Shevchenko, L.; Kan, L.; Bondareva, L.; et al. A Technology for Obtaining Doubled Haploids in Microspore Cultures of the Brassicaceae Family (Guidelines); VNIISSOK: Moscow, Russia, 2016; ISBN 9785901695715. [Google Scholar]


| Species | Accession Name | Accession Type | Source |
|---|---|---|---|
| Spring rape (Brassica napus var. napus) | Ratnik | cultivar | VNIIMK |
| Hurma | breeding accession | Astra | |
| European radish (Raphanus sativus L. subsp. sativus convar. radicula) | RBK (pink-red with white tip) | cultivar | FSBSI FSVC |
| Sarepta Mustard (Brássica júncea (L.) Czern) | Sudarushka | breeding accession | FSBSI FSVC |
| 72 | breeding accession | FSBSI FSVC | |
| Red cabbage (B. oleracea L. convar. capitata (L.) Alef. var. capitata (L.) f. rubra (L.) Thell.) | 428 | breeding accession | FSBSI FSVC |
| 439 | breeding accession | FSBSI FSVC | |
| White head cabbage (B. oleracea L. convar. capitata L. Alef. var. capitata (L.) f. alba DC.) | Parus | cultivar | FSBSI FSVC |
| Genotype | Bud Size, mm | Ratio of Debris Particles Per Pc to Microspores Per Pc in the Preparation | Two-Way ANOVA Factors/The Contribution of the Influence 5, % | ||
|---|---|---|---|---|---|
| Isolation Method No. 3 | Isolation Method No. 2 | Isolation Method No. 1 | |||
| spring rapeseed ‘Ratnik’ | 2.5–3.0 | 1.59 1 a 3/B 4 | 3.12 a/B | 9.01 a/A | bud size *** 2/18% isolation method ***/68% bud size x isolation method ***/10% random factors/4% |
| 3.0–3.5 | 1.56 a/C | 3.09 a/B | 8.36 a/A | ||
| 3.5–4.0 | 1.21 a/C | 2.92 a/B | 5.33 b/A | ||
| 4.0–4.5 | 0.56 a/C | 1.27 b/B | 3.26 c/A | ||
| European radish ‘RBK’ | 2.0–2.5 | 0.59 a/B | 11.45 a/A | 13.60 b/A | bud size ***/9% isolation method ***/64% bud size x isolation method ***/25% random factors/2% |
| 2.5–3.0 | 0.28 a/C | 3.95 c/B | 16.31 b/A | ||
| 3.0–3.5 | 0.38 a/C | 6.40 bc/B | 40.47 a/A | ||
| 3.5–4.0 | 0.46 a/B | 3.72 c/B | 35.33 a/A | ||
| 4.0–4.5 | 0.52 a/C | 8.30 ab/B | 12.06 b/A | ||
| Genotype | Bud Size, mm | Isolation Method No. 2 Embryoids Pcs/ Petri Dish | Isolation Method No. 3 Embryoids Pcs/ Petri Dish | Two-Way ANOVA Factors |
|---|---|---|---|---|
| spring rapeseed ‘Ratnik’ | 3.0–3.5 | 0.0 1 b 3/B 4 | 378.0 b/A | bud size *** 2 isolation method *** bud size x isolation method *** |
| 3.5–4.0 | 295.0 a/B | 522.7 a/A | ||
| 4.0–4.5 | 0.0 b/B | 37.3 c/A | ||
| spring rapeseed ‘Hurma’ | 2.0–2.5 | 1099.5 b/A | 1177.5 b/A | bud size *** isolation method ** bud size x isolation method * |
| 2.5–3.0 | 1304.5 a/A | 1311.5 a/A | ||
| 3.0–3.5 | 268.7 c/B | 627.0 c/A | ||
| European radish ‘RBK’ | 3.0–3.5 | 0.3 a/B | 7.0 a/A | bud size ns isolation method ** bud size x isolation method ** |
| 3.5–4.0 | 2.7 a/A | 3.3 ab/A | ||
| 4.0–4.5 | 0.7 a/A | 1.3 b/A | ||
| Sareptian mustard ‘Sudarushka’ | 2.5–2.9 | 6.3 b/B | 16.0 ab/A | bud size ** isolation method ** bud size x isolation method ns |
| 2.9–3.3 | 15.7 a/A | 22.7 a/A | ||
| 3.3–3.7 | 1.7 b/B | 11.3 b/A | ||
| Sareptian mustard breeding accession No. 72 | 2.0–2.5 | 1.0 b/B | 9.0 b/A | bud size *** isolation method *** bud size x isolation method ** |
| 2.5–3.0 | 3.0 a/B | 22.7 a/A | ||
| 3.0–3.5 | 0.0 b/B | 9.7 b/A | ||
| red cabbage breeding accession No. 428 | 3.5–4.0 | 0.0 -/B | 11.0 b/A | bud size *** isolation method *** bud size x isolation method *** |
| 4.0–4.5 | 0.0 -/B | 16.0 a/A | ||
| 4.5–5.0 | 0.0 -/- | 0.0 -/- | ||
| red cabbage breeding accession No. 439 | 3.5–4.0 | 1.7 b/A | 0.3 c/B | bud size *** isolation method *** bud size x isolation method *** |
| 4.0–4.5 | 10.3 a/B | 31.3 a/A | ||
| 4.5–5.0 | 0.0 b/B | 6.0 b/A | ||
| white cabbage ‘Parus’ | 4.0–4.5 | 2.3 b/A | 5.7 bc/a | bud size *** isolation method *** bud size x isolation method *** |
| 4.5–5.0 | 7.0 a/A | 10.0 b/A | ||
| 5.0–5.5 | 8.7 a/B | 23.0 a/A | ||
| 5.5–6.0 | 0.0 b/B | 2.0 c/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozar, E.V.; Kozar, E.G.; Domblides, E.A. Effect of the Method of Microspore Isolation on the Efficiency of Isolated Microspore Culture In Vitro for Brassicaceae Family. Horticulturae 2022, 8, 864. https://doi.org/10.3390/horticulturae8100864
Kozar EV, Kozar EG, Domblides EA. Effect of the Method of Microspore Isolation on the Efficiency of Isolated Microspore Culture In Vitro for Brassicaceae Family. Horticulturae. 2022; 8(10):864. https://doi.org/10.3390/horticulturae8100864
Chicago/Turabian StyleKozar, Elena V., Elena G. Kozar, and Elena A. Domblides. 2022. "Effect of the Method of Microspore Isolation on the Efficiency of Isolated Microspore Culture In Vitro for Brassicaceae Family" Horticulturae 8, no. 10: 864. https://doi.org/10.3390/horticulturae8100864
APA StyleKozar, E. V., Kozar, E. G., & Domblides, E. A. (2022). Effect of the Method of Microspore Isolation on the Efficiency of Isolated Microspore Culture In Vitro for Brassicaceae Family. Horticulturae, 8(10), 864. https://doi.org/10.3390/horticulturae8100864






