Multivariate Discrimination of Some Grapevine Cultivars under Drought Stress in Iran
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Parameters
2.3. Relative Water Content
2.4. Photosynthetic Pigments
2.5. Electrolyte Leakage
2.6. Lipid Peroxidation and Malondialdehyde Concentration
2.7. Proline Content
2.8. Soluble Sugar Total Content
2.9. Antioxidant Enzyme Activity Assay
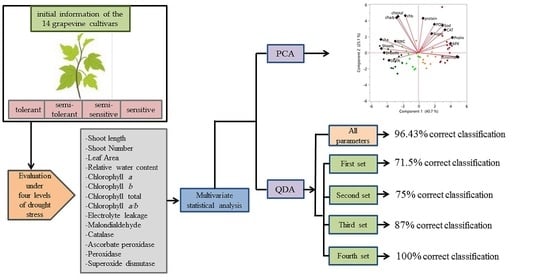
2.10. Statistical Analysis
3. Results
3.1. Response of Grapevine Cultivars to Drought Stress
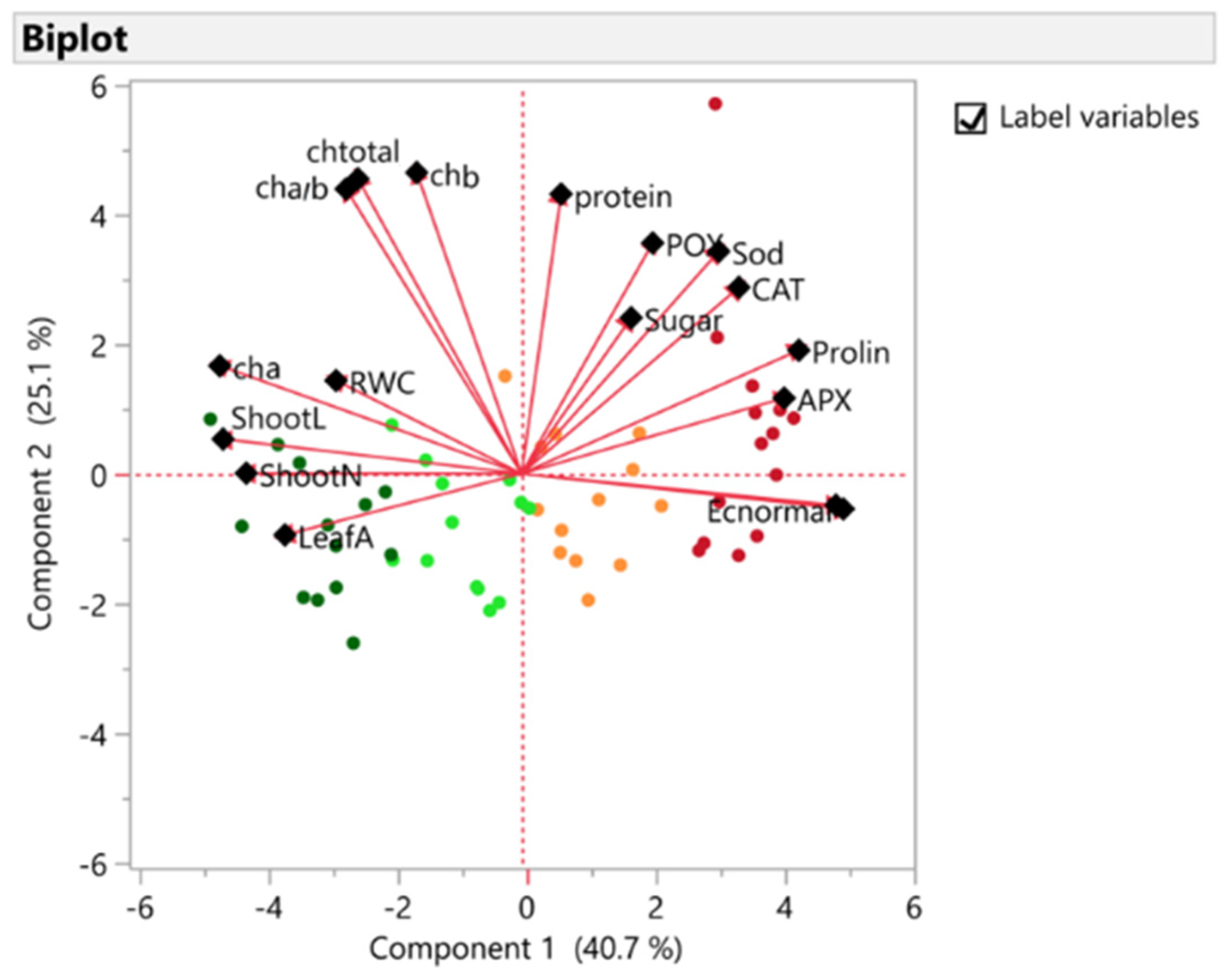
3.2. Principal Components Analysis
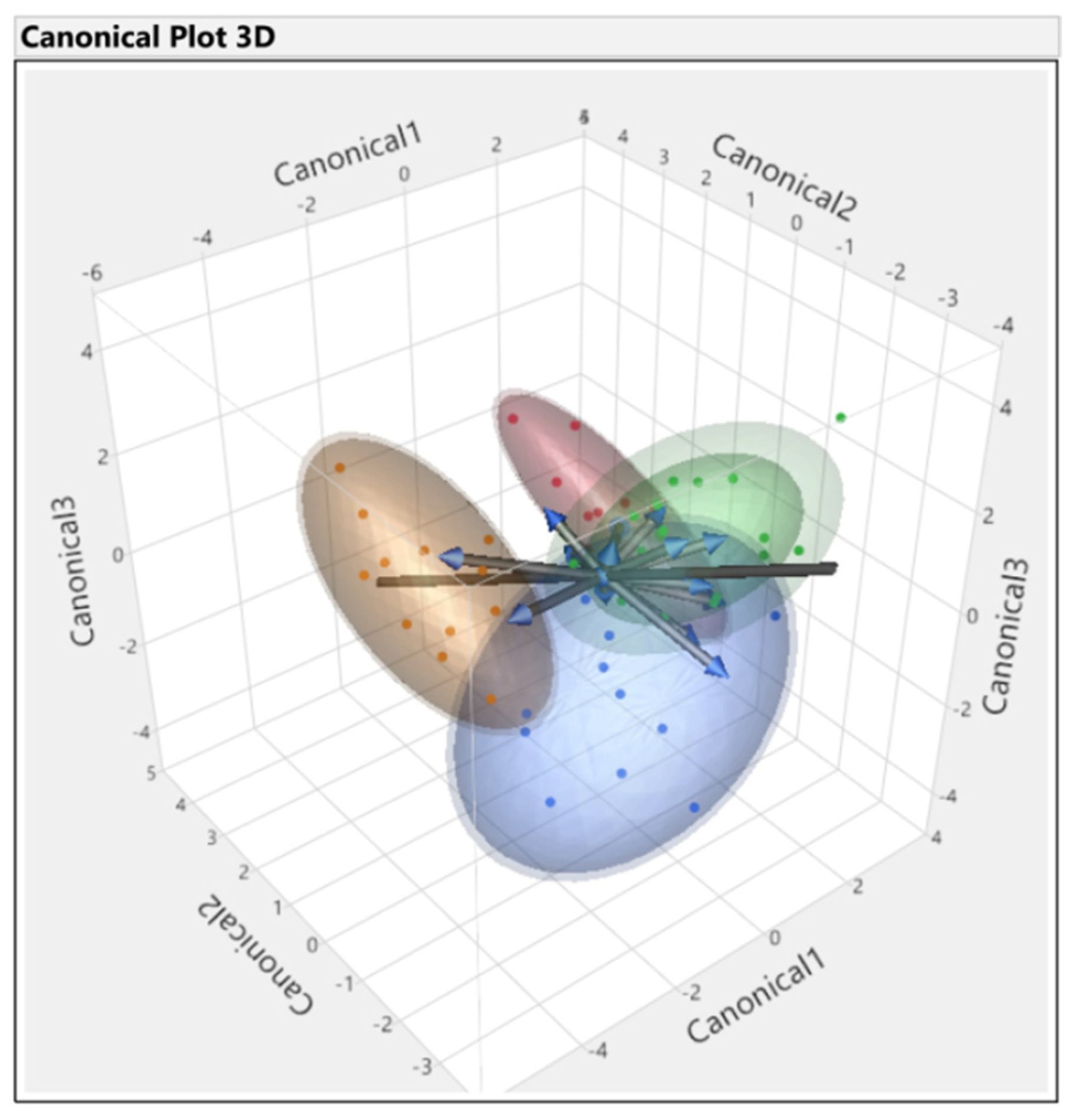
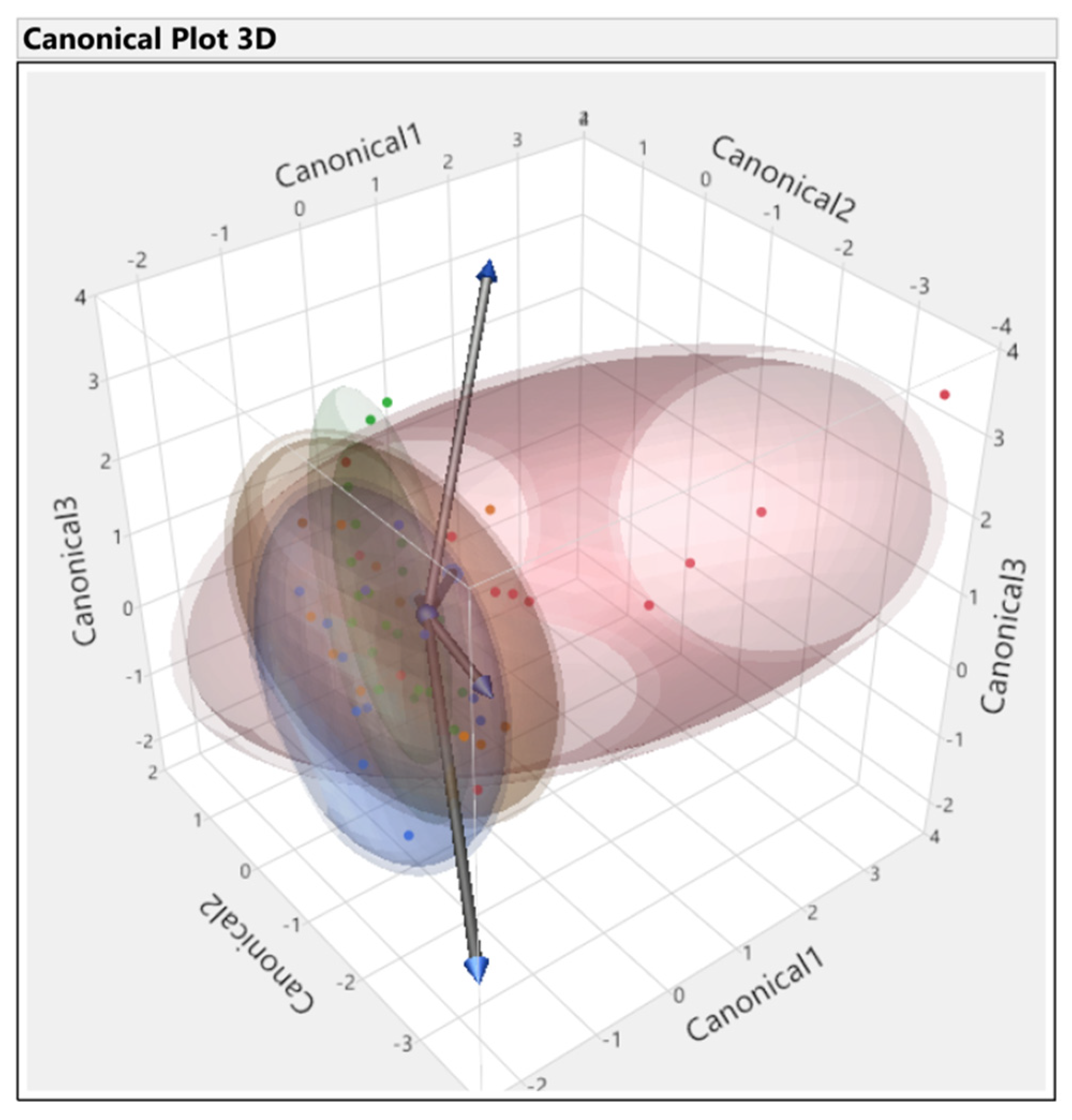
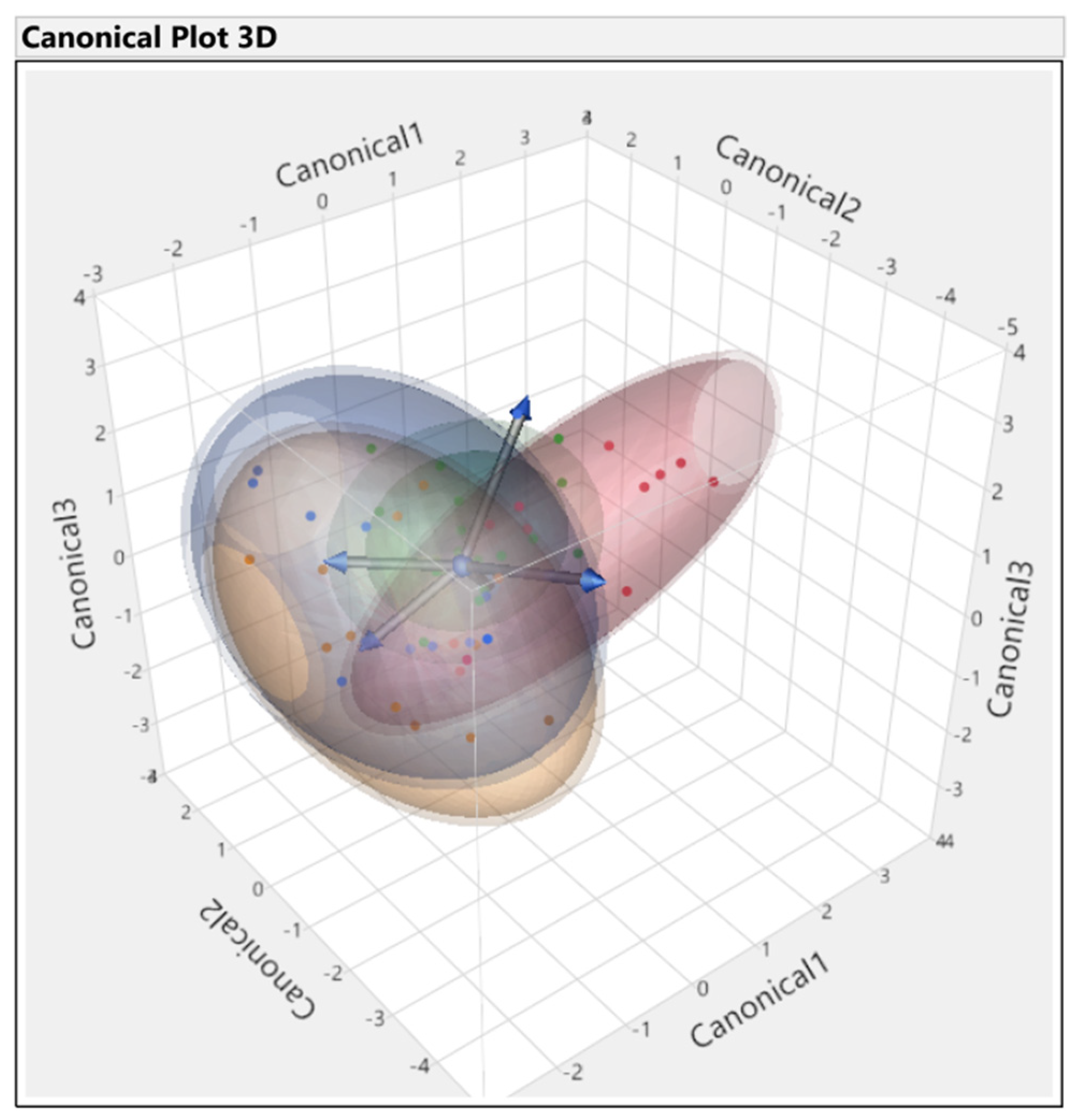
3.3. Discriminant Analysis
3.3.1. First Set
3.3.2. Second Set
3.3.3. Third Set
3.3.4. Fourth Set
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D. Plant drought stress: Effects, mechanisms, and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Liu, F.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul. 2013, 71, 31–40. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Mukherjee, A.K. Salt stress-induced cytosolute accumulation, antioxidant response, and membrane deterioration in three rice cultivars during early germination. Seed Sci. Technol. 2002, 30, 279–287. [Google Scholar]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, M.J.; Santos, T.; Regalado, P.A.; Rodrigues, L.M.; Lopes, M.C. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Appl. Biol. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Suklje, K.; Antalick, G.; Coetzee, Z.; Schmidtke, L.; Cesnik, H.B.; Brandt, J.; du Toit, W.; Lisjak, K.; Deloire, A. Effect of leaf removal and ultraviolet radiation on the composition and sensory perception of Vitis vinifera L. cv. Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2014, 20, 223–233. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants, and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Alston, J.M.; Sambucci, O. Grapes in the World Economy. In The Grape Genome. Compendium of Plant Genomes; Cantu, D., Walker, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- FAO-OIV Focus. Table and Dried Grapes; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Amiry, S.; Esmaiili, M.; Alizadeh, M. Classification of adulterated honeys by multivariate analysis. Food Chem. 2017, 224, 390–397. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in Araucaria Angustifolia. Braz. J. Plant Physiol. 1999, 11, 69–75. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids. In Pigments of Photosynthetic Biomembranes; Wilchek, M., Bayer, E.A., Eds.; Methods in Enzymology; Academic Press: Cambridge, UK, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Gohari, G.; Zareei, E.; Rostami, H.; Panahirad, S.; Kulak, M.; Farhadi, H.; Amini, M.; del Carmen Martinez-Ballesta, M.; Fotopoulos, V. Protective effects of cerium oxide nanoparticles in grapevine (Vitis vinifera L.) cv. Flame Seedless under salt stress conditions. Ecotoxicol. Environ. Saf. 2021, 220, 112402. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid penoxidation. Arch. Biochemical. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Woldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Watanabe, S.; Kojima, K.; Ide, Y. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult. 2000, 63, 199. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalase and Peroxidases. Method Enzymol. 1955, 11, 764–775. [Google Scholar] [CrossRef]
- In, B.C.; Motomura, S.; Inamoto, K.; Doi, M.; Mori, G. Multivariate analysis of relations between preharvest environmental factors, postharvest morphological and physiological factors, and vase life of cut Asami Redroses. J. Jpn. Soc. Hortic. Sci. 2007, 76, 66–72. [Google Scholar] [CrossRef][Green Version]
- Giannopolitis, C.N.; Reis, S.K. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Sci. Direct. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Deming, S.N.; Michotte, Y.; Kaufman, L. Principal components and factor analysis. In Chemometrics: A Textbook; Elsevier: Amsterdam, The Netherlands, 1988; pp. 339–369. [Google Scholar]
- Bari, L.R.; Ghanbari, A.; Darvishzadeh, R.; Giglou, M.T.; Baneh, H.D. Discernment of grape rootstocks base on their response to salt stress using selected characteristics in combination with chemometric tools. Food Chem. 2021, 365, 130–408. [Google Scholar] [CrossRef]
- Deka, D.; Singh, A.K.; Singh, A.K. Effect of Drought Stress on Crop Plants with Special Reference to Drought Avoidance and Tolerance Mechanisms: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2703–2721. [Google Scholar] [CrossRef]
- Colom, M.R.; Vazzana, C. Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping lovegrass plants. Environ. Exp. Bot. 2003, 49, 135–144. [Google Scholar] [CrossRef]
- Tsegay, D.; Amsalem, D.; Almeida, M.; Crandles, M. Responses of grapevine rootstocks to drought stress. Int. J. Plant Physiol. Biochem. 2014, 6, 1–6. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Xiang, D.-B.; Peng, L.-X.; Zhao, J.-L.; Zou, L.; Zhao, G.; Song, C. Effect of drought stress on yield, chlorophyll contents, and photosynthesis in Tartary buckwheat (Fagopyrum tataricum). J. Food Agric. Environ. 2013, 11, 1358–1363. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Doupis, G.; Chartzoulakis, K.; Patakas, A. Differences in antioxidant mechanisms in grapevines subjected to drought and enhanced UVB radiation. Emir. J. Food Agric. 2012, 24, 607–613. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2018, 62, 314–320. [Google Scholar] [CrossRef]
- Altinci, N.T.; Cangil, R. Drought Tolerance of Some Wine Grape Cultivars under In Vitro Conditions. J. Agric. Fac. Gaziosmanpasa Univ. 2019, 36, 145–151. [Google Scholar] [CrossRef]
- Karimi, S.; Hojati, S.; Eshghi, S.; Moghaddam, R.N.; Jandoust, S. Magnetic exposure improves the tolerance of Figure ‘Sabz’ explants to drought stress induced in vitro. Sci. Hortic. 2012, 137, 95–99. [Google Scholar] [CrossRef]
- Savee, R.; Adillon, J. Comparison between plant water relations of in vitro plants and rooted cuttings of kiwifruit. Acta Hortic. 1990, 282, 53–57. [Google Scholar] [CrossRef]
- Labudda, M. Lipid peroxidation is a biochemical marker for oxidative stress during drought. An effective tool for plant breeding. E-Wydawnictwo 2013, 1–12. [Google Scholar]
- Harish, C.; Baig Pandey, M.J.; Chandra, A.; Bhatt, R.K. Drought stress-induced changes in lipid peroxidation and antioxidant system in genus Avena. J. Environ. Biol. 2010, 31, 435–440. [Google Scholar]
- Wang, S.; Liang, D.; Li, C.; Hao, Y.; Ma, F.; Shu, H. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef]
- Prochozkova, D.; Jan, S.; Abd-Allah, E.F.; Ahmad, P. Water stress in grapevine (Vitis vinifera L.). In Water Stress and Crop Plants: A Sustainable Approach, 1st ed.; Ahmad, P., Ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2016; Volume 2. [Google Scholar]
- Kamangar, A.; Haddad, R. Effect of Water Stress and Sodium Silicate on Antioxidative Response in Different Grapevine (Vitis vinifera L.) Cultivars. J. Agr. Sci. Technol. 2016, 18, 1859–1870. [Google Scholar]
- Asada, K. The water–cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Zhou, F.; Ding, C. Exogenous ascorbic acid and glutathione alleviate oxidative stress induced by salt stress in the of Oryza sativa L. Z. Für Nat. C 2014, 69, 226–236. [Google Scholar] [CrossRef]
- Salazar-Parra, C.; Aguirreolea, J.; Sanchez-Daz, M.; Irigoyen, J.J.; Morales, J. Climate change (elevated CO2, elevated temperature and moderate drought) triggers the antioxidant enzymes’ response of grapevine cv. Tempranillo, avoiding oxidative damage. Physiol. Plant 2012, 144, 99–110. [Google Scholar] [CrossRef]
- Mirzaee, M.; Moieni, A.; Ghanati, F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two Canola (Brassica napus L.) Cultivars. J. Agri. Sci. Technol. 2013, 15, 593–602. [Google Scholar]
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J.S. Warming and drought differentially influence the production and resorption of elemental and metabolic nitrogen pools in Quercus rubra. Glob. Change Biol. 2015, 21, 4177–4195. [Google Scholar] [CrossRef]
- Corso, M.; Bonghi, C. Grapevine rootstock effects on abiotic stress tolerance. Plant Sci. Today 2014, 1, 108–113. [Google Scholar] [CrossRef]



| Symbol | Genotype | Type of Use | Firmness | Skin Color | Berry Shape | Ripening | Seed |
|---|---|---|---|---|---|---|---|
| 1 | Garashiligh | Grape juice | Soft | Dark blue | Round | Medium | Seeded |
| 1 | Tukilgan | Grape juice | Soft | Light yellow | Oval shaped | Medium | Seeded |
| 1 | Sahebi | Soft | Red | Oval shaped | Late | Seeded | |
| 2 | Rasmi | Fresh use | Firm | Yellow-green | Round | Late | Seeded |
| 2 | Aldarag | Grape juice | Soft | Yellow-green | Oval shaped | Late | Seeded |
| 2 | Shahani | Fresh use, grape juice | Medium | Dark blue | Round | Medium | Seeded |
| 2 | Seirak pusteh | Fresh use, grape juice | Medium | Yellow-green | Round | Late | Seeded |
| 2 | Khalili | Fresh use, raisins | Medium | yellow | Oval shaped | Early | Seeded |
| 3 | Copake Bogan | Grape juice | Medium | Yellow-green | Ovate | Late | Seeded |
| 3 | Noras | Fresh use | Medium | Yellow-green | Ovate | Early | Seeded |
| 3 | Kechi Amjaie | Fresh use, Grape juice | Soft | Yellow | Oblong | Late | Seeded |
| 4 | Aghshiligh | Grape juice | Soft | Yellow-green | Round | Late | Seeded |
| 4 | Keshmeshi Tabrizi | Grape juice | Medium | Yellow-green | Oval shaped | Early | Seeded |
| 4 | Keshmeshi | Fresh use, raisins | Medium | Yellow | Elliptic | Medium | Seedless |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Shoot L | −0.83 | 0.09 | 0.16 |
| Shoot N | −0.77 | 0.001 | 0.09 |
| Leaf A | −0.66 | −0.17 | 0.32 |
| RWC | −0.52 | 0.25 | 0.004 |
| Chl a | −0.84 | 0.30 | −0.01 |
| Chl b | −0.29 | 0.84 | −0.37 |
| Chl a/b | −0.45 | 0.82 | −0.27 |
| Chl total | −0.49 | 0.79 | −0.31 |
| EL | 0.90 | −0.09 | −0.06 |
| MDA | 0.88 | −0.08 | −0.10 |
| Proline | 0.77 | 0.34 | −0.06 |
| Sugar | 0.30 | 0.43 | 0.46 |
| Protein | 0.11 | 0.78 | 0.30 |
| CAT | 0.60 | 0.52 | 0.11 |
| APX | 0.73 | 0.21 | −0.42 |
| POX | 0.36 | 0.64 | 0.38 |
| Sod | 0.55 | 0.62 | 0.28 |
| Source | Count | Number Misclassified | Percent Misclassified | Entropy RSquare | −2LogLikelihood |
|---|---|---|---|---|---|
| All parameters | 56 | 2 | 3.5 | 0.90 | 14.5 |
| First set | 56 | 16 | 28.5 | 0.57 | 64.7 |
| Second set | 56 | 10 | 17.8 | 0.45 | 83.5 |
| Third set | 56 | 13 | 23.2 | 0.64 | 54.6 |
| Fourth set | 56 | 0 | 0.0 | 0.98 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahim, S.; Ghanbari, A.; Naji, A.M.; Shokohian, A.A.; Maleki Lajayer, H.; Gohari, G.; Hano, C. Multivariate Discrimination of Some Grapevine Cultivars under Drought Stress in Iran. Horticulturae 2022, 8, 871. https://doi.org/10.3390/horticulturae8100871
Fahim S, Ghanbari A, Naji AM, Shokohian AA, Maleki Lajayer H, Gohari G, Hano C. Multivariate Discrimination of Some Grapevine Cultivars under Drought Stress in Iran. Horticulturae. 2022; 8(10):871. https://doi.org/10.3390/horticulturae8100871
Chicago/Turabian StyleFahim, Somayyeh, Alireza Ghanbari, Amir Mohammad Naji, Ali Akbar Shokohian, Hasan Maleki Lajayer, Gholamreza Gohari, and Christophe Hano. 2022. "Multivariate Discrimination of Some Grapevine Cultivars under Drought Stress in Iran" Horticulturae 8, no. 10: 871. https://doi.org/10.3390/horticulturae8100871
APA StyleFahim, S., Ghanbari, A., Naji, A. M., Shokohian, A. A., Maleki Lajayer, H., Gohari, G., & Hano, C. (2022). Multivariate Discrimination of Some Grapevine Cultivars under Drought Stress in Iran. Horticulturae, 8(10), 871. https://doi.org/10.3390/horticulturae8100871