Abstract
Tree peony (Paeonia ostii) is an ornamental flowering plant that is generally recalcitrant to establishment of a mature somatic embryo regeneration system in vitro. Glucose-6-phosphate translocator (GPT) plays an important regulatory role in embryogenesis of plants. In this study, PoGPT1 was cloned, and a bioinformatic analysis and functional verification of the gene were performed. The results showed that PoGPT1 encoded a polypeptide of 392 amino acids, which was a basic non-secreted hydrophobic transmembrane protein, and was mainly localized in the plastids. PoGPT1 was highly expressed in tree peony leaves, and its transcript abundance increased with the progression of zygotic embryo development. Overexpression of PoGPT1 caused up-regulation of leafy cotyledon 1 (PoLEC1), somatic embryogenesis receptor-like kinase (PoSERK), and agamous-like15 (PoAGL15) in tree peony callus. In addition, PoGPT1 overexpression promoted the increase in indole-3-acetic acid (IAA), 5-deoxystrigol (5DS), and brassinolide (BL) contents, especially of IAA, but reduced the contents of abscisic acid (ABA), 6-benzyladenosine (BARP), and 1-aminocyclopropanecarboxylic acid (ACC). The present research showed that PoGPT1 synergistically regulated the contents of endogenous hormones and expression levels of embryogenesis-related genes to promote the embryonic development of tree peony. The results provide theoretical and technical support for the establishment of a tree peony embryogenic callus formation and subsequent research on somatic embryogenesis.
1. Introduction
Tree peony (Paeonia sect. Moutan) is among the oldest and most culturally important horticultural and medicinal plants in China. It is known for its large and colorful flowers, and is widely regarded as the national flower of China [1,2,3,4,5]. To date, tree peony has been propagated mainly using asexual methods, such as plant division and grafting. However, these methods have a low propagation efficiency, which limits large-scale plant production [6,7,8,9]. Tissue culture methods have the potential to solve this problem, but there are some problems when using such methods to produce tree peony plantlets by direct organogenesis. For example, it is difficult to induce explants to form roots, and a large proportion of the roots that do form are of poor quality, with a disconnect between the adventitious root and the stem. This results in low survival rates of plantlets at the transplanting stage [6,10,11,12]. Somatic (non-zygotic) embryos develop from tissue or somatic cells that form proembryogenic masses, and have the same ability as zygotic embryos to germinate and develop into a complete plant [13,14]. Thus, tree peony plantlet regeneration via the somatic embryogenesis pathway may overcome the aforementioned problems.
Previous studies of embryogenic callus culture and somatic embryo regeneration of tree peony have shown that the regeneration ability of tree peony somatic embryos is strongly associated with the explant genotype and hormones [10,11,15]. However, the embryogenic cell induction rate of tree peony is low and the differentiation of somatic embryos from proembryogenic masses is poor [16]. Even if seedlings are formed, many irregularities are observed, such as plant deformity and nonconnection of the root duct and sieve tubes [6,17]. Thus, an efficient and stable somatic embryo regeneration system for tree peony has not been previously reported. In essence, the low efficiency of the transformation from non-embryogenic callus to embryogenic callus is a major restrictive factor in the early stage of somatic embryo development [10,12,16]. Early-stage embryos are heterotrophic tissues that absorb, transport, and assimilate carbohydrates from plastids in non-photosynthetic cells [18,19]. GPT1, the gene encoding glucose-6-phosphate translocator 1 (GPT1), is mainly expressed in heterotrophic tissues, where its product mediates glucose metabolism and transport between source and sink cells [20,21]. GPT1 also participates in starch and fatty acid synthesis and/or the oxidative pentose phosphate pathway to produce nicotinamide adenine dinucleotide phosphate (NADPH) [22,23,24], as well as playing an important role in the synthesis, storage, and transportation of materials essential for embryonic structure and development [25,26]. Previous research has shown that GPT1 is essential for pollen fitness and successful reproduction during pollen maturation [27,28]. The distribution of sugars and auxin is severely affected in the gpt1 homozygous mutant of Arabidopsis thaliana, resulting in embryonic death [26,29]. Therefore, GPT1 is crucial for early embryo development. However, it is unknown whether GPT1 plays a related regulatory role through glucose metabolism and transport in the process of tree peony embryogenic callus induction.
In this study, we cloned PoGPT1 and analyzed its function. Our findings showed that PoGPT1 is expressed in different organs and during the process of embryogenic callus induction in tree peony. Given that somatic embryogenesis receptor-like kinase (SERK), leafy cotyledon 1 (LEC1), and agamous-like15 (AGL15) are crucial genes in plant somatic embryo development [30,31,32], we analyzed the regulatory effects of PoGPT1 on these genes and endogenous hormones. Homologous overexpression of PoGPT1 significantly affected the transcript levels of PoSERK, PoLEC1, and PoAGL15, and the contents of endogenous hormones. Our analyses show that the expression of PoGPT1 is strongly associated with auxin content. These results provide insight into the role of PoGPT1 during embryogenic callus induction in tree peony.
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
Tree peony (Paeonia ostii) was used as the test material. All plants were grown in a nursery at the Henan Agricultural University in Zhengzhou, China. The plants were approximately 10 years old, cultivated in sandy loam soil, and of healthy, vigorous growth.
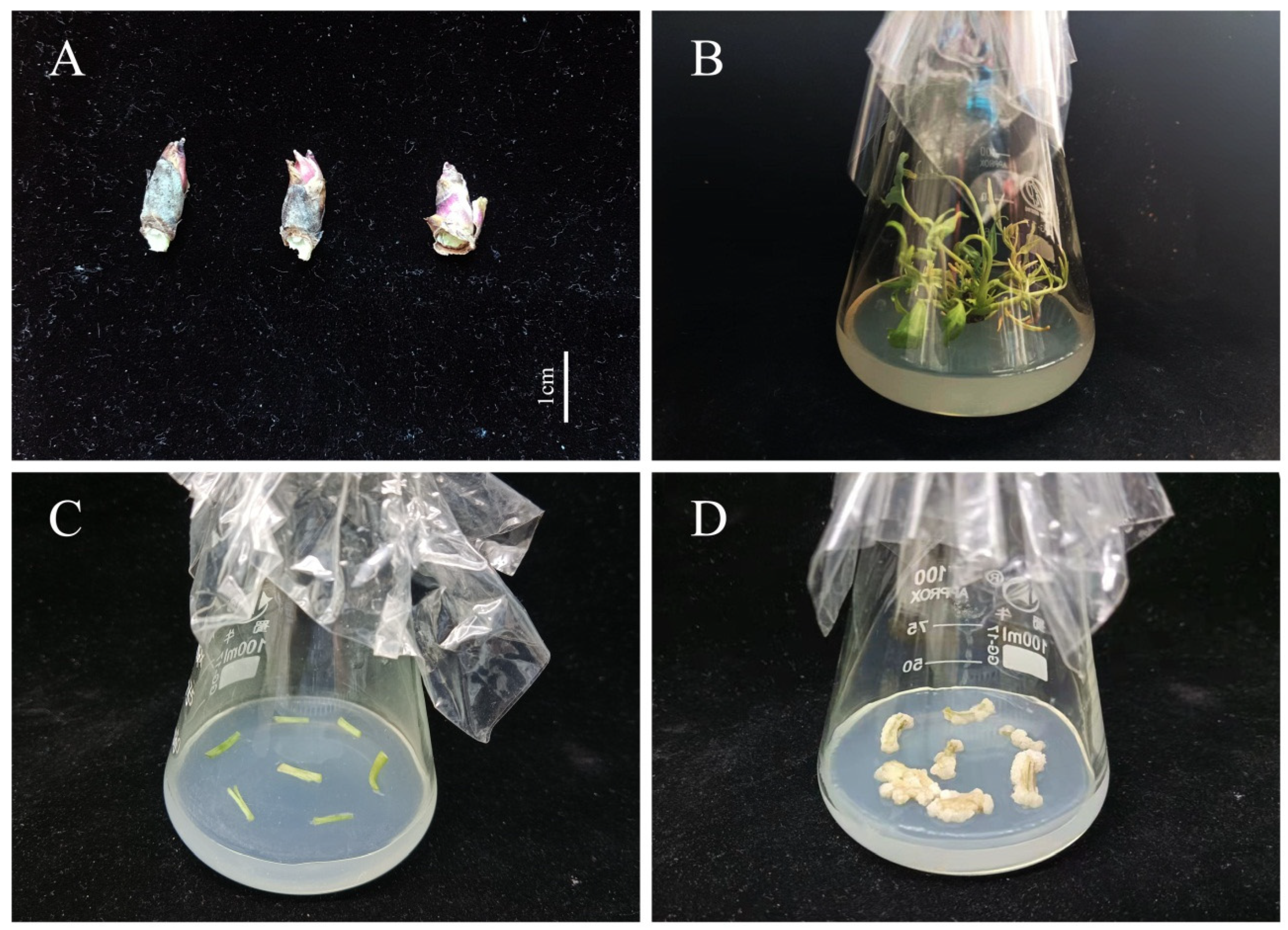
Our team’s previous research found that 5, 60, 65, 75, 90, and 110 days were the key stages (5 days): proembryo, 60 days: globular embryo, 65 days: heart-shaped embryo, 75 days: torpedo embryo, 90 days: cotyledon embryo, 110 days: mature embryo) of zygotic embryo development [33], so the pods were collected at 5, 60, 65, 75, 90, and 110 days after pollination, from the beginning of pod development until full maturity (from April to August, 2021). The pericarp of the pod was removed after collection, and the RNA was isolated specifically from developing embryos. In addition, roots, stems, leaves, flowers, and fully mature seeds were collected on the same days as specified for pods. The different fragments of the plant and zygotic embryos were used for real-time quantitative PCR (RT-qPCR) spatiotemporal expression analysis. Axillary buds (Figure 1A) were selected for total RNA extraction and gene cloning. The samples were quickly frozen in liquid nitrogen and stored in a −80 °C refrigerator (Thermo Forma 991, Thermo Fisher Scientific, Shanghai, China). Each sample comprised 0.5 g tissue with three replicates. The RNAiso Plus Kit (TaKaRa, Dalian, China) was used to extract total RNA from the tree peony materials.Axillary buds were sterilized on clean bench (SW-CJ-2F, Sujing Group, Suzhou Antai Air Technology Co., Ltd., Suzhou, China) [6], and then selected for tissue culture on plantlet induction medium (MS + Ca2+(WPM) + 6-BA 0.3 mg·L−1 + IBA 0.5 mg·L−1 + PVP 1.0 g·L−1 + Sucrose 30 g·L−1 + Agar 7 g·L−1, pH 5.8). After 30 d of culture (Figure 1B), the stems of plantlets in vitro were collected for induction of non-embryonic callus (1/2 MS + Ca2+(WPM) + 6-BA 1.0 mg·L−1 + 2.4-D 1.0 mg·L−1 + PVP 1.0 g·L−1 + LH 0.5 g·L−1 + Sucrose 30 g·L−1 + Agar 7 g·L−1, pH 5.8) (Figure 1C). The calli were used for transformation after 35 d of culture (Figure 1D).
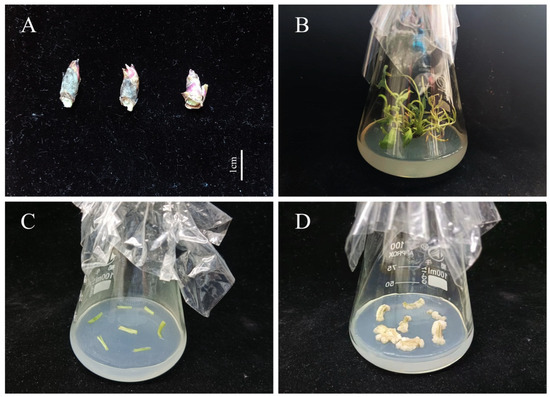
Figure 1.
Induction of non-embryogenic callus in the tree peony ((A), Axillary buds; (B), Plantlets in vitro; (C), Callus induction; (D), Non-embryonic callus). Scale bar: 1 cm.
The plantlets and calli were cultured in glass Erlenmeyer flasks (100 mL, Sichuan Shubo Group Co., Ltd., Sichuan, China) in a tissue culture room at the Laboratory of Ornamental Plants, Henan Agricultural University, Zhengzhou, China, under the following conditions: air temperature: 23 ± 1 °C; relative humidity: 70 ± 5%; photoperiod: 16 h light/8 h dark, with photosynthetically active radiation (45 ± 2 μmol·m−2·s−1) supplied by plant growth fluorescent lamps (TLD-type, Philips Co., Shanghai, China).
2.2. Cloning and Analysis of GPT1
Total RNA was extracted from 0.5 g of the tree peony materials (stems and leaves) using the modified CTAB method [34]. The cDNA was synthesized using the Prime Script RT Reagent Kit (TaKaRa). Gene-specific primers (Table 1) were designed to clone the full-length coding sequence (CDS) of PoGPT1 (GenBank: ON392713). BioXM2.7 was used to translate the open reading frame (ORF) of PoGPT1. The physicochemical properties and conserved domains of the PoGPT1 protein were estimated using analytical tools accessible on the NCBI website (https://www.ncbi.nlm.nih.gov/ (accessed on 20 June 2021)). The online software SMART (http://smart.embl-heidelberg.de (accessed on 20 June 2021)) was used for domain analysis. DNAMAN8 was used for multiple sequence alignment, and MEGA7.0 was used to construct phylogenetic trees with the neighbor-joining method.

Table 1.
Summary of primers used in the study.
2.3. Subcellular Localization Analysis
The online software WoLF PSORT (https://www.genscript.com/wolf-psort.html (accessed on 8 October 2022)) was used for subcellular localization prediction of PoGPT1. The ORF of PoGPT1 (without the stop codon) was amplified with gene-specific primers (Table 1) and cloned into the pCAMBIA1300 vector to generate 35S::PoGPT1:GFP. Agrobacterium-mediated transformation of Nicotiana benthamiana leaf cells was used to investigate subcellular localization of its coding proteins [35]. The GFP fluorescence was observed at 40× magnification with a confocal laser scanning microscope (A1+, Nikon, Shanghai, China). The filter settings for visualization of the fluorescence were as follows: chloroplast fluorescence: excitation light wavelength 640 nm, emission light wavelength 675 nm; GFP fluorescence: excitation light wavelength 488 nm, emission light wavelength 510 nm; DPAI: excitation light wavelength 340 nm, emission light wavelength 488 nm. The software Fiji was used for co-localization quantification analysis.
2.4. Plasmid Construction and Transformation of Tree Peony Callus
To construct the overexpression vector, the ORF of PoGPT1 (without the stop codon) was amplified with gene-specific primers (Table 1) and cloned into pCAMBIA1300 to generate 35S::PoGPT1. The complete vector was verified by sequencing, and the construct was transformed into Agrobacterium tumefaciens strain LBA4404. The pCAMBIA1300 vector was transformed into A. tumifaciens cells separately as the control (CK). Nontransformed callus was used as the blank control (WT). The A. tumefaciens cells were used to transform calli of tree peony and were removed before expression analysis. RT-qPCR positive identification and expression level was used to prove that PoGPT1 containing callus are really transgenic on day 5, and the absence of vir gene in the PoGPT1 containing callus was confirmed by gene sequence analysis and gel electrophoresis. The specific protocol was based on our previous research results [36]. The transformed calli were analyzed to determine gene transcript levels and endogenous hormone contents, and paraffin-embedded thin sections were observed. The positive transgenic calli were cultured in embryogenic induction medium (1/2 MS + Ca2+(WPM) + 6-BA 0.5 mg·L−1 + TDZ 0.5 mg·L−1 + PVP 1.0 g·L−1 + LH 0.5 g·L−1 + Sucrose 20 g·L−1 + Agar 7 g·L−1, pH 5.8) for 20 days.
2.5. Determination of Plant Hormone Content
The transgenic calli with different genotypes (CK and 35S::PoGPT1) were finely ground in liquid nitrogen, and the powder was transferred to 10 mL centrifuge tubes. The contents of endogenous plant hormones (abscisic acid, ABA; 1-aminocyclopropanecarboxylic acid, ACC; 6-benzyladenosine, BARP; indole-3-acetic acid, IAA; 5-deoxystrigol, 5DS; and brassinolide, BL) in the transformed calli were determined using ultra-high-performance liquid chromatography (LC-30A, Shimadzu, Kyoto, Japan)–mass spectrometry (Triple TOF 5600+, AB SCIEX, Foster City, CA, USA) following a previously described method [6]. The plant hormone standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). The extraction and measurement methods were as described in our previous study [6].
2.6. Morphological and Anatomical Observation
The transgenic calli with different genotypes (CK, WT and 35S::PoGPT1) were transferred to somatic embryogenesis induction medium (1/2 MS + Ca2+(WPM) + 6-BA 0.5 mg·L−1 + TDZ 0.5 mg·L−1 + PVP 1.0 g·L−1 + LH 0.5 g·L−1 + Sucrose 20 g·L−1 + Agar 7 g·L−1, pH 5.8) and cultured for 20 d. Embryogenic calli were collected for morphological and anatomical observations. After fixation in formalin/acetic acid/alcohol = 1/1/18 (v/v/v), paraffin embedded sections (five sections of each callus were selected for observation and analyzed; thickness, 10 μm; prepared by Wuhan Service Biotechnology Co., Ltd., Wuhan, China) were examined to observe the cellular structure of the different transgenic calli. There were three calli where dissected in each transgenic calli.
2.7. Real-Time Quantitative PCR (RT-qPCR) Analysis
Expression of the somatic embryogenesis-related genes PoLEC1 (GenBank: ON454840), PoSERK (GenBank: KF876175), and PoAGL15 (GenBank: ON454841) was analyzed to verify the establishment of embryonic properties of the calli by RT-qPCR. Forward and reverse primers were designed using Primer-BLAST online tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/primertool.cgi (accessed on 20 June 2021)). A tree peony β-tubulin gene (EF608942) was used as the internal reference gene (Table 1). Analyses of gene transcript levels were conducted using the SYBR Premix Ex Taq™ II Kit (TaKaRa) in accordance with the manufacturer’s instructions. A standard two-step method was used with three biological replicates [37,38]. The quantitative analysis was performed on a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The relative transcript levels were calculated using the ∆∆Ct method.
2.8. Statistical Analysis
Analysis of variance (ANOVA) was performed with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Significant differences between means were detected using Duncan’s multiple range test at p ≤ 0.05.
3. Results
3.1. Sequence Analysis of PoGPT1
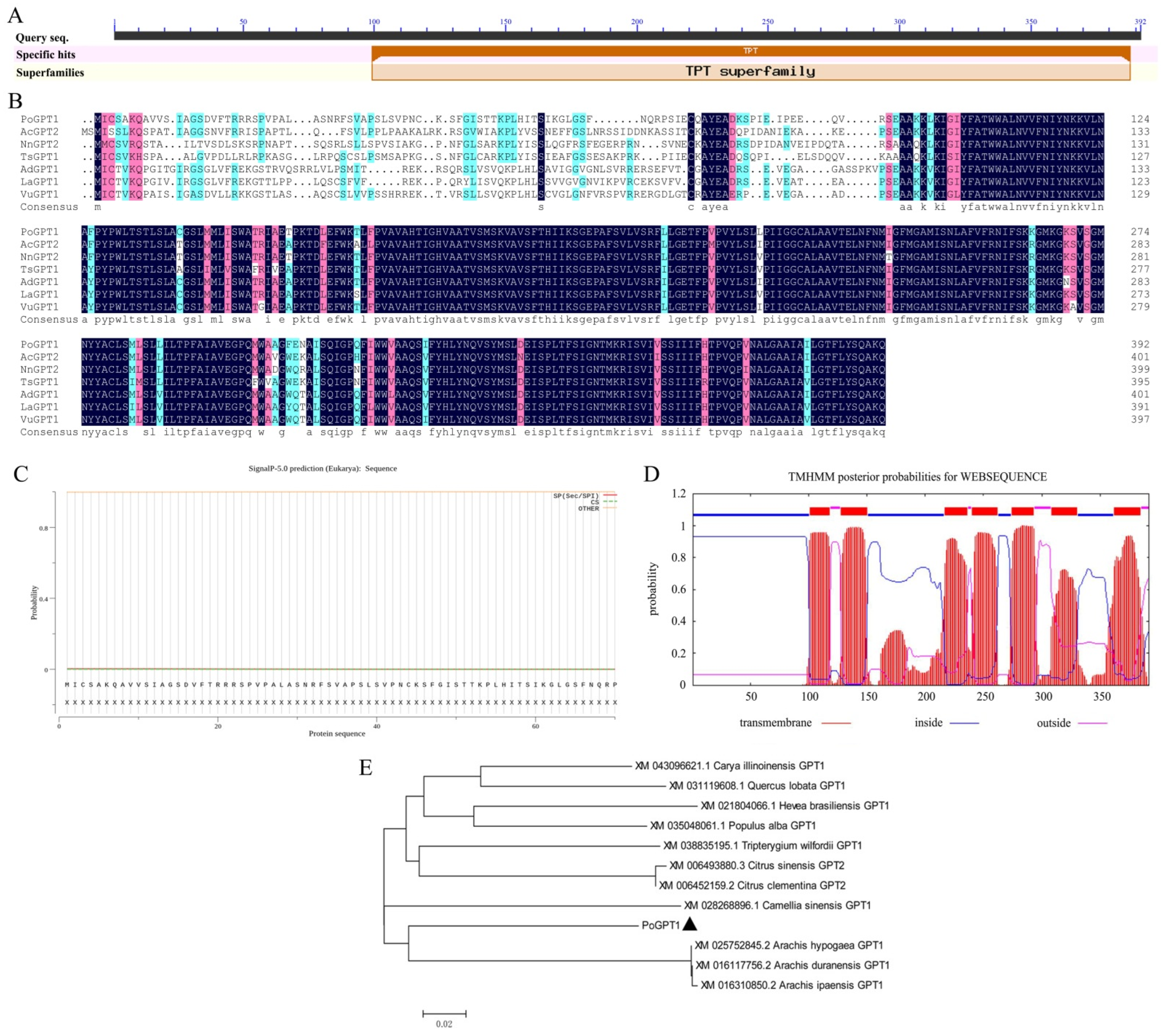
The ORF of PoGPT1 was 1179 bp, encoding a polypeptide of 392 amino acids with a predicted molecular weight of 42.80 kDa. The amino acid sequence included a highly conserved triose phosphate translocator (TPT) superfamily domain (Figure 2A,B).
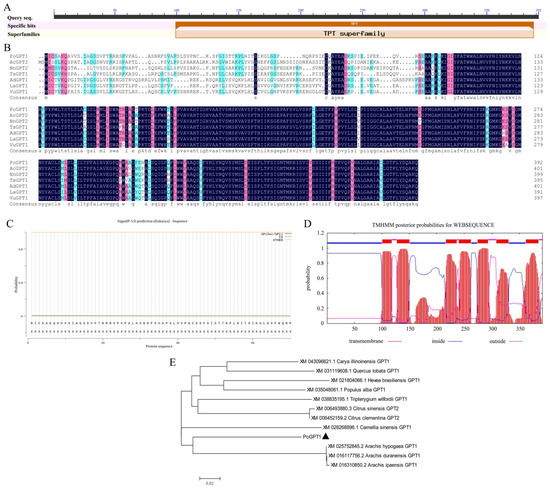
Figure 2.
Protein structure and phylogenetic analysis of PoGPT1 ((A), Conserved domains; (B), Sequence alignment, the color of amino acids from dark to light indicates their similarity from high to low; (C), Signal peptide analysis; (D), Transmembrane domain analysis; (E), Phylogenetic analysis).
The online prediction analysis revealed that PoGPT1 lacked a signal peptide, indicating that it was a non-secretory protein (Figure 2C). The transmembrane domain prediction analysis revealed that PoGPT1 was a typical transmembrane protein with seven transmembrane domains (Figure 2D). The physicochemical properties of the predicted PoGPT1 protein were analyzed using online software; the protein instability index of 49.04, fat solubility index of 102.04, and total average hydrophilicity index of 0.464 indicated that PoGPT1 was an unstable hydrophobic liposoluble protein. The predicted isoelectric point of the protein was 9.4. The protein contained more basic amino acid residues than acidic residues; thus, it was an alkaline protein. It showed high homology with homologs in Carya cathayensis, Camellia sinensis, citrus, oak, and rubber tree. Therefore, the gene was designated PoGPT1 (Figure 2B,E).
3.2. Subcellular Localization of PoGPT1
Subcellular localization of PoGPT1 was predicted according to the k-nearest neighbor classification algorithm by WoLF PSORT online analysis software, and the results showed that the predicted scores of PoGPT1 in plastids, vacuoles and chloroplasts were 6, 4 and 3, respectively.
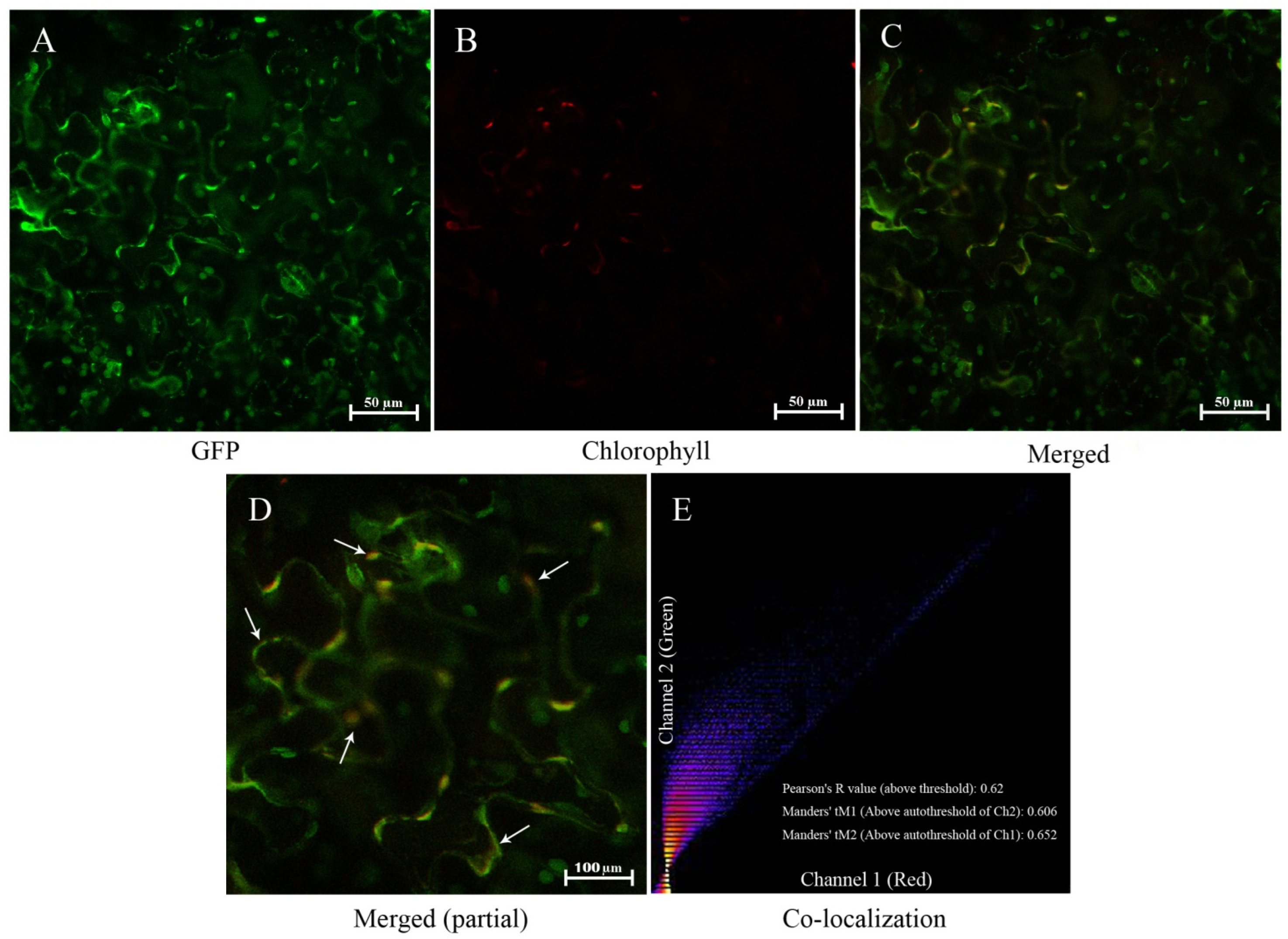
To further confirm the location of PoGPT1, the GFP-expressing vector for analyzing its subcellular localization was constructed and transfected into the lower epidermal cells of N. benthamiana leaves. Green fluorescent signal and red autofluorescence were emitted from the plastids in the leaf cells (Figure 3A–C). The green fluorescent signal from the GFP-PoGPT1 construct substantially overlapped with the red autofluorescence emitted from the plastids (Figure 3D), and the Pearson’s correlation coefficient and Manders’ colocalization coefficients were all above 0.6 (Figure 3E). At the same time, fluorescence signal overlap was found in the plasmatic membrane (Figure 3D), which may be caused by PoGPT1 possessing six transmembrane domains. In conclusion, PoGPT1 was mainly localized in plastids.
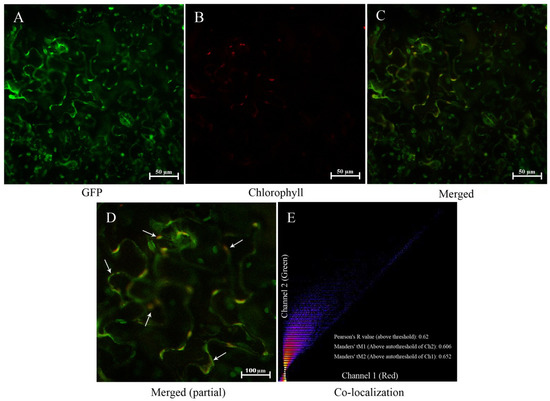
Figure 3.
Subcellular localization of PoGPT1 ((A), GFP; (B), Chlorophyll; (C), Merged; (D), Merged (partial); (E), Co-localization). Nicotiana benthamiana leaf lower epidermis cells were infected with the 35::PoGPT1-GFP construct mediated by Agrobacterium tumefaciens strain GV3101. Green fluorescence indicates the fusion protein location. Scale bar: 50 μm, 100 μm.
3.3. Spatiotemporal Expression Analysis of PoGPT1
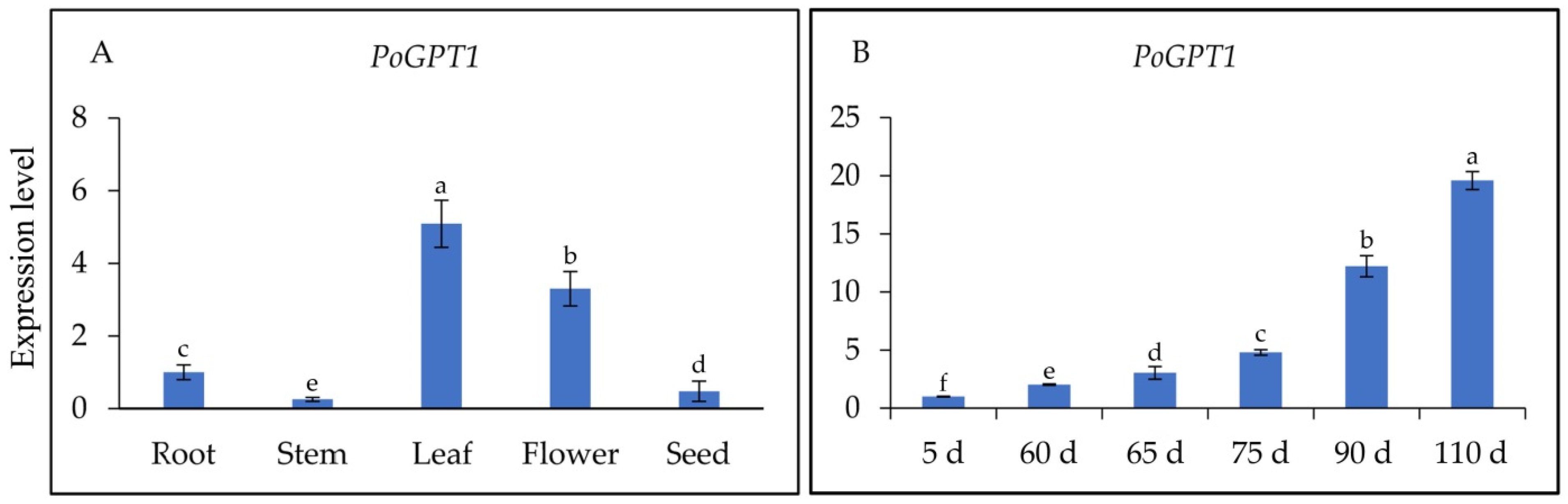
To determine the spatiotemporal-specific expression patterns of PoGPT1 and its transcript levels in different organs of tree peony and at different stages of embryonic development, RT-qPCR analysis was performed on cDNAs synthesized from RNA isolated from the roots, stems, leaves, flowers, and seeds, and from pods at different developmental stages. The PoGPT1 transcript abundances were ranked, from highest to lowest, as follows: leaf > flower > root > seed > stem (Figure 4A). The RT-qPCR analyses also revealed a gradual increase in the abundance of PoGPT1 transcripts during zygotic embryo development (Figure 4B).
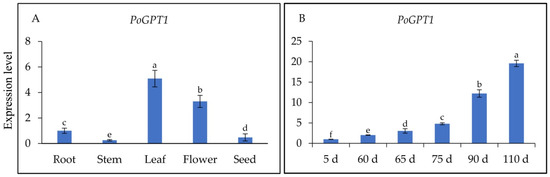
Figure 4.
Spatiotemporal expression analysis of PoGPT1 in tree peony ((A), Expression levels in different tissues of tree peony; (B), Expression levels at different stages of tree peony zygotic embryo development). Vertical bars indicate standard error (n = 3). Different letters indicate significant differences using the Duncan’s Multiple Range Test (p ≤ 0.05).
3.4. RT-qPCR Analysis of PoGPT1 and Genes Associated with Somatic Embryogenesis
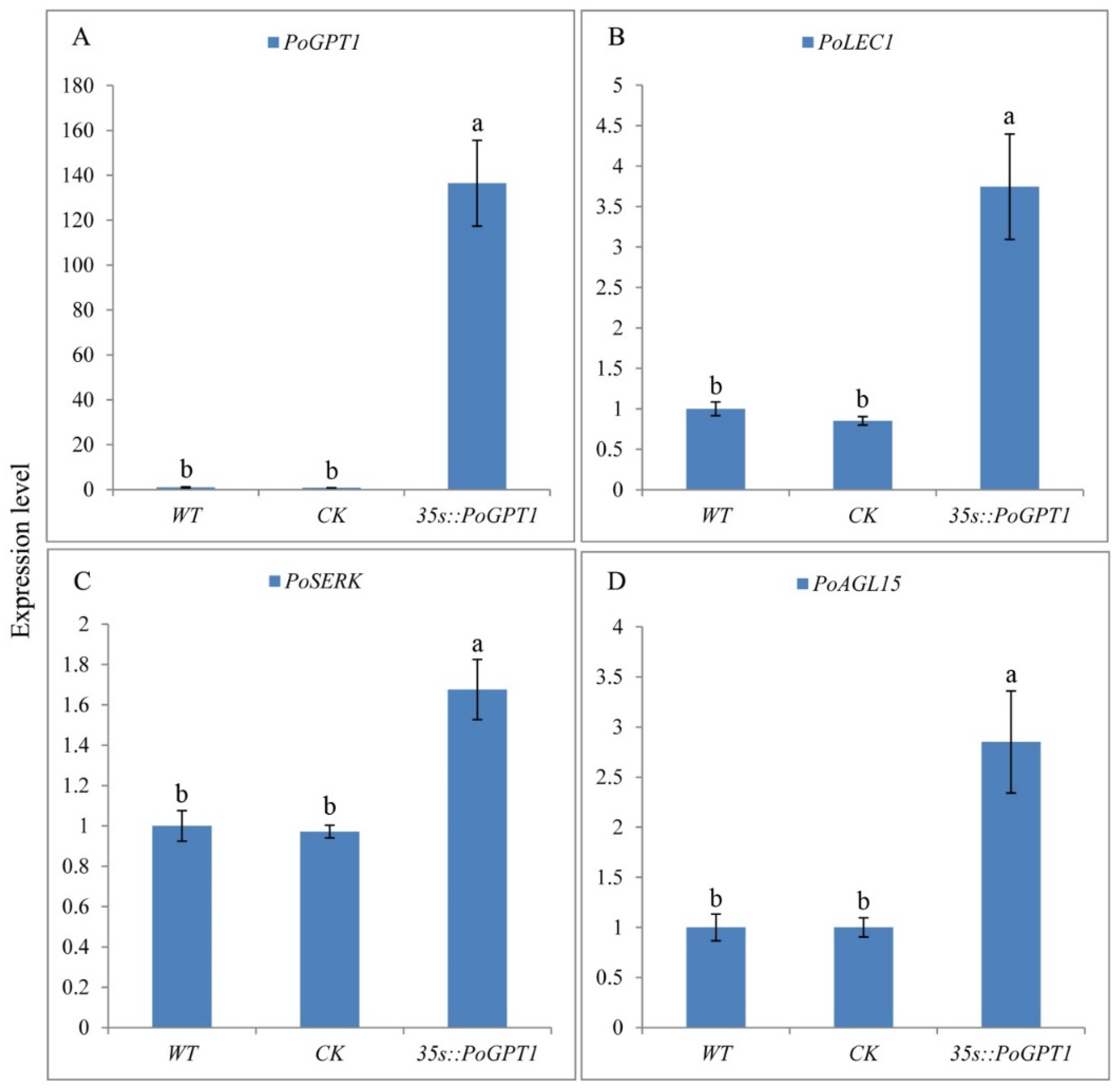
The PoGPT1 overexpression vector pCAMBIA1302-PoGPT1 was transformed into tree peony calli by Agrobacterium infection. PoGPT1 was expressed in both WT and CK callus, but the expression level was relatively low, and there was no significant difference between them. Significantly increased expression of PoGPT1 was confirmed in the transgenic calli compared with that in WT and CK calli (Figure 5A). Further RT-qPCR analyses confirmed significant up-regulation of PoLEC1 (Figure 5B), PoSERK (Figure 5C), and PoAGL15 (Figure 5D) in the transgenic calli overexpressing PoGPT1. The empty vector pCAMBIA1302 had no significant effect on the expression of these genes in tree peony calli.
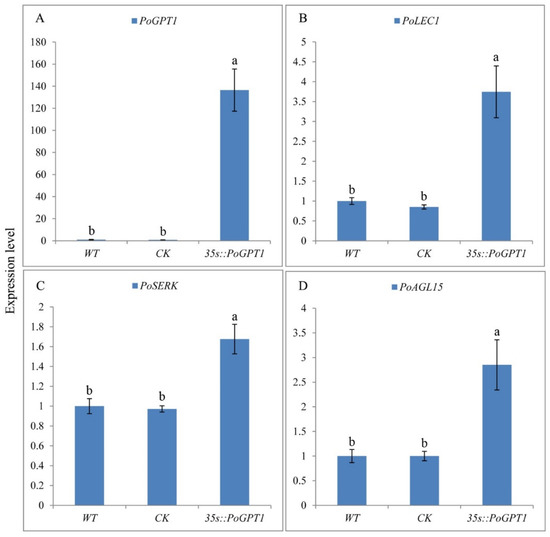
Figure 5.
RT-qPCR positive identification and expression level of embryogenesis-related genes in PoGPT1-overexpressing transgenic calli of tree peony ((A), Expression level of PoGPT1; (B), Expression level of PoLEC1; (C), Expression level of PoSERK; (D), Expression level of PoAGL15). Vertical bars indicate standard error (n = 3). Different letters indicate significant differences using the Duncan’s Multiple Range Test (p ≤ 0.05).
3.5. Anatomical Observations
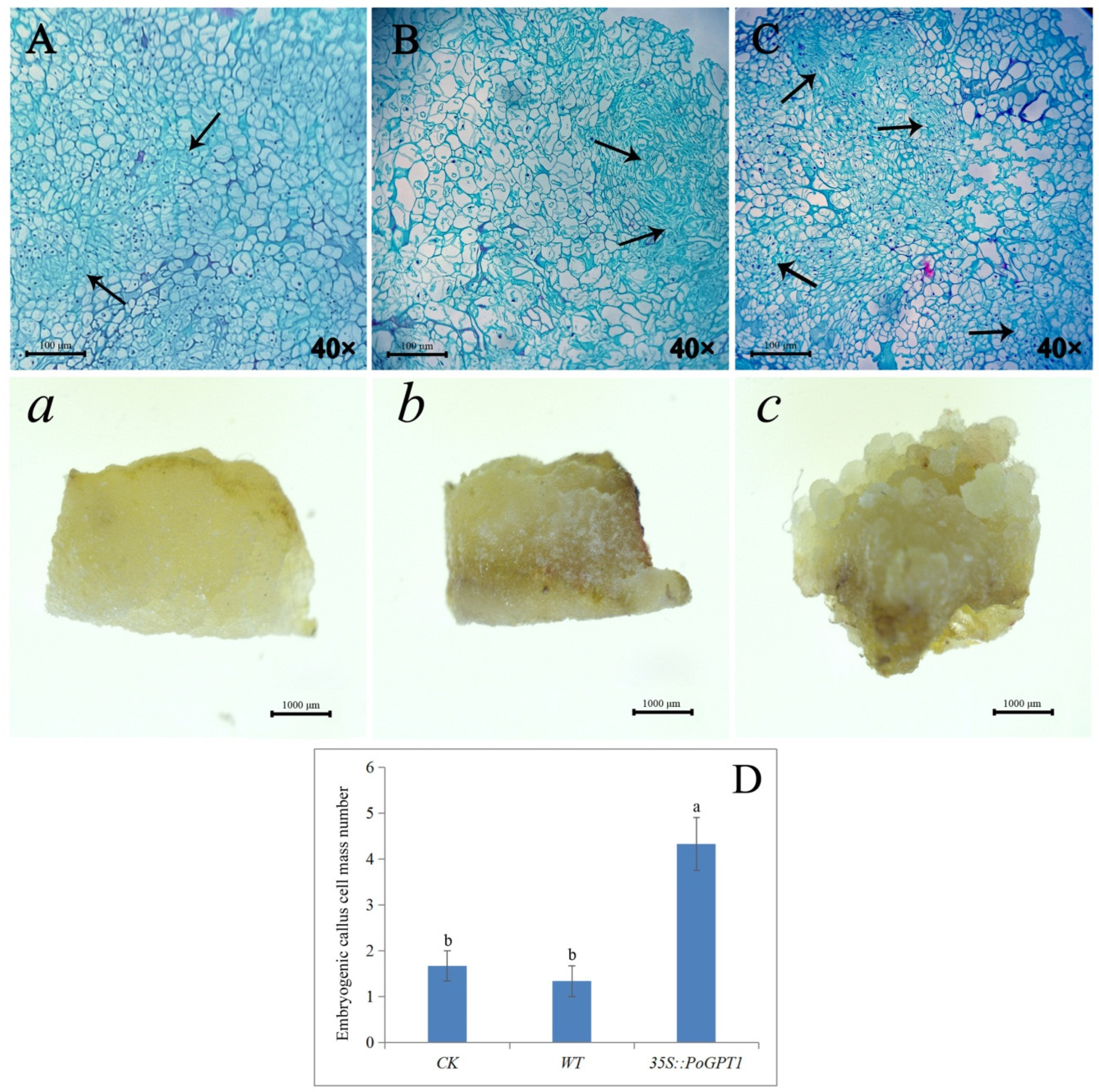
Observation of paraffin-embedded sections revealed that the transgenic calli overexpressing PoGPT1 comprised more compact cells and a large embryogenic cell mass compared with the WT and CK calli after 25 d of culture (Figure 6A–C, a–c), indicating that increased PoGPT1 expression effectively promoted the formation of embryogenic cell clusters in tree peony calli. It showed a low embryogenesis in WT, and there was no difference in the number of embryogenic cell masses observed between the WT and CK calli, which were significantly lower than that of 35s::PoGPT1 (Figure 6D).
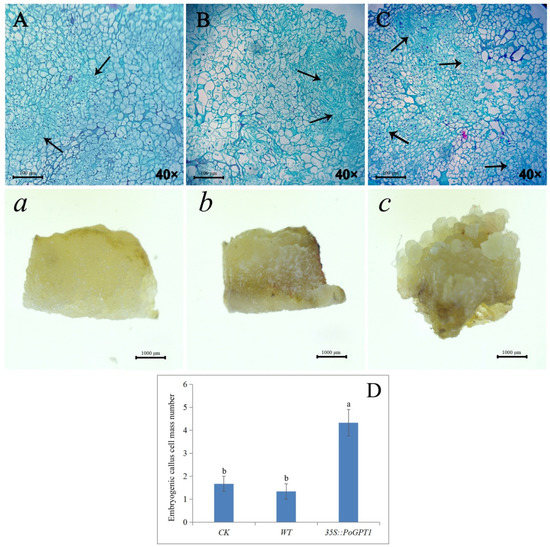
Figure 6.
Phenotypic observation, paraffin-embedded sections and of PoGPT1-overexpressing transgenic callus of tree peony ((A), (a), WT: nontransformed callus; (B), (b), CK: pCAMBIA1302; (C), (c), 35s::PoGPT1; (D): Embryogenic callus cell mass quantitative analysis. Vertical bars indicate standard error (n = 3). Different letters indicate significant differences using the Duncan’s Multiple Range Test (p ≤ 0.05). Black arrow indicates embryogenic callus mass. Scale bar: 100 μm, 1000 μm).
3.6. Endogenous Plant Hormone Contents
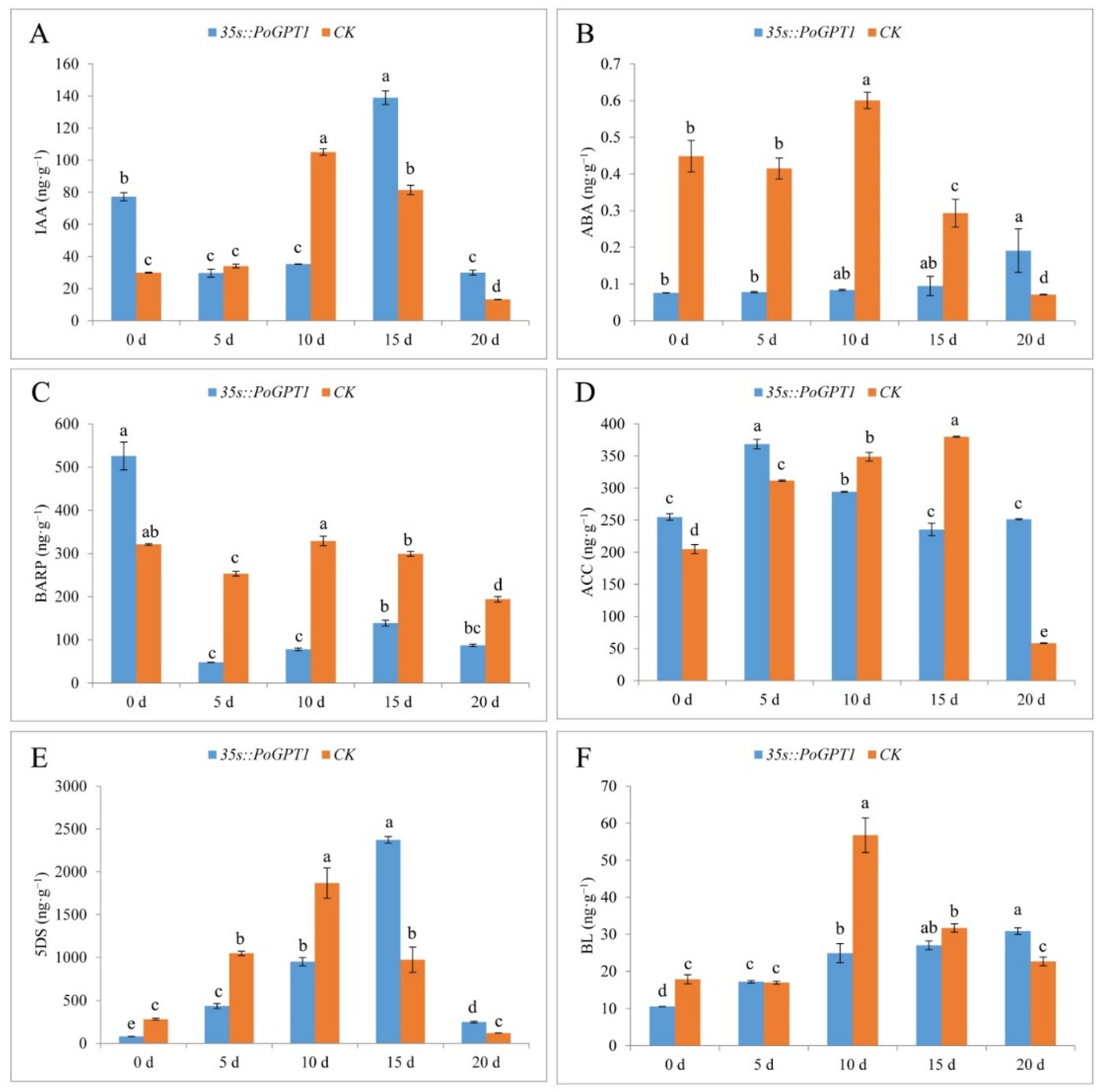
Given that no anatomical difference was observed between WT and CK calli, CK with the same transformation background as 35s::PoGPT1 was used as the control to analyze the effect of PoGPT1 overexpression on endogenous hormone contents of tree peony calli in all the following studies. Overexpression of PoGPT1 differentially affected the contents of various endogenous hormones during embryogenesis in tree peony calli (Figure 7). Overexpression of PoGPT1 significantly increased IAA content on days 0, 15 and 20, but CK only had significantly higher IAA content on day 10 (Figure 7A). The ABA content in transformed calli gradually increased during the culture period (Figure 7B). The ABA content was significantly lower than that of the CK from day 0 to day 15, then increased on day 20. In contrast, the BARP content was significantly higher in transformed calli than in the CK on day 0, then decreased sharply and remained at a lower level than that of the CK from day 5 to day 20 (Figure 7C). The ACC content was higher in transformed calli than in the CK at days 0, 5, and 20, but was lower than that of the CK on days 10 and 15 (Figure 7D). The 5DS content first increased and then decreased in the transformed calli (Figure 7E). The 5DS content was lower in the transformed calli than in the CK from day 0 to day 10, then increased sharply to peak on day 15 at a level significantly higher than that of the CK before decreasing. The BL content in the transformed calli gradually increased over time, but was lower in the transformed calli than in the CK at all time points except for day 5 and 20, when it was higher than that of the CK (Figure 7F).
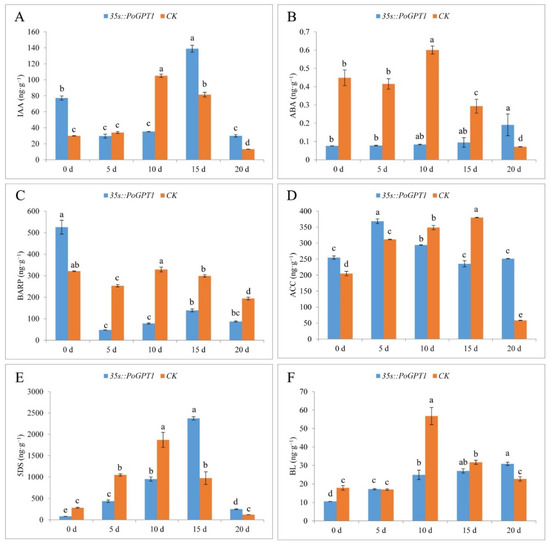
Figure 7.
Endogenous plant hormone contents in tree peony calli ((A), IAA; (B), ABA; (C), BARP; (D), ACC; (E), 5DS; (F), BL). Vertical bars indicate standard error (n = 3). Different letters indicate significant differences using the Duncan’s Multiple Range Test (p ≤ 0.05).
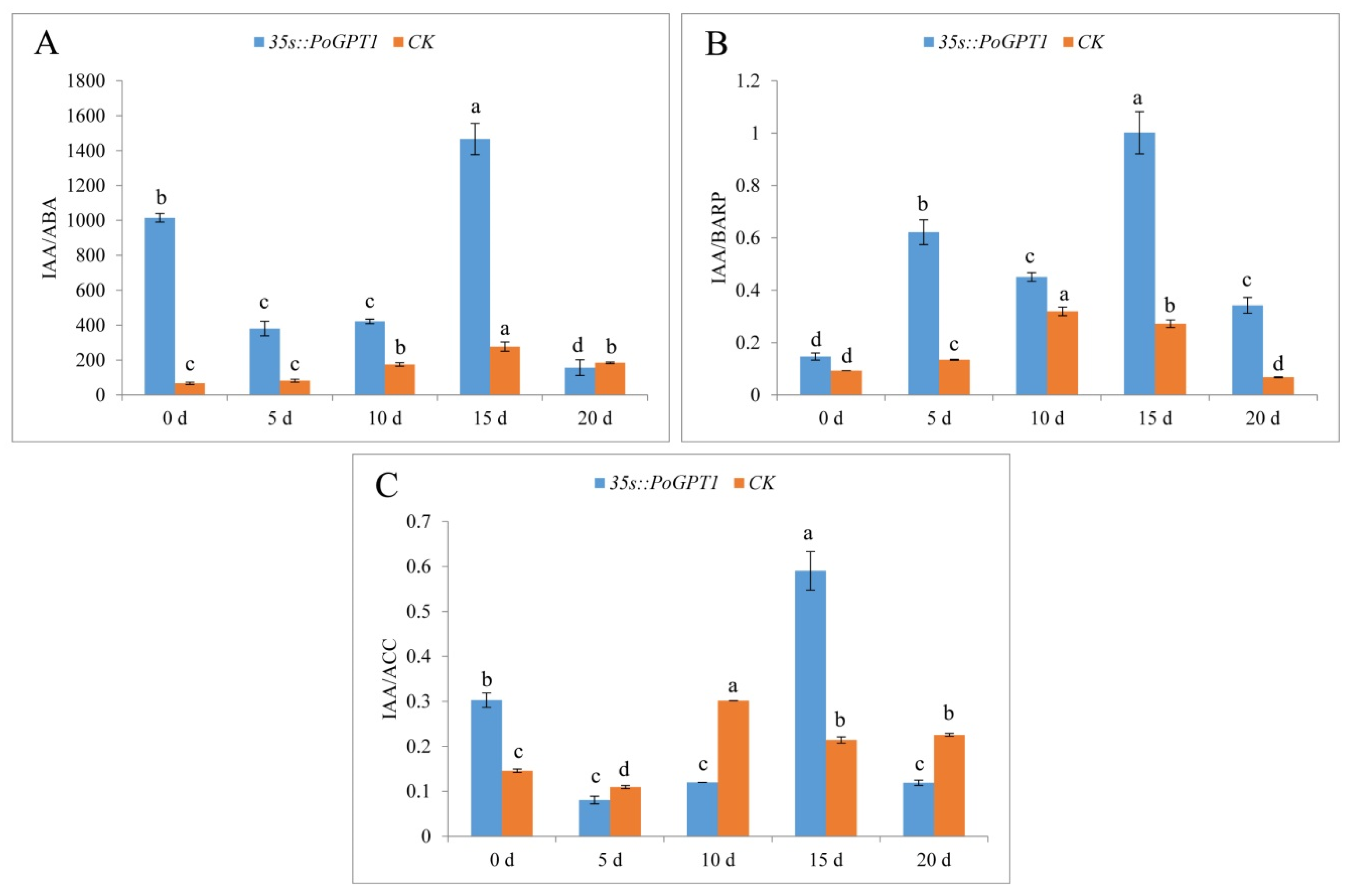
Plant embryo development was regulated by multiple hormones. Therefore, we measured and analyzed the ratio of IAA and some other hormones (Figure 8). Both the IAA/ABA and IAA/BARP ratios were higher in calli overexpressing PoGPT1 than in the CK (Figure 8A,B). The ratio values of these plant hormones peaked on day 15. In contrast, the IAA/ACC ratio was significantly higher than that in the CK only on days 0 and 15, and peaked on day 15 (Figure 8C).
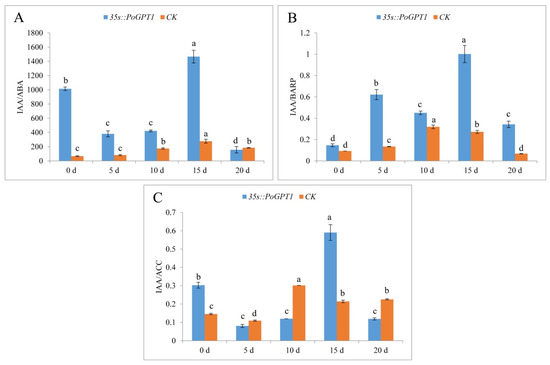
Figure 8.
Endogenous plant hormone ratios in tree peony calli ((A), IAA/ABA; (B), IAA/BARP; (C), IAA/ACC). Vertical bars indicate standard error (n = 3). Different letters indicate significant differences using the Duncan’s Multiple Range Test (p ≤ 0.05).
4. Discussion
Embryogenic callus formation and somatic embryogenesis are the process of embryo regeneration in which somatic cells develop into complete plants, without the involvement of sexual reproduction [39,40,41,42]. The photosynthetic system has not yet been established at the early stage of plant embryogenic callus formation, so the growth and differentiation of plant cells and tissues may be more reliant on heterotrophic processes [29]. Most of the glucose taken up by plant cells is phosphorylated to form glucose-6-phosphate (G6P) by hexokinase, and then G6P is translocated from the cytosol into the plastids by GPT. Inside the plastid, G6P is converted into starch and fatty acids or enters the oxidative pentose phosphate pathway [43,44,45]. Because GPT1 play an important role in both glucose metabolism and transport in heterotrophic tissues, it is a crucial participant in the regulation of plant growth and embryonic development [21,25]. Preliminary studies have observed many irregularities, such as delayed development of heart-shaped embryos, plant deformities, and poor root development, during tree peony embryogenic callus formation and mocropropagation, which might be associated with metabolite synthesis and metabolism, endogenous hormone contents, and embryogenesis-related gene regulation [6,7,8,10,11]. Given that GPT1 is an important nutrient transport factor [25], it might perform an important role in the regulation of tree peony embryogenic callus formation. In the present study, the PoGPT1 gene was successfully cloned, and bioinformatic analyses revealed that the 1179 bp ORF encodes a polypeptide of 392 amino acids. The PoGPT1 protein was predicted to be a basic non-secreted hydrophobic transmembrane protein with seven transmembrane domains. Subcellular localization analysis confirmed that the protein was mainly localized to the plastids, consistent with the results of previous studies on GPT proteins [19,26].
Previous investigations have observed that GPT1 is highly expressed in pollen, that there are significant temporal-specific differences in its expression during pollen development, and that it is important for both male and female gametogenesis [27,29]. In the present study, we detected high transcript levels of PoGPT1 in the leaves and flowers, and a gradual increase in its transcript levels during embryo development of tree peony. These results showed that PoGPT1 was highly expressed in vegetative and reproductive organs and tissues of tree peony, and that it was co-expressed with other important embryogenesis-related genes during embryonic development. These expression patterns may reflect that PoGPT1 is required to provide metabolic substrates and energy sources during the growth and development of embryos in tree peony [19,25].
Embryonic development is a complex process coordinated by multiple genes, especially SERK, LEC1, and AGL15 [31,46,47]. Previous research has shown that GPT1 is required for early embryonic development [25,26]. Therefore, we generated tree peony calli overexpressing PoGPT1 to explore its effect on key genes in embryogenesis. We observed that overexpression of PoGPT1 resulted in significant up-regulation of PoLEC1, PoSERK, and PoAGL15, all of which are involved in embryogenesis. In addition, overexpression of PoGPT1 resulted in a significant increase in the embryogenic callus cell mass, indicating that it may have the ability to enhance somatic embryogenesis in tree peony.
Embryo induction and development relies on interactions among endogenous hormones [48,49]. Endogenous auxin, cytokinin, ethylene, ABA, jasmonates, and gibberellins are involved in the acquisition of embryogenic competence by somatic cells [41,49,50]. Among these hormones, auxin is the most important endogenous hormone for embryogenic callus induction, and high IAA and low ABA contents have been shown to favor embryogenic callus formation [40,51,52]. In the present study, we observed that overexpression of PoGPT1 significantly decreased the contents of ABA and BARP in the embryogenic calli of tree peony, significantly increased the contents of IAA and 5DS on day 15, and led to a continuous increase in BL content. We also observed that overexpression of PoGPT1 resulted in significant increase in the IAA/ABA and IAA/BARP ratios, and increase in the IAA/ABA, IAA/BARP, and IAA/ACC ratios at day 15 of culture. The transcript level of PoGPT1 was positively correlated with the contents of endogenous IAA and other associated hormones, consistent with the reported role of AtGPT1 in the polar transport of auxin [26]. We determined that the transcript levels of PoLEC1, PoSERK, and PoAGL15 were associated with changes in hormone contents. These findings suggest that PoGPT1 could directly or indirectly regulate the changes in the content of related endogenous hormones, coordinate the expression of embryogenesis-related genes, and participate in regulating the process of embryogenic calli in tree peony.
5. Conclusions
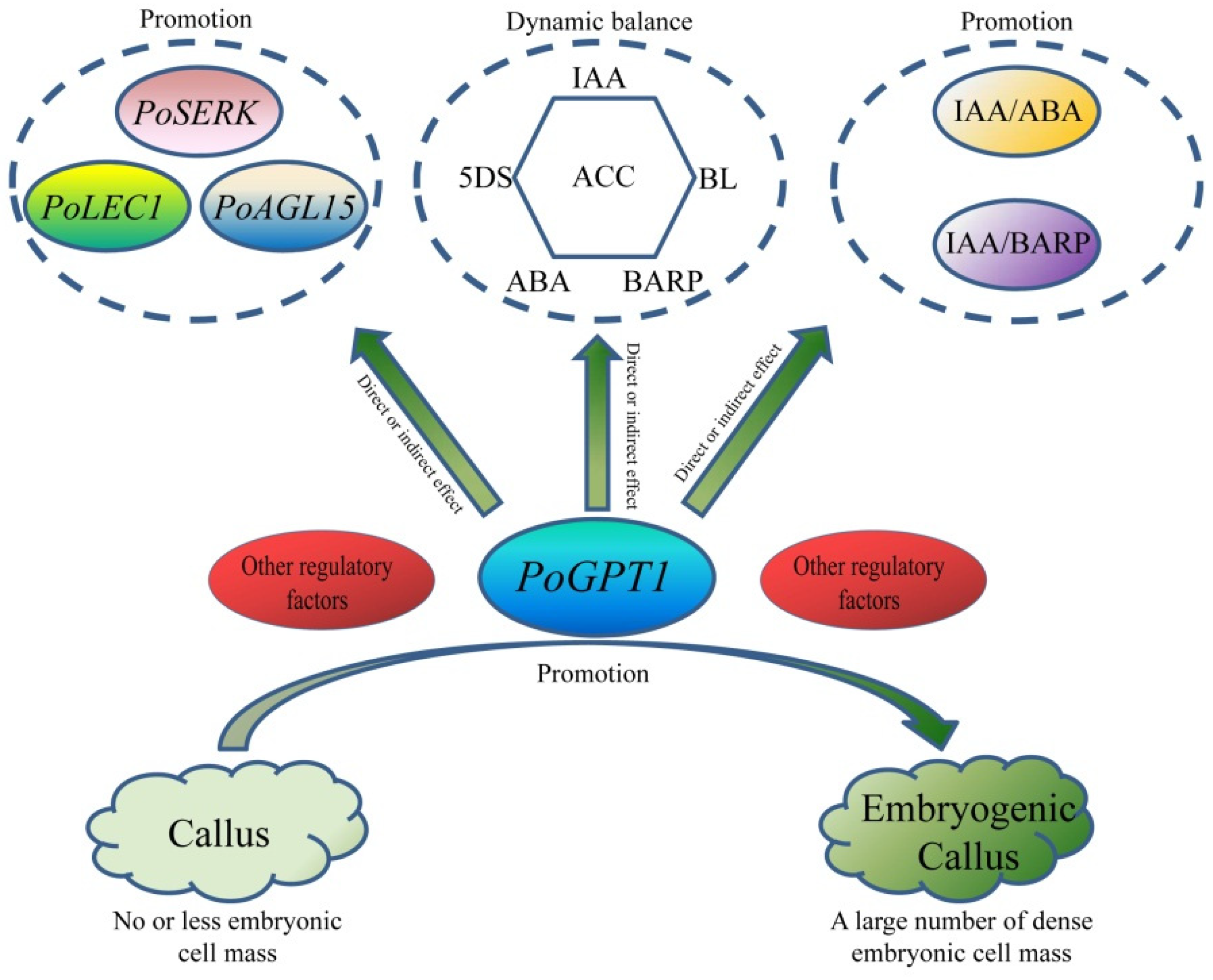
The present results show that PoGPT1 is mainly localized to the plastids, and is highly expressed in tree peony leaves. Overexpression analyses showed that it can directly or indirectly up-regulate expression of embryogenesis-related genes in tree peony callus, enhance embryogenic callus formation, and mediate dynamic changes in IAA, ABA, BARP, ACC, 5DS, and BL contents to regulate the early embryonic development of tree peony. On the basis of these results, we speculate that PoGPT1 might coordinate the expression of endogenous hormones, especially IAA, thereby stimulating the remodeling of totipotency of somatic cell and promoting the establishment of embryogenesis (Figure 9). Further research is required to determine how this process is regulated and the specific mechanism of action.
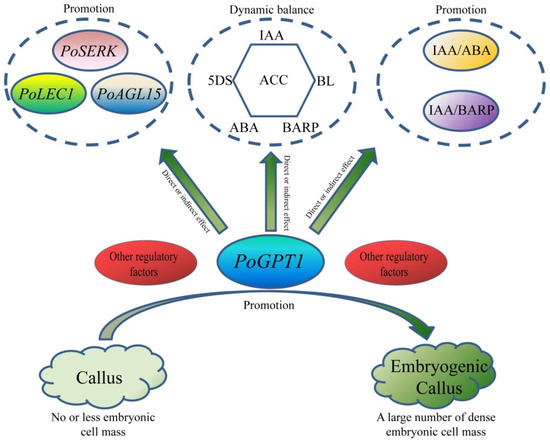
Figure 9.
Model map of PoGPT1 promotion of embryogenic callus formation in tree peony (Paeonia ostii).
Author Contributions
Study conception and design: Z.W. and S.H.; data collection: Y.S. (Yinglong Song), L.S., Y.S. (Yuke Sun) and D.H.; analysis and interpretation of results: Y.S. (Yinglong Song), W.S. and Y.S. (Yuxiao Shen); draft manuscript preparation: Y.S. (Yinglong Song) and W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key R&D Program of China (Grant No. 2020YFD1000503), the Natural Science Foundation of China (Grant No. 32001353), and the Science and Technology Program of Shanghai (Grant No. 21DZ1202000).
Data Availability Statement
Data supporting the reported results will be available and provided upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
IAA, indole-3-acetic acid; ABA, abscisic acid; BARP, 6-benzyladenosine; ACC, 1-aminocyclopropanecarboxylic acid; 5DS, 5-deoxystrigol; BL, brassinolide; GPT1, glucose-6-phosphate translocator; RT-qPCR, quantitative reverse-transcription PCR; MS, Murashige–Skoog medium; WPM, woody plant medium; 6-BA, 6-benzylaminopurine; IBA, 3-indolebutyric acid; 2,4-D, 2,4-dichlorophenoxyacetic acid; TDZ, thidiazuron; PVP, polyvinylpyrrolidone; LH, lactalbumin hydrolysate; CTAB, cetyltrimethylammonium Ammonium Bromide.
References
- Wang, Y.J.; Dong, C.L.; Xue, Z.Y.; Jin, Q.J.; Xu, Y.C. De novo transcriptome sequencing and discovery of genes related to copper tolerance in Paeonia ostii. Gene 2016, 576, 126–135. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, C.; Wang, X.Q.; Guo, J.; Dong, L. Ethylene-influenced development of tree peony cut flowers and characterization of genes involved in ethylene biosynthesis and perception. Postharvest Biol. Technol. 2017, 125, 150–160. [Google Scholar] [CrossRef]
- Zhang, H.F.; Li, X.F.; Wu, K.; Wang, M.K.; Liu, P.; Wang, X.S.; Deng, R.X. Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii. Molecules 2017, 22, 5. [Google Scholar] [CrossRef]
- Gao, J.; Xue, J.Q.; Xue, Y.Q.; Liu, R.; Ren, X.X.; Wang, S.L.; Zhang, X.X. Transcriptome sequencing and identification of key callus browning-related genes from petiole callus of tree peony (Paeonia suffruticosa cv. Kao) cultured on media with three browning inhibitors. Plant Physiol. Biochem. 2020, 149, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.S.; Zhang, W.B.; Chang, Y.T.; Ma, Y.; Deng, Y.Y.; Fan, K.K.; Zhang, X.; Jiang, Z.H.; Hu, T. A Preliminary Investigation on the Functional Validation and Interactions of PoWOX Genes in Peony (Paeonia ostii). Horticulturae 2022, 8, 266. [Google Scholar] [CrossRef]
- Shang, W.Q.; Wang, Z.; He, S.L.; Liu, Y.P.; Fu, Z.Z. Research on the relationship between phenolic acids and rooting of tree peony (Paeonia suffruticosa) plantlets in vitro. Sci. Hortic. 2017, 224, 53–60. [Google Scholar] [CrossRef]
- Du, Y.M.; Cheng, F.Y.; Zhong, Y. Induction of direct somatic embryogenesis and shoot organogenesis and histological study in tree peony (Paeonia sect. Moutan). Plant Cell Tissue Organ Cult. 2020, 141, 557–570. [Google Scholar] [CrossRef]
- Ren, X.X.; Liu, Y.; Jeong, B.R. Callus induction and browning suppression in tree peony Paeonia ostii ‘Fengdan’. Hortic. Environ. Biotechnol. 2020, 61, 591–600. [Google Scholar] [CrossRef]
- Fu, Z.Z.; Xu, M.L.; Wang, H.J.; Li, Y.M.; Wang, L.M.; Gao, J.; Zhang, J.; Yuan, X.; Zhang, H.C. Analysis of the transcriptome and related physiological indicators of tree peony (Paeonia suffruticosa Andr.) plantlets before and after rooting in vitro. Plant Cell Tissue Organ Cult. 2021, 147, 529–543. [Google Scholar] [CrossRef]
- Zhu, X.T.; Li, X.Q.; Ding, W.J.; Jin, S.H.; Wang, Y. Callus induction and plant regeneration from leaves of peony. Hortic. Environ. Biotechnol. 2018, 59, 575–582. [Google Scholar] [CrossRef]
- Wen, S.S.; Chen, L.; Cheng, F.Y.; Tian, R.N. Correction to: Micropropagation of tree peony (Paeonia sect. Moutan): A review. Plant Cell Tissue Organ Cult. 2020, 141, 15. [Google Scholar] [CrossRef]
- Zhang, R.L.; Wang, X.B.; Shao, L.M.; Li, D.Q.; Xia, Y.P.; Zhang, J.P. Tissue culture and genetic transformation system construction of Paeonia lactiflora and Paeonia suffruticosa. Plant Physiol. J. 2021, 57, 235–247. [Google Scholar] [CrossRef]
- Karami, O.; Rahimi, A.; Mak, P.; Horstman, A.; Boutilier, K.; Compier, M.; Zaal, B.; Offringa, R. An Arabidopsis AT-hook motif nuclear protein mediates somatic embryogenesis and coinciding genome duplication. Nat. Commun. 2020, 12, 2508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, M.; Li, M.Q.; Li, P.; Zhou, R.; Jiang, F.L.; Wu, Z. Changes in Endogenous Hormone Contents and Related Gene Expression during Somatic Embryogenesis of Garlic. Acta Bot. Boreali-Occident. Sin. 2021, 41, 1175–1187. [Google Scholar] [CrossRef]
- He, G.M.; Cheng, F.Y.; Li, P. Preliminary studies on culture in vitro of ovule and immature embryo of two tree peony cultivars. Acta Hortic. Sin. 2006, 1, 185. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, D.J.; He, S.L.; Mao, C.C.; Meng, X.Y.; He, D. Effects of different treatments on physiological biochemical properties of early somatic embryogenesis of Paeonia suffruticosa Andr. J. Henan Agric. Sci. 2018, 47, 105–111. [Google Scholar] [CrossRef]
- Shen, M.M.; Wang, Q.; Yu, X.N.; Teixeira da Silva, J.A. Micropropagation of herbaceous peony (Paeonia lactiflora Pall.). Sci. Hortic. 2012, 148, 30–38. [Google Scholar] [CrossRef]
- Flügge, U.-I. Phosphate translocators in plastids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 27–45. [Google Scholar] [CrossRef]
- Baune, M.C.; Lansing, H.; Fischer, K.; Meyer, T.; Charton, L.; Linka, N.; von Schaewen, A. The Arabidopsis plastidial glucose-6-phosphate transporter GPT1 is dually targeted to peroxisomes via the endoplasmic reticulum. Plant Cell 2020, 32, 1703–1726. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, B.; Fischer, K.; Hilpert, B.; Schubert, S.; Gutensohn, M.; Weber, A.; Flügge, U.-I. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: The glucose 6-phosphate/phosphate antiporter. Plant Cell 1998, 10, 105–117. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Kim, J.H.; Lee, W.J.; Jin, J.Y.; Kim, J.; Hong, S.W.; Lee, H. At Myb56 regulates anthocyanin levels via the modulation of AtGPT2 expression in response to sucrose in Arabidopsis. Mol. Cells 2018, 41, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Knappe, S.; Flügge, U.-I.; Fischer, K. Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substratebinding site. Plant Physiol. 2003, 131, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.H.; Häusler, R.E.; Fettke, J.; Herbst, K.; Niewiadomski, P.; Gierth, M.; Bell, K.; Steup, M.; Flügge, U.-I.; Schneider, A. The role of plastidial glucose-6-phosphate/phosphate translocators in vegetative tissues of Arabidopsis thaliana mutants impaired in starch biosynthesis. Plant Biol. 2010, 12, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Dyson, B.C.; Allwood, J.W.; Feil, R.; Xu, Y.; Miller, M.; Bowsher, C.G.; Goodacre, R.; Lunn, J.E.; Johnson, G.N. Acclimation of metabolism to light in Arabidopsis thaliana: The glucose 6-phosphate/phosphate translocator GPT2 directs metabolic acclimation. Plant Cell Environ. 2015, 38, 1404–1417. [Google Scholar] [CrossRef]
- Andriotis, V.M.E.; Pike, M.J.; Bunnewell, S.; Hills, M.J.; Smith, A.M. The plastidial glucose-6-phosphate/phosphate antiporter GPT1 is essential for morphogenesis in Arabidopsis embryos. Plant J. 2010, 64, 128–139. [Google Scholar] [CrossRef]
- Zhang, M.M.; Xu, X.W.; Zheng, Y.P.; Zhang, Y.; Deng, X.X.; Lou, S.; Wu, Q.P.; Xu, J.; Zhang, S.Q. Expression of a plastid-localized sugar transporter in the suspensor is critical to embryogenesis. Plant Physiol. 2020, 185, 1021–1038. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Deng, X.X.; Qu, A.; Zhang, M.M.; Tao, Y.; Yang, L.Y.; Liu, Y.D.; Xu, J.; Zhang, S.Q. Regulation of pollen lipid body biogenesis by MAP kinases and downstream WRKY transcription factors in Arabidopsis. PLoS Genet. 2018, 14, e1007880. [Google Scholar] [CrossRef]
- Qu, A.L.; Xu, Y.; Yu, X.X.; Si, Q.; Xu, X.W.; Liu, C.H.; Yang, L.L.; Zheng, Y.P.; Zhang, M.M.; Zhang, S.Q.; et al. Sporophytic control of anther development and male fertility by glucose-6-phosphate/phosphate translocator 1 (OsGPT1) in rice. J. Genet. Genom. 2021, 48, 695–705. [Google Scholar] [CrossRef]
- Niewiadomski, P.; Knappe, S.; Geimer, S.; Fischer, K.; Schulz, B.; Unte, U.S.; Rosso, M.G.; Ache, P.; Flügge, U.-I.; Schneider, A. The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell 2005, 17, 760–775. [Google Scholar] [CrossRef]
- Pérez-Pascual, D.; Jiménez-Guillen, D.; Villanueva-Alonzo, H.; Souza-Perera, R.; Godoy-Hernández, G.; Zúñiga-Aguilar, J.J. Ectopic expression of the Coffea canephora SERK1 homolog-induced differential transcription of genes involved in auxin metabolism and in the developmental control of embryogenesis. Physiol. Plant 2018, 163, 530–551. [Google Scholar] [CrossRef]
- Tvorogova, V.E.; Lutova, L.A. Genetic regulation of zygotic embryogenesis in Angiosperm plants. Russ. J. Plant Physiol. 2018, 65, 1–14. [Google Scholar] [CrossRef]
- Zheng, Q.L.; Zheng, Y.M.; Ji, H.H.; Burnie, W.; Perry, S.E. Gene regulation by the AGL15 transcription factor reveals hormone interactions in somatic embryogenesis. Plant Physiol. 2016, 172, 2374–2387. [Google Scholar] [CrossRef] [PubMed]
- Li, L.D. Observation and Transcriptome Analysis of Tree Peony Embryo Development. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2019. [Google Scholar] [CrossRef]
- Honaas, L.; Kahn, E. A practical examination of RNA isolation methods for European pear (Pyrus communis). BMC Res. Notes 2017, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Shen, P. The preliminary study on establishment of genetic transformation system of callus of Paeonia. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2014. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201801&filename=1017253701.nh (accessed on 20 June 2021).
- Kralik, P.; Ricchi, M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Forootan, A.; Sjöback, R.; Björkman, J.; Sjögreen, B.; Linz, L.; Kubista, M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol. Detect. Quantif. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Zimmerman, J.L. Somatic embryogenesis: A model for early development in higher plants. Plant Cell 1993, 5, 1411–1423. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Prinsen, E.; Ayaydin, F.; Miskolczi, P.; Potters, G.; Asard, H.; Van Onckelen, H.A.; Dudits, D.; Fehér, A. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol. 2002, 129, 1807–1819. [Google Scholar] [CrossRef]
- Rose, R.J. Somatic embryogenesis in the Medicago truncatula model: Cellular and molecular mechanisms. Front. Plant Sci. 2019, 10, 267. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis—stress-induced remodeling of plant cell fate. BBA-Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Fernie, A.R. The spatial organization of metabolism within the plant cell. Annu. Rev. Plant Biol. 2013, 64, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Renato, M.; Boronat, A.; Azcon-Bieto, J. Respiratory processes in non-photosynthetic plastids. Front. Plant Sci. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.A.; Paul, M.J.; Foyer, C.H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. BBA-Gene Regul. Mech. 2016, 1857, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Maillot, P.; Lebel, S.; Schellenbaum, P.; Jacques, A.; Walter, B. Differential regulation of SERK, LEC1-like and pathogenesis-related genes during indirect secondary somatic embryogenesis in grapevine. Plant Physiol. Biochem. 2009, 47, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Joshi, S.; Tian, R.; Junior, R.D.; Chakrabarti, M.; Perry, S.E. The MADS-domain factor AGAMOUS-Like18 promotes somatic embryogenesis. Plant Physiol. 2022, 188, 1617–1631. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Bangerth, F. Endogenous hormone concentrations and embryogenic callus development in wheat. Plant Cell Tissue Organ Cult. 2001, 67, 37–46. [Google Scholar] [CrossRef]
- Kępczyńska, E.; Orłowska, A. Profiles of endogenous ABA, bioactive GAs, IAA and their metabolites in Medicago truncatula Gaertn. non-embryogenic and embryogenic tissues during induction phase in relation to somatic embryo formation. Planta 2021, 253, 67. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodriguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.O.; Avilez, J.; De-laPena, C.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Etienne, H.; Sotta, B.; Montoro, P.; Miginiac, E.; Carrona, M.P. Relations between exogenous growth regulators and endogenous indole-3-acetic acid and abscisic acid in the expression of somatic embryogenesis in Hevea brasiliensis (Müll. Arg.). Plant Sci. 1993, 88, 91–96. [Google Scholar] [CrossRef]
- Ivanova, A.; Velcheva, M.; Denchev, P.; Atanassov, A.; van Onckelen, H.A. Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol. Plant 1994, 92, 85–89. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).