Biomass, Phenolic Compounds, Essential Oil Content, and Antioxidant Properties of Hyssop (Hyssopus officinalis L.) Grown in Hydroponics as Affected by Treatment Type and Selenium Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Design of Experiments
2.2. Plant Harvesting and Sample Preparation
2.3. Determination of Selenium in Hyssop
2.4. Determination of Phenolic Compounds
2.4.1. Extraction of Phenolic Compounds
2.4.2. Determination of Total Contents of Hydroxycinnamic Acids, Flavonoids, and Phenolic Compounds
2.4.3. High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) Analysis of Individual Phenolic Compounds
2.5. Isolation and Determination of Essential Oil
2.6. Determination of Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Se Application on Plant Yield and Se Concentration in Hyssop
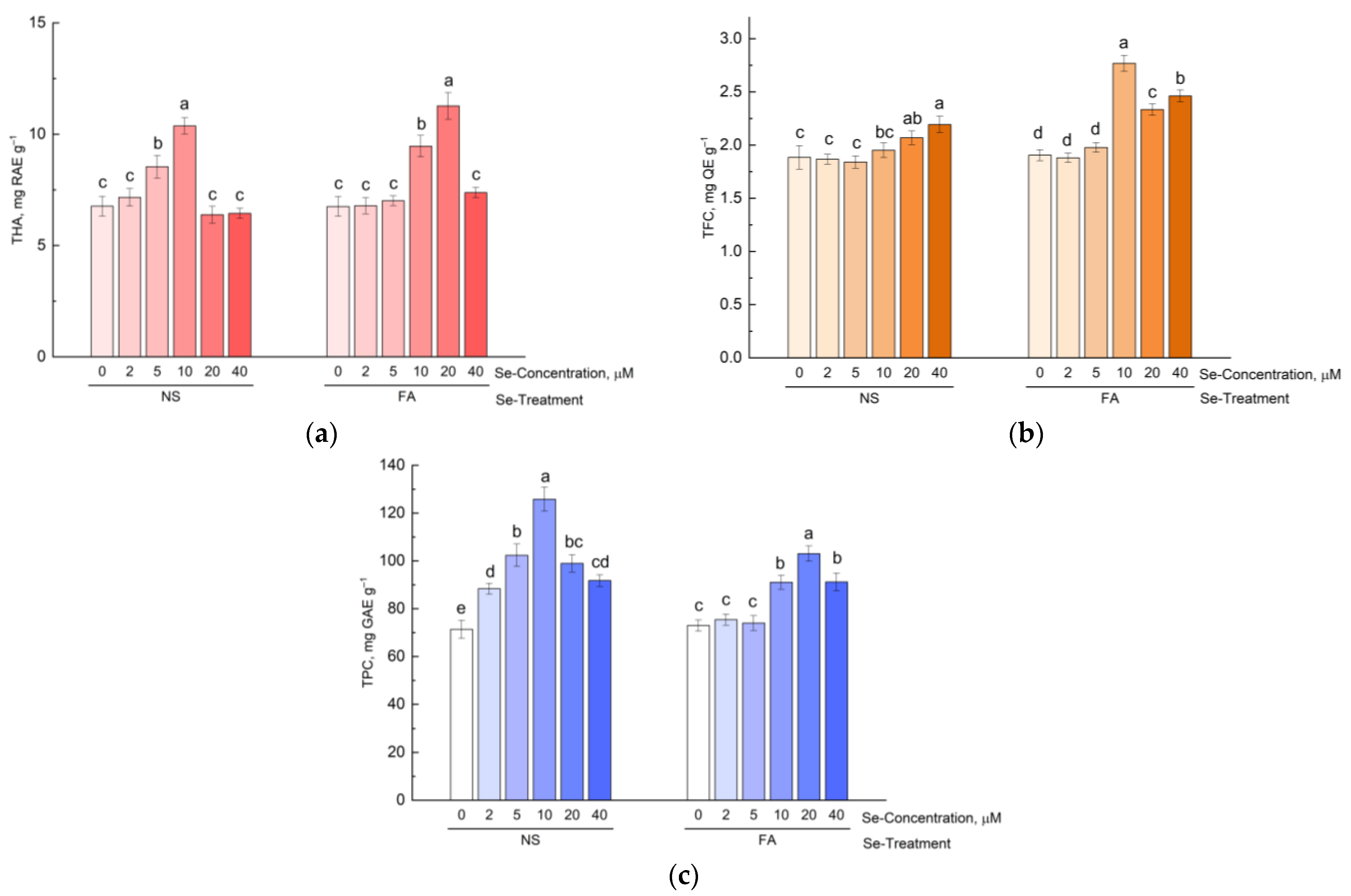
3.2. Effect of Se Application on Content of Phenolic Compounds in Hyssop
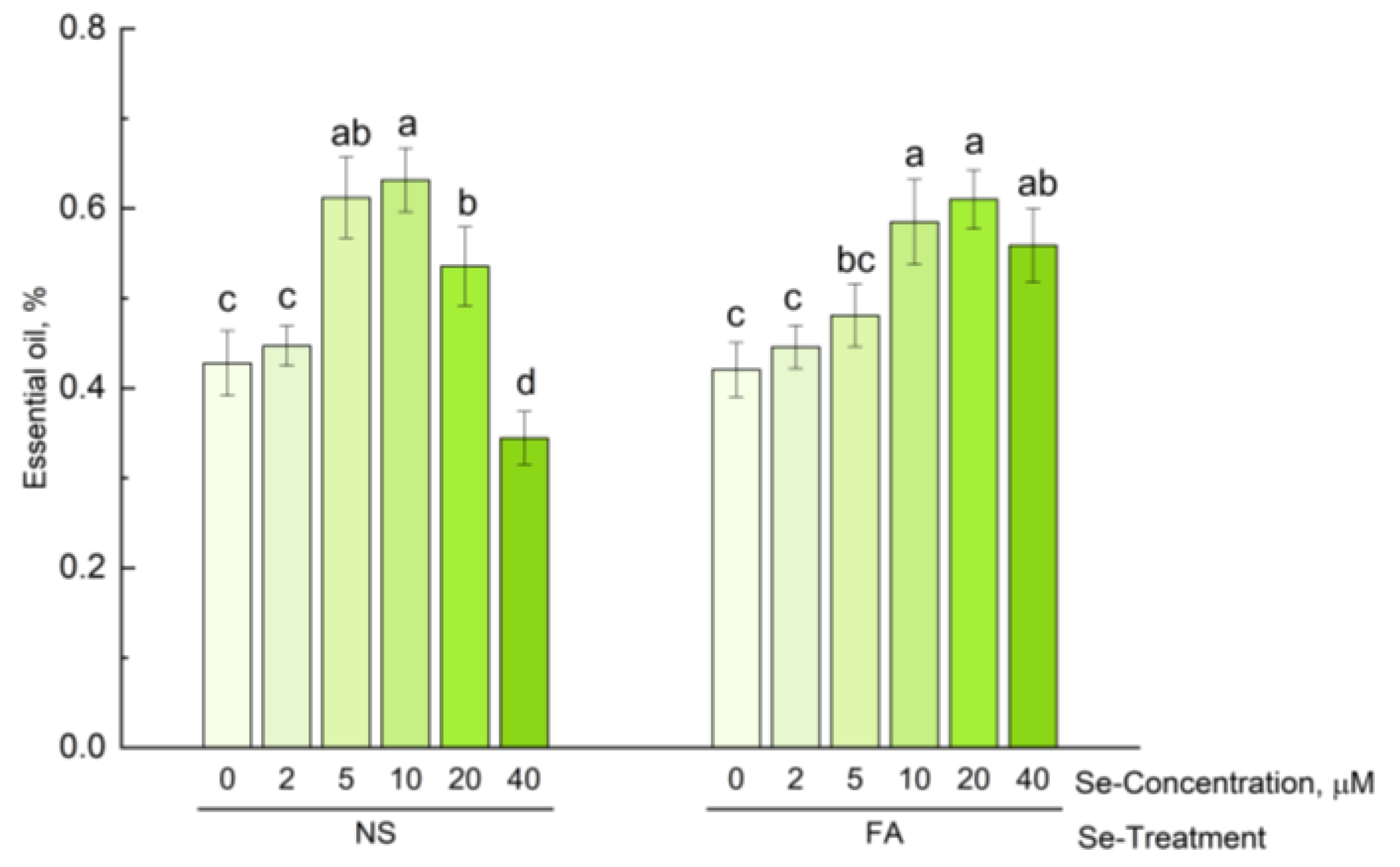
3.3. Effect of Se Application on Content of Essential oil in Hyssop
3.4. Effect of Se Application on Antioxidant Activity of Hyssop Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borrelli, F.; Pagano, E.; Formisano, C.; Piccolella, S.; Fiorentino, A.; Tenore, G.C.; Izzo, A.A.; Rigano, D.; Pacifico, S. Hyssopus officinalis subsp. aristatus: An unexploited wild-growing crop for new disclosed bioactives. Ind. Crops Prod. 2019, 140, 111594. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Echeverria Guevara, M.P.; Paganetto, G.; Grandini, A.; Maresca, I.; Menghini, L.; Di Martino, L.; Marengo, A.; Tacchini, M. Wild Italian Hyssopus officinalis subsp. aristatus (Godr.) Nyman: From Morphological and Phytochemical Evidences to Biological Activities. Plants 2021, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Khammar, A.A.; Moghaddam, M.; Asgharzade, A.; Sourestani, M.M. Nutritive Composition, Growth, Biochemical Traits, Essential Oil Content and Compositions of Salvia officinalis L. Grown in Different Nitrogen Levels in Soilless Culture. J. Soil Sci. Plant Nutr. 2021, 21, 3320–3332. [Google Scholar] [CrossRef]
- Sgherri, C.; Cecconami, S.; Pinzino, C.; Navari-Izzo, F.; Izzo, R. Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 2010, 123, 416–422. [Google Scholar] [CrossRef]
- Khan, S.; Purohit, A.; Vadsaria, N. Hydroponics: Current and future state of the art in farming. J. Plant Nutr. 2021, 44, 1515–1538. [Google Scholar] [CrossRef]
- Kalinichenko, L.V. Hyssop—A new crop for hydroponics. Gavrish 2013, 5, 40–41. Available online: http://gavrish-journal.ru/images/En/gavrish_2013_5.pdf (accessed on 5 October 2022).
- Maia, J.; Leite, R.S.; Feres, C.I.M.e.A.; Jones, K.M.P. Hydroponic Medicinal Plants: A Review of the Literature. Rev. Bionorte 2014, 3, 31–41. Available online: https://www.revistabionorte.com.br/arquivos_up/artigos/a69.pdf (accessed on 5 October 2022).
- Aghaei, K.; Ghasemi Pirbalouti, A.; Mousavi, A.; Badi, H.N.; Mehnatkesh, A. Effects of foliar spraying of l-phenylalanine and application of bio-fertilizers on growth, yield, and essential oil of hyssop [Hyssopus officinalis L. subsp. Angustifolius (Bieb.)]. Biocatal. Agric. Biotechnol. 2019, 21, 101318. [Google Scholar] [CrossRef]
- Akoumianaki-Ioannidou, A.; Kapama, D.; Mpantouna, A.; Moustakas, N.K. Cadmium Effects on Hyssop (Hyssopus officinalis L.) Morphology and Cd Uptake in Relation to Substrate Acidity/Alkalinity. Not. Bot. Horti Agrobot. 2019, 47, 1394–1399. [Google Scholar] [CrossRef]
- Jman Redzic, S. Wild Edible Plants and Their Traditional Use in the Human Nutrition in Bosnia-Herzegovina. Ecol. Food Nutr. 2006, 45, 189–232. [Google Scholar] [CrossRef]
- Kizil, S.; Güler, V.; Kirici, S.; Turk, M. Some agronomic characteristics and essential oil composition of Hyssop (Hyssopus officinalis L.) under cultivation conditions. Acta Sci. Pol. Hortorum Cultus. Ogrod. 2016, 15, 193–207. [Google Scholar]
- Venditti, A.; Bianco, A.; Frezza, C.; Conti, F.; Bini, L.M.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Damiano, S.; Lupidi, G.; et al. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis subsp. aristatus (Godr.) Nyman from central Italy. Ind. Crops Prod. 2015, 77, 353–363. [Google Scholar] [CrossRef]
- Rashidi, S.; Eikani, M.H.; Ardjmand, M. Extraction of Hyssopus officinalis L. essential oil using instant controlled pressure drop process. J. Chromatogr. A 2018, 1579, 9–19. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus Essential Oil: An Update of Its Phytochemistry, Biological Activities, and Safety Profile. Oxid Med. Cell. Longev. 2022, 2022, 8442734. [Google Scholar] [CrossRef]
- Wesolowska, A.; Jadczak, D. Comparison of the Chemical Composition of Essential Oils Isolated from Hyssop (Hyssopus officinalis L.) with Blue, Pink and White Flowers. J. Essent. Oil Bear. Plants 2018, 21, 938–949. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Hristov, A.N. Lavender and hyssop productivity, oil content, and bioactivity as a function of harvest time and drying. Ind. Crops Prod. 2012, 36, 222–228. [Google Scholar] [CrossRef]
- Özer, H.; Sökmen, M.; Güllüce, M.; Adigüzel, A.; Kilic, H.; Şahin, F.; Sökmen, A.; Bariş, Ö. In-vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of Hyssopus officinalis L. ssp. Angustifolius. Ital. J. Food Sci. 2006, 18, 73–83. [Google Scholar]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Fathiazad, F.; Mazandarani, M.; Hamedeyazdan, S. Phytochemical analysis and antioxidant activity of Hyssopus officinalis L. from Iran. Adv. Pharm. Bull. 2011, 1, 63–67. [Google Scholar] [CrossRef]
- Schiavon, M.; dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium Fertilization Alters the Chemical Composition and Antioxidant Constituents of Tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.Z.; Park, B.-J.; Kang, H.-M.; Lee, Y.-T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Wang, Y.; Ying, Z.; Bian, Z.; Zhu, W.; Liu, W.; Yang, L.; Jiang, D. How Exogenous Selenium Affects Anthocyanin Accumulation and Biosynthesis-Related Gene Expression in Purple Lettuce. Polish J. Environ. Stud. 2017, 26, 717–722. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of Selenium Enrichment and Type of Application on Yield, Functional Quality and Mineral Composition of Curly Endive Grown in a Hydroponic System. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.A.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Kannan, S. Foliar Fertilization for Sustainable Crop Production BT—Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Lichtfouse, E., Ed.; Springer: Dordrecht, Netherlands, 2010; pp. 371–402. [Google Scholar]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Fernandez, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and partitioning of selenium in basil (Ocimum basilicum L.) plants grown in hydroponics. Sci. Hortic. 2017, 225, 271–276. [Google Scholar] [CrossRef]
- Skrypnik, L.; Styran, T.; Savina, T.; Golubkina, N. Effect of Selenium Application and Growth Stage at Harvest on Hydrophilic and Lipophilic Antioxidants in Lamb’s Lettuce (Valerianella locusta L. Laterr.). Plants 2021, 10, 2733. [Google Scholar] [CrossRef]
- Tavakoli, S.; Enteshari, S.; Yousefifard, M. Investigation of the effect of selenium on growth, antioxidant capacity and secondary metabolites in Melissa officinalis. Iran. J. Plant Physiol. 2020, 10, 3125–3134. [Google Scholar]
- Kurkova, T.; Skrypnik, L.; Zalieckienėe, E. Features of plant material pre-treatment for selenium determination by atomic absorption and fluorimetric methods. Chemija 2008, 19, 40–43. [Google Scholar]
- Štefan, M.B.; Rodríguez, J.V.; Blažeković, B.; Kindl, M.; Vladimir-Knežević, S. Total hydroxycinnamic acids assay: Prevalidation and application on Lamiaceae species. Food Anal. Methods 2014, 7, 326–336. [Google Scholar] [CrossRef]
- Sevket, A.L.P.; Ercisli, S.; Jurikova, T.; Cakir, O.; Gozlekci, S. Bioactive content of rose hips of different wildly grown Rosa dumalis genotypes. Not. Bot. Horti Agrobot. 2016, 44, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Mohamadpoor, H.; Bajalan, I.; Malekpoor, F. Chemical Compositions and Antioxidant Activity of Essential Oils from Inflorescences of Two Landraces of Hyssop [Hyssopus officinalis L. subsp. angustifolius (Bieb.)] Cultivated in Southwestern, Iran. J. Essent. Oil Bear. Plants 2019, 22, 1074–1081. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta—Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H. Selenium in plants. In Progress in Botany; Luettge, U., Ed.; Springer: Heidelberg, Germany, 2015; pp. 93–107. [Google Scholar]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Habibi, G.; Ghorbanzade, P.; Abedini, M. Effects of selenium application on physiological parameters of Melissa officinalis L. plants. Iran. J. Med. Aromat. Plants 2016, 32, 698–714. [Google Scholar]
- Mezeyová, I.; Hegedusova, A.; Andrejiová, A.; Hegedus, O.; Golian, M. Phytomass and content of essential oils in Ocimum basilicum after foliar treatment with selenium. J. Int. Sci. Publ. 2016, 4, 19–27. [Google Scholar]
- Hawrylak-Nowak, B. Enhanced selenium content in sweet basil (Ocimum basilicum L.) by foliar fertilization. Veg. Crop. Res. Bull. 2008, 69, 63–72. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Deyton, D.E.; Kopsell, D.E. Selenization of Basil and Cilantro Through Foliar Applications of Selenate-selenium and Selenite-selenium. HortSci. Horts 2009, 44, 438–442. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Skrypnik, L.; Golovin, A.; Savina, T. Effect of salicylic acid on phenolic compounds, antioxidant and antihyperglycemic activity of Lamiaceae plants grown in a temperate climate. Front. Biosci. 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Groth, S.; Budke, C.; Neugart, S.; Ackermann, S.; Kappenstein, F.-S.; Daum, D.; Rohn, S. Influence of a Selenium Biofortification on Antioxidant Properties and Phenolic Compounds of Apples (Malus domestica). Antioxidants 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium Biofortification in Radish Enhances Nutritional Quality via Accumulation of Methyl-Selenocysteine and Promotion of Transcripts and Metabolites Related to Glucosinolates, Phenolics, and Amino Acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef] [Green Version]
- Põldma, P.; Moor, U.; Tõnutare, T.; Herodes, K.; Rebane, R. Selenium treatment under field conditions affects mineral nutrition, yield and antioxidant properties of bulb onion (Allium cepa L.). Acta Sci. Pol. Hortorum Cultus 2013, 12, 167–181. [Google Scholar]
- Linling, L.I.; Jie, Y.U.; Honghui, Y.; Sanxing, Z.H.A.; Kun, D.; Xian, X.; Yanyan, L.U.O.; Cheng, S.; Cheng, H. High-density kinetic analysis of the metabolomic and transcriptomic response of Ginkgo biloba flavonoids biosynthesis to selenium treatments. Not. Bot. Horti Agrobot. 2019, 47, 792–803. [Google Scholar] [CrossRef] [Green Version]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef]
- Ghasemian, S.; Masoudian, N.; Saeid Nematpour, F.; Safipour Afshar, A. Selenium nanoparticles stimulate growth, physiology, and gene expression to alleviate salt stress in Melissa officinalis. Biologia 2021, 76, 2879–2888. [Google Scholar] [CrossRef]
- Khalid, K.A. Evaluation of Salvia officinalis L. Essential Oil under Selenium Treatments. J. Essent. Oil Res. 2011, 23, 57–60. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M. Selenium and salt interactions in sage (Salvia officinalis L.): Growth and yield, chemical content, ion uptake. Ind. Crops Prod. 2021, 171, 113855. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, G.P.; Park, K.W. Status of selenium contents and effect of selenium treatment on essential oil contents in several Korean herbs. Hortic. Sci. Technol. 2001, 19, 384–388. [Google Scholar]
- Jalali, M.; Abdollahi Moghaddam, H.; Sohrabi, F. Effect of concentration and type of selenium application on the quantity and quality of essential oil of Lemon verbena (Lippia citriodora L.). J. Plant Res. (Iran. J. Biol.) 2021, 35, 496–510. [Google Scholar]
- Nazari, M.R.; Abdossi, V.; Hargalani, F.Z.; Larijani, K. Antioxidant potential and essential oil properties of Hypericum perforatum L. assessed by application of selenite and nano-selenium. Sci. Rep. 2022, 12, 6156. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh Rostam Kalaei, M.; Abdossi, V.; Danaee, E. Evaluation of foliar application of selenium and flowering stages on selected properties of Iranian Borage as a medicinal plant. Sci. Rep. 2022, 12, 12568. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, M.; Antić-Mladenović, S.; Ristić, M.; Maksimović, S.; Blagojević, S. Influence of selenium on the yield and quality of chamomile (Chamomilla recutita (L.) Rausch.). Rostl. Výroba 2000, 46, 123–126. [Google Scholar]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of Sodium Selenate Improves Dietary Mineral Contents and Antioxidant Capacity of Culinary Herb Microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef]
| Se Treatment | Se Concentration, µM | Plant Height cm | FW Shoots g plant−1 | DW Shoots g plant−1 | Se Concentration µg g−1 |
|---|---|---|---|---|---|
| Nutrient solution | 0 | 29.3 ± 2.3 a | 4.92 ± 0.09 a | 1.34 ± 0.07 a | 0.013 ± 0.002 e |
| (NS) | 2 | 31.4 ± 2.2 a | 4.99 ± 0.18 a | 1.37 ± 0.05 a | 0.992 ± 0.055 e |
| 5 | 30.9 ± 2.8 a | 5.20 ± 0.35 a | 1.39 ± 0.09 a | 7.83 ± 0.32 d | |
| 10 | 32.6 ± 3.15 a | 5.00 ±0.46 a | 1.38 ± 0.13 a | 18.23 ± 1.12 c | |
| 20 | 34.4 ± 3.35 a | 5.06 ± 0.34 a | 1.41 ± 0.10 a | 26.33 ± 2.11 b | |
| 40 | 31.0 ± 2.6 a | 4.99 ± 0.29 a | 1.38 ± 0.08 a | 38.35 ± 1.49 a | |
| Foliar application | 0 | 29.7 ± 3.1 a | 4.82 ± 0.39 a | 1.33 ± 0.11 a | 0.013 ± 0.002 e |
| (FA) | 2 | 31.9 ± 3.5 a | 5.03 ± 0.23 a | 1.37 ± 0.10 a | 0.513 ± 0.038 de |
| 5 | 33.7 ± 2.4 a | 5.06 ± 0.20 a | 1.38 ± 0.04 a | 2.48 ± 0.10 d | |
| 10 | 35.8 ± 3.4 a | 5.01 ± 0.25 a | 1.40 ± 0.09 a | 9.69 ± 0.61 c | |
| 20 | 31.3 ± 2.7 a | 5.15 ± 0.31 a | 1.39 ± 0.08 a | 19.48 ± 1.44 b | |
| 40 | 32.9 ± 3.1 a | 5.16 ± 0.18 a | 1.42 ± 0.08 a | 31.37 ± 1.92 a |
| Se Treatment | Se Concentration, µM | Content of Individual Phenolic Compounds, mg g−1 | ||||
|---|---|---|---|---|---|---|
| Rosmarinic Acid | Chlorogenic Acid | Caffeic Acid | Protocatechuic Acid | Isoquercitrin | ||
| Nutrient solution | 0 | 2.44 ± 0.10 d | 0.71 ± 0.04 d | 0.093 ±0.005 c | 0.120 ± 0.004 c | 0.036 ± 0.001 c |
| (NS) | 2 | 2.39 ± 0.15 d | 0.70 ± 0.06 d | 0.092 ± 0.005 c | 0.121 ± 0.005 c | 0.036 ± 0.002 c |
| 5 | 3.25 ± 0.14 b | 0.85 ± 0.02 c | 0.107 ± 0.007 b | 0.128 ± 0.003 c | 0.038 ± 0.001 c | |
| 10 | 3.64 ± 0.12 a | 1.16 ± 0.08 ab | 0.129 ± 0.004 a | 0.130 ± 0.004 c | 0.039 ± 0.002 c | |
| 20 | 3.18 ± 0.17 b | 1.20 ± 0.06 a | 0.130 ± 0.003 a | 0.218 ± 0.010 b | 0.043 ± 0.002 b | |
| 40 | 2.78 ± 0.18 c | 1.03 ± 0.07 b | 0.105 ± 0.008 bc | 0.272 ± 0.013 a | 0.047 ± 0.002 a | |
| Foliar application | 0 | 2.44 ± 0.12 c | 0.70 ± 0.04 c | 0.091 ± 0.005 c | 0.119 ± 0.007 d | 0.036 ± 0.001 b |
| (FA) | 2 | 2.41 ± 0.11 c | 0.71 ± 0.03 c | 0.092 ± 0.005 c | 0.124 ± 0.007 d | 0.037 ± 0.001 b |
| 5 | 2.59 ± 0.09 c | 0.74 ± 0.07 c | 0.096 ± 0.006 c | 0.122 ± 0.005 d | 0.036 ± 0.002 b | |
| 10 | 3.27 ± 0.14 b | 1.12 ± 0.04 b | 0.132 ± 0.004 b | 0.164 ± 0.013 c | 0.039 ± 0.001 ab | |
| 20 | 3.82 ± 0.11 a | 1.31 ± 0.07 a | 0.146 ± 0.010 a | 0.227 ± 0.010 b | 0.042 ± 0.004 a | |
| 40 | 3.16 ± 0.16 b | 0.99 ± 0.07 b | 0.122 ± 0.005 b | 0.251 ± 0.012 a | 0.041 ± 0.003 a | |
| Se Treatment | Se Concentration, µM | Antioxidant Activity, mg TE g−1 | ||
|---|---|---|---|---|
| DPPH | ABTS | FRAP | ||
| Nutrient solution | 0 | 17.45 ± 0.88 d | 27.64 ± 0.66 d | 13.70 ± 0.55 c |
| (NS) | 2 | 17.98 ± 0.28 d | 25.92 ± 1.39 d | 13.89 ± 0.43 c |
| 5 | 23.02 ± 0.88 c | 40.49 ± 1.47 b | 23.58 ± 1.32 a | |
| 10 | 30.35 ± 1.14 a | 51.38 ± 1.10 a | 22.25 ± 1.19 ab | |
| 20 | 25.76 ± 0.99 b | 38.19 ± 1.39 b | 20.74 ±0.93 b | |
| 40 | 24.83 ± 0.93 bc | 33.75 ± 0.97 c | 20.23 ± 1.09 b | |
| Foliar application | 0 | 17.80 ± 0.52 d | 28.68 ± 1.33 c | 13.83 ± 0.32 c |
| (FA) | 2 | 17.99 ± 0.48 d | 27.71 ± 1.09 c | 13.81 ± 0.38 c |
| 5 | 18.08 ± 0.37 d | 26.36 ± 0.87 c | 14.03 ± 0.55 c | |
| 10 | 23.79 ± 0.82 c | 44.24 ± 0.76 a | 19.02 ± 0.44 b | |
| 20 | 34.09 ± 0.81 a | 45.67 ± 0.96 a | 27.11 ± 1.35 a | |
| 40 | 29.44 ± 0.49 b | 39.87 ± 1.32 b | 20.21 ± 0.63 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrypnik, L.; Feduraev, P.; Styran, T.; Golovin, A.; Katserov, D.; Nebreeva, S.; Maslennikov, P. Biomass, Phenolic Compounds, Essential Oil Content, and Antioxidant Properties of Hyssop (Hyssopus officinalis L.) Grown in Hydroponics as Affected by Treatment Type and Selenium Concentration. Horticulturae 2022, 8, 1037. https://doi.org/10.3390/horticulturae8111037
Skrypnik L, Feduraev P, Styran T, Golovin A, Katserov D, Nebreeva S, Maslennikov P. Biomass, Phenolic Compounds, Essential Oil Content, and Antioxidant Properties of Hyssop (Hyssopus officinalis L.) Grown in Hydroponics as Affected by Treatment Type and Selenium Concentration. Horticulturae. 2022; 8(11):1037. https://doi.org/10.3390/horticulturae8111037
Chicago/Turabian StyleSkrypnik, Liubov, Pavel Feduraev, Tatiana Styran, Anton Golovin, Dmitriy Katserov, Sofia Nebreeva, and Pavel Maslennikov. 2022. "Biomass, Phenolic Compounds, Essential Oil Content, and Antioxidant Properties of Hyssop (Hyssopus officinalis L.) Grown in Hydroponics as Affected by Treatment Type and Selenium Concentration" Horticulturae 8, no. 11: 1037. https://doi.org/10.3390/horticulturae8111037
APA StyleSkrypnik, L., Feduraev, P., Styran, T., Golovin, A., Katserov, D., Nebreeva, S., & Maslennikov, P. (2022). Biomass, Phenolic Compounds, Essential Oil Content, and Antioxidant Properties of Hyssop (Hyssopus officinalis L.) Grown in Hydroponics as Affected by Treatment Type and Selenium Concentration. Horticulturae, 8(11), 1037. https://doi.org/10.3390/horticulturae8111037










