Abstract
Hyssop (Hyssopus officinalis L.) is an aromatic plant that is rich in various biologically active compounds and is widely used as a natural preservative and flavoring agent in the food and cosmetic industry and as a folk medicine against certain respiratory diseases. Despite the fact that selenium is not an essential trace element for plants, in a certain range of concentrations it can not only improve plant growth, but also increase the content of nutrients and biologically active compounds in plants. In the present study, the effect of treatment type (in a nutrient solution (NS) or by foliar application (FA)) and selenium (Se) concentration (2.0, 5.0, 10.0, 20.0, 40.0 μM) on the biomass, phenolic compounds, essential oil content, and antioxidant properties of hyssop grown in hydroponics was studied. Neither a toxic nor a beneficial effect of Se addition on the plants was observed. Selenium treatment significantly increased Se concentration in hyssop up to 38.35 μg g−1 (NS) and 34.86 μg g−1 (FA). The effect of selenium on the content of phenolic compounds, essential oil, and the antioxidant activity of hyssop depended on the applied selenium concentration. Hyssop treated with 10 μM Se (NS) and with 20 μM Se (FA) had a higher total content of hydroxycinnamic acids and higher contents of rosmarinic and chlorogenic acids, as well as a higher total content of phenolic compounds, higher content of essential oil, and higher antioxidant activity compared to other experimental plants. The results confirm the feasibility of selenium treatment of hyssop without reducing its production in order to enhance its nutritional and pharmaceutical properties.
1. Introduction
Hyssop (Hyssopus officinalis L.) is a half-shrub of the Lamiaceae family. Hyssop plants are typical xerophytes adapted to drought, and they grow in large amounts on dry, rocky, calcareous soils in Europe, southwestern and central Asia, North Africa, and northwestern India [1]. This species is also cultivated in Asia, China, central and southern Europe, and North America by both generative and vegetative propagation [2].
In soilless culture, the regulated environment of the greenhouse and mineral uptake allows for faster plant growth, improving productivity, and reducing the production cycle in several plant species of the Lamiaceae family [3,4,5]. Hydroponic systems enable individuals and commercial growers to grow fresh herbs and green vegetables throughout the year [6]. Currently, growing hyssop in hydroponics is not particularly widespread. However, previous studies have shown that hyssop can become a promising crop for growing in hydroponics, which will help expand the range of green and spicy crops with high nutritional properties in the food market [7,8].
Hyssop is widely used in folk and modern medicine as an expectorant and diuretic; has a beneficial effect in the treatment of gastrointestinal complications, inflammation of the larynx, asthma, bronchitis, and herpes; and accelerates wound healing [9]. Young shoots and leaves of hyssop have a ginger–sage aroma and a very pleasant bitter–spicy taste. They are used in salads and in spice mixtures for cooking meat, fish, and vegetable dishes [10,11,12].
The medicinal and nutritional properties of hyssop are associated with the presence of a whole complex of biologically active compounds of various chemical structures in its leaves. To date, quite a lot of articles have been published on the study of the effectiveness of essential oil extraction from hyssop and the analysis of the chemical composition of essential oil and its biological activity [13,14,15,16]. Hyssop essential oil is used as a natural preservative and flavoring agent in the food and cosmetic industry. It has been shown to have antiseptic, antifungal, antiviral (especially against HIV), antitumor, antispasmodic, and antioxidant properties [14]. The main components of hyssop essential oil are pinocamphene, isopinocamphene, β-pinene, 1,8-cineole, myrtenol, linalool, pinocarvone, methyl eugenol, and limonene [12,16,17,18]. Phenolic compounds are another important group of hyssop phytocomponents. Phenolic acids (rosmarinic, ferulic, caftaric, gentisic, caffeic, chlorogenic, and p-coumaric acids), flavonol derivatives (isoquercitrin, rutin, quercitrin, isorhamnetin), and flavones (apigenin 7-O-β-D-glucuronide, luteolin) were identified among phenolic compounds in hyssop [1,19,20]. The phenolic compounds listed above make a major contribution to the antioxidant properties of hyssop extracts.
The quantitative and qualitative composition of phytochemicals in plants can vary widely and depends both on the conditions of growth or cultivation and on varietal characteristics. Mineral nutrition of plants is one of the main factors affecting plant metabolism and the level of secondary metabolites. Although selenium is not an essential trace element for plants, there are some facts confirming the positive effect of selenium on various processes of plant life [21,22]. In addition, many studies have shown that the treatment of plants with selenium led to an increase in the content of phenolic compounds, essential oil, and antioxidant activity in basil [23,24], spinach [25], wheat microgreen [26], lettuce [27], curly endive [28], and some other plant species [29]. However, in many of the works listed above, it was shown that the effect of selenium application on the secondary metabolism of plants strongly depended on its concentration. Hyssop does not belong to the group of plants that can accumulate high concentrations of selenium. For these plants, it is very important to control the dose of selenium during treatment. High doses of selenium can have quite a toxic effect and cause the death of the plant [30].
Depending on how plants absorb nutrients, fertilization techniques can be categorized as either root fertilization or foliar fertilization. Foliar application is increasingly used to alleviate micronutrient deficiencies in plants [31,32]. However, the efficiency of foliar application depends on the rate of assimilation of microelements and their mobility in the plant organs [33]. It has been demonstrated in earlier research on the biofortification of different plants with selenium that foliar application results in a significant increase in selenium concentration in plant tissues [29]. These results suggest that plants may absorb selenium through their leaf surface. However, depending on the characteristics of the leaf surface, the efficiency of this process can vary significantly between various plant species.
As far as is known, no information about the effect of selenium application on the growth parameters and Se accumulation in hyssop was found in the literature. Due to this reason, the levels of selenium concentration (2.0, 5.0, 10.0, 20.0, and 40.0 µM) for the present study were chosen based on our previous studies and on the literature data for the other plant species of the Lamiaceae family [23,34,35,36]. In addition to the study of selenium accumulation and its impact on hyssop growth, this research was undertaken to study the content of phenolic compounds, essential oil, and antioxidant activity of hyssop as affected by types of selenium application (in a nutrient solution or foliar treatment) and selenium concentration (2.0, 5.0, 10.0, 20.0, and 40.0 µM).
2. Materials and Methods
2.1. Plant Materials and Design of Experiments
The experiment was carried out in the greenhouse of Immanuel Kant Baltic Federal University (Kaliningrad, Russia) from 6 June 2022 to 8 August 2022. Hyssop seeds (Hyssopus officinalis L. cv Lekar) were planted in perlite and watered with a half-strength nutrient solution. Four weeks after sowing, when the plants had developed four real leaves, they were transferred to 2 L pots with nutrient solution (four plants per pot). In the first week of adaptation, the plants were grown on a half-strength nutrient solution. After a week, the plants were transferred to a full-strength nutrient solution and treated with selenium. Two types of treatment were investigated in the experiment: the addition of selenium to a nutrient solution and foliar application. A solution of sodium selenate (Na2SeO4) in water was used as a source of selenium. Five selenium levels were studied (2.0, 5.0, 10.0, 20.0, and 40.0 µM). The nutrient solution was renewed and a selenium foliar treatment was carried out every week. Foliar application was performed manually in the amount of 20 mL of Se solution per pot (i.e., 5 mL per plant). Plants growing on a nutrient solution without selenium were used as a control, and the concentrations of all other nutrients were as follows: 6.3 mM N–NO3−, 0.7 mM N–NH4+, 1.0 mM P–H2PO4−, 1.0 mM S–SO42−, 2.7 mM K+, 2.45 mM Ca2+, 1.0 mM Mg2+, 50 μM Fe2+–Fe-EDTA, 30 μM B–BO3−, 4 μM Mn2+, 1 μM Cu2+, 1 μM Mo–MoO42−, 2 μM Zn2+. The following salts were used for the preparation of the nutrition solution: KNO3 (0.7 mM), NH4NO3 (0.7 mM), Ca(NO3)2·4H2O (2.45 mM), KH2PO4 (1 mM), KCl (1 mM), MgSO4·7H2O (1 mM), Fe-Na-EDTA (50 μM), H3BO3 (30 μM), MnCl2·4H2O (4 μM), CuSO4·5H2O (1 μM), Na2MoO4·2H2O (1 μM), ZnSO4·7H2O (2 μM). Control plants in the experiment with selenium foliar treatment were sprayed with an appropriate amount of distilled water. The pH and electrical conductivity values were 5.8 ± 0.1 and 2.2 ± 0.1 dSm−1, respectively. Monitoring and adjustment of these parameters were carried out every 2 days.
The following conditions were maintained in the greenhouse during the growing season: humidity 70%; day 22 °C, 16 h; night 18 °C, 8 h; photosynthetically active radiation 440 µmol (m−2 s−1). The nutrient solution was constantly aerated by using a compressor (Hailea ACO 388d), which pumped air to each pot through microtubes.
Four replications for each Se concentration (0, 2.0, 5.0, 10.0, 20.0, and 40.0 µM) and for each type of treatment (nutrient solution and foliar application) were conducted. There were a total of 48 pots in the experiment. The pots were arranged in a totally randomized design.
2.2. Plant Harvesting and Sample Preparation
Hyssop plants were harvested four weeks after the initial treatment with selenium. The height and mass of the shoots were measured. One part of the shoots of each plant was dried at 60 °C and used to determine the dry mass of plants and the content of selenium; another part of the shoots was dried at 40 °C and used for the essential oil extraction The rest of the shoots were placed in liquid nitrogen 5–10 min after collection and frozen at −80 °C. Later, the plant material was lyophilized, crushed, and used for the analysis of phenolic compounds and antioxidant activity.
2.3. Determination of Selenium in Hyssop
Selenium concentration was determined in dried hyssop shoots by atomic absorption spectrometry with the generation of HG-AAS hydrides (SpectrAA 220 FS with a prefix for generation of vapors of hydrides VGA 77, Varian) after mineralization of plant samples by autoclave decomposition under pressure [37]. Glass test tubes containing all chemical reagents without plant raw materials or a standard selenium solution were used as controls. Selenium concentration was expressed in micrograms per gram of dry weight.
2.4. Determination of Phenolic Compounds
2.4.1. Extraction of Phenolic Compounds
Using 70% ethanol as solvent, phenolic compounds were extracted from crushed and lyophilized plant material. One gram of plant material was mixed with 40 mL of 70% ethanol in a round-bottom flask. The flask was then heated in a water bath at 60 °C under reflux for one hour. Following that, the mixture was filtered into a measuring flask. The extraction procedure was repeated three times. The filtrates were combined. The end volume of extract was brought to 100 mL with 70% ethanol.
2.4.2. Determination of Total Contents of Hydroxycinnamic Acids, Flavonoids, and Phenolic Compounds
Determination of the total content of hydroxycinnamic acids was carried out according to [38]. The reaction mixture consisted of 1.0 mL of plant extract or standard (rosmarinic acid), 2 mL of 0.5 M HCl, and 2 mL of Arno’s reagent. Arno’s reagent was prepared by dissolving 10 g of NaNO2 and 10 g of Na2MoO4 in 100 mL of water. After incubation for five minutes, 2 mL of 8.5% NaOH solution was added to the reaction mixture. The total volume of the reaction mixture was 10 mL. The absorption was measured at 505 nm by UV–VIS-NIR spectrophotometer (UV-3600, Shimadzu Co., Kyoto, Japan). The total content of hydroxycinnamic acids was calculated using a calibration curve and expressed in milligrams of rosmarinic acid per gram of dry weight (mg RAE g−1).
The total content of flavonoids was measured by spectrophotometry using AlCl3 according to [39]. The reaction mixture consisted of 100 μL of extract or standard (quercetin), 4 mL of distilled water, and 300 μL of a 5% NaNO2 solution. After incubation for five minutes, 300 µL of a 10% AlCl3 solution was added to the mixture and incubated for another 6 min. Following that, 2 mL of a 1 M NaOH solution was added. The total volume of the mixture was adjusted to 10 mL with distilled water. The absorption was measured at 510 nm by UV–VIS-NIR spectrophotometer (UV-3600, Shimadzu Co., Kyoto, Japan). The total flavonoid content was calculated using a calibration curve and then expressed in milligrams of quercetin equivalent (QE) per gram of dry weight (mg QE g−1).
The total content of phenolic compounds was determined by the Folin–Ciocâlteu assay [40]. In short, 100 µL of plant extract or standard (gallic acid) was mixed with 300 µL of 0.2 M Folin–Ciocâlteu reagent in a test tube and incubated for 10 min at room temperature in the dark. Further, 6 mL of a 7.5% Na2CO3 solution was added to the mixture and incubated for 60 min at room temperature in the dark. The absorption was measured at 765 nm by UV–VIS-NIR spectrophotometer (UV-3600, Shimadzu Co., Kyoto, Japan). The total content of phenolic compounds was expressed in milligrams of gallic acid equivalent per gram of dry weight (mg GAE g−1).
2.4.3. High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) Analysis of Individual Phenolic Compounds
The extracts prepared as indicated above were concentrated using a rotary evaporator and dissolved in one milliliter of 70 ethanol prior to high-performance liquid chromatography analysis. After centrifugation at 4500× g for 15 min, the supernatant was passed through a 0.22 m syringe filter. The separation of substances was carried out on a Shimadzu LC-20 Prominence chromatograph with a Shimadzu SPD20MA (Shimadzu Co., Kyoto, Japan) diode matrix detector and a reversed-phase Phenomenex Luna column (C18 250 × 4.6 mm, 5 µm). A mixture of solvents was used as a mobile phase, namely water/formic acid 99.5/0.5 (solvent A) and acetonitrile (solvent B). Gradient mode was used during separation: 0 min—95% A, 5% B; 3 min—88% A, 12% B; 46 min—75% A, 25% B; 49.5 min—10% A, 90% B; 52 min—10% A, 90% B; 52.7 min—95% A, 5% B; 59 min—95% A, 5 % B. The injection volume was 10 µL; the flow rate was 0.85 mL min−1; the column temperature was 40 °C. The 180–900 nm wavelength range was used for detection. By comparing the chromatograms’ peak retention times and UV spectra to the corresponding values of chromatographically pure standards, individual phenolic compounds were identified. Chromatograms were processed in the LabSolutions program. The content of individual phenolic compounds was calculated using calibration curves plotted in the concentration range of 10–100 µg mL−1. The following standards were used in the study: 3,4-dihydroxybenzoic acid, caftaric acid, chicoric acid, chlorogenic acid, p-coumaric acid, ellagic acid, gallic acid, rosmarinic acid, sinapic acid, trans-caffeic acid, luteolin 7-O-glucoside, apigenin 7-O-glucoside, apigenin 7-O-glucuronide, baicalin, catechin, diosmin, isorhamnetin, kaempferol 3-O-glucoside, quercetin 3-O-rutinoside, and quercetin 3-β-D-glucoside. All standards were purchased from Sigma-Aldrich (SigmaAldrich Rus, Moscow, Russia).
2.5. Isolation and Determination of Essential Oil
Using a Clevenger apparatus, essential oil from dried hyssop shoots was extracted by hydrodistillation for 4 h [41]. The yield of essential oil was determined by the gravimetric method and calculated in percentage of plant dry weight.
2.6. Determination of Antioxidant Activity
To determine the antioxidant activity, extracts prepared as described in Section 2.4.1 were used. The ability of the extracts to react with radicals of 2,2′-azino-bis(3-ethylbenzthiazolino-6-sulfonic acid (ABTS) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) and their reducing power in the reaction with Fe(III)-2,4,6-tripyridyl-s-triazine complex (FRAP) were used to determine their antioxidant activity.
In the DPPH assay, the reaction mixture consisted of 20 µL of the extract or standard solution and 300 µL of a 0.1 mM solution of DPPH. After incubation in the dark at room temperature for 30 min, a decrease in optical density compared to the control was measured at 515 nm using a CLARIOstar microplate reader (BMG Labtech GmbH, Ortenberg, Germany) [42].
For the ABTS assay, a pre-prepared solution of ABTS radical was used. ABTS radical was generated by mixing aliquots of a 7.0 mM ABTS solution and 2.45 mM potassium persulfate solution. The solution was incubated in the dark at room temperature for 16 h. The reaction mixture consisted of 20 µL of extract or standard and 300 µL of the prepared ABTS·+ radical cation solution. The reaction mixture was incubated for 15 min at 37 °C in the dark. Following that, the optical density was measured at 734 nm using a CLARIOstar microplate reader (BMG Labtech GmbH, Ortenberg, Germany) [42].
In the FRAP assay, a freshly prepared reagent consisting of 0.3 M acetate buffer (pH 3.6), 10 mM solution of 2,4,6-tripyridyl-s-triazine in 40 mM HCl, and 20 mM solution of FeCl3 × 6H2O in the ratio 10:1:1 was used. The reaction was started by mixing 300 μL of the reagent and 20 μL of the extract or standard solution. The reaction mixture was incubated for 10 min at 37 °C in the dark. An increase in optical density compared to the control was measured at 593 nm using a CLARIOstar microplate reader (BMG Labtech GmbH, Ortenberg, Germany) [42].
Solutions of Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) of known concentration were used as a standard solution in DPPH, ABTS, and FRAP assays. The results of the analyses are expressed in milligrams of Trolox equivalents per gram of dry weight (mg TE g−1).
2.7. Statistical Analysis
The obtained data were statistically processed using OriginPro 2019b (OriginLab Corporation, Northampton, MA, USA). Statistical processing of data was carried out only by taking into account pot replications (n = 4). Before ANOVA, data were checked for normality by the Shapiro–Wilks test and the homogeneity of variance. These tests did not reveal restrictions for processing the data by ANOVA. All data were statistically analyzed by a one-way ANOVA for each type of Se treatment separately. The significance of the difference among means was determined based on the post hoc Tukey’s honest significant difference (HSD) test at a significance level of p ≤ 0.05 (n = 4). The data in tables and graphs are reported as mean ± SD (standard deviation).
3. Results and Discussion
3.1. Effect of Se Application on Plant Yield and Se Concentration in Hyssop
The study of the effect of different selenium concentrations (2–40 µM) on the height of shoots and biomass of hyssop cultivated by the hydroponic method showed that there were no significant changes in growth parameters compared to the control (Table 1). The absence of the selenium effect was found both when selenium was added to the nutrient solution and when selenium was applied by foliar treatment (Table 1). It is believed that in non-selenium-accumulator plants, the toxic effect of this trace element is associated with the inclusion of selenium in sulfur-containing amino acids and peptides instead of sulfur, which leads to a violation of their functions. In addition, selenium causes both oxidative and nitrosative stresses, thereby disrupting metabolism and damaging cellular structures [43,44]. In the present research, the treatment of plants with selenium at a concentration of 40 µM did not lead to a decrease in hyssop yield. In addition, no stimulating effect of lower concentrations on plant growth was shown. Similar results were obtained in [34], the authors of which found that the addition of selenium to the nutrient solution at concentrations of 4, 8, and 12 mg Se L−1 (i.e., 50, 100, and 150 µM) had no significant effect on the accumulation of biomass by basil plants. However, in the research on the possibility of selenium biofortification of lettuce, it was shown that a selenium concentration of 6 µM had a beneficial effect on plant growth, and a concentration of 20 µM or higher was toxic to plants and led to a decrease in biomass accumulation [45]. Thus, it can be assumed that the threshold of Se toxicity varies greatly depending on the type of plant, and to establish the limits of hyssop tolerance to this trace element, additional research is required to study the selenium effect in a wider range of concentrations.

Table 1.
Effect of type and concentration of Se application on plant height, biomass, and selenium concentration in hyssop.
The control (untreated with selenium) plants were characterized by a low concentration of selenium in shoots, which was 0.013 µg g−1 (Table 1). Hyssop treatment with selenium even at a low concentration (2 µM) increased the concentration of this trace element by 76 times (when selenium was added to the nutrient solution) and by 39 times (in the case of foliar treatment). Selenium concentration in the treated plants significantly depended on the treatment method as well. When selenium was added to the nutrient solution, hyssop contained an average of 1.5 times more selenium compared to plants treated in a foliar way (Table 1). Increased selenium levels in plants can be achieved through soil and foliar applications, soaking seeds in selenium solution prior to planting, seed dressing, or hydroponically growing plants in selenium-rich nutrient solutions [34]. An increase in Se concentration in leaf tissues and shoots when Se was added to the nutrient solution was observed in some species of plants of the Lamiaceae family, in particular in basil [23,34,46] and lemon balm [47]. Foliar treatment was also previously used to increase selenium concentration in basil [48,49,50]. It is believed that the results of spraying strongly depend on the characteristics of leaves and fruit surface, such as the presence of hairs, characteristics of the epicarp, the chemical composition of epicuticular wax, or the deposition of wax scales [51]. In addition, since the main route of selenate transfer in plants is the xylem, and the absorption of mineral elements through the leaf surface is low due to the presence of cuticles, density, and degree of stomata opening; therefore, the selenium content found during selenium absorption by roots is generally higher than that during foliar fertilization [34], which is confirmed by the results of the present research. It is worth noting, however, that foliar treatment makes it possible to maintain a sufficiently low level of selenium in plants to avoid posing a health risk.
3.2. Effect of Se Application on Content of Phenolic Compounds in Hyssop
Phenolic compounds are a group of phytochemicals found as secondary metabolites in various plants. They are of universal interest because of the wide range of biological activity they possess and their positive impact on human health [52]. Due to the high content of phenolic compounds, plants of the Lamiaceae family have always been considered a source of valuable natural substances for healthcare [53,54]. In this research, the effect of hyssop treatment with selenium on the total content of hydroxycinnamic acids, flavonoids, and phenolic compounds, as well as on the content of individual phenolic compounds, was studied.
A significantly higher content of hydroxycinnamic acids compared to the control was observed in hyssop plants grown on a nutrient medium with the addition of 5 and 10 µM of selenium, and in plants sprayed with selenium at concentrations of 10 and 20 µM (Figure 1a).
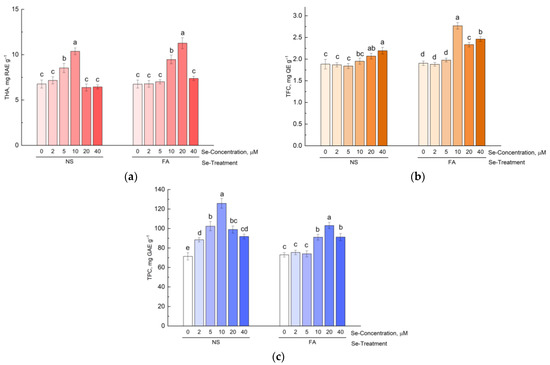
Figure 1.
Effect of type and concentration of Se application on (a) total hydroxycinnamic acid content, (b) total flavonoid content, and (c) total phenolics content in hyssop. Different letters indicate significant differences among plants due to selenium concentration according to Tukey HSD test (p ≤ 0.05, n = 4). NS, nutrient solution; FA, foliar application; THA, total hydroxycinnamic acids; TFC, total flavonoid content; TPC, total phenolic content.
The maximum total content of flavonoids was observed in hyssop plants treated with selenium at a concentration of 10 µM in a foliar way (Figure 1b). It is worth noting that, compared with hydroxycinnamic acids, stimulation of flavonoid accumulation compared with the control was observed at higher concentrations of selenium, namely when adding 20 and 40 µM of selenium to the nutrient solution and 10–40 µM by foliar treatment.
When selenium was added to the nutrient solution, the total content of phenolic compounds also increased. For all studied concentrations (2–40 µM), the total content of phenolic compounds was significantly higher in the treated plants compared to the control (Figure 1c). At the same time, the maximum total content of selenium was established in plants grown on a nutrient medium with the addition of 10 µM of selenium. Foliar treatment with selenium also had a stimulating effect on the accumulation of phenolic compounds, but only when using higher concentrations of selenium (10–40 µM) (Figure 1c).
Using high-performance liquid chromatography with a diode array detector (HPLC-DAD), the content of rosmarinic, chlorogenic, caffeic, and protocatechuic acids, as well as a flavonol derivative, isoquercitrin (Table 2), was determined in hyssop. The content of the studied individual phenolic compounds was dominated by rosmarinic acid. The rosmarinic acid content was 2.5–3.0 times higher than that of chlorogenic acid, and an order of magnitude higher than the content of other phenolic compounds. The results obtained in this research are consistent with previously obtained data for plants of the Lamiaceae family, for many species of which rosmarinic acid is the main phenolic compound [53].

Table 2.
Effect of type and concentration of Se application on the content of individual phenolic compounds in hyssop.
The effect of selenium concentration on the content of individual phenolic compounds was similar to the effect of Se concentration on the total content of individual groups of phenolic compounds. The maximum content of rosmarinic acid was found in plants grown with the addition of 10 µM of selenium to the nutrient solution and foliar treatment with selenium at a concentration of 20 µM (Table 2). Treatment with selenium at concentrations of 5–40 µM (nutrient solution) and 10–40 µM (foliar application) resulted in a significant increase in the content of chlorogenic and caffeic acids in hyssop plants compared with the control. Significant stimulation of protocatechuic acid and isoquercitrin accumulation was observed when using higher concentrations of selenium (20–40 µM), regardless of the treatment type.
To date, a sufficient amount of experimental data has been obtained confirming the stimulating effect of selenium on the accumulation of secondary metabolites of a phenolic nature. In particular, an increase in the total content of phenolic compounds in the treatment of plants with selenium is shown when processing basil [23,46], apples [55], lettuce [27], spinach [25], and others with this trace element. It should be noted that the effect of selenium on individual phenolic compounds may be different. Thus, in [56], it was shown that the content of caffeic acid, sinapic acid, kaempferol-3-O-arabinoside-7-O-rhamnoside, kaempferol-3-ramnosil glucoside, and kaempferol-3,7-diramnoside in the leaves of radish sprayed with selenium increased. On the contrary, the content of kaempferol-3-glucoside and ferulic acid was lower in comparison with the control plants. In addition, some authors note that, as a rule, the dependence of the accumulation of phenolic compounds on selenium concentration has a U-shaped dose-response, in which an increase in the content of phenolic compounds was only observed at optimal concentrations of exogenous selenium [57]. A similar relationship was revealed in the present research, in particular with regard to the effect of different selenium concentrations on the total content of hydroxycinnamic acids and phenolic compounds, as well as on the content of rosmarinic, chlorogenic, and caffeic acids (Figure 1a,c, Table 2). The maximum content of these compounds was observed at medium concentrations of selenium (10 μM and 20 μM for the selenium addition to the nutrient solution and foliar treatment, respectively). However, it should be noted that the mechanism of selenium action on the secondary metabolism of plants remains poorly understood. Several studies have shown that the selenium treatment of plants leads to an increase in the expression of certain genes involved in the metabolism of phenolic compounds (phenylalanine-ammonia-lyase, chalcone synthase, flavonol synthase, rosmarinic acid synthase, etc.), but the ways through which selenium can regulate these genes are not clear yet [58,59,60].
3.3. Effect of Se Application on Content of Essential oil in Hyssop
Selenium treatment led to an increase in the essential oil content in hyssop shoots (Figure 2). When selenium was added to the nutrient solution, a higher level of essential oil content compared to control plants was observed even at 5 µM, whereas with non-root treatment, a significant increase was only observed at 10 µM. It is worth noting, however, that when 40 µM of selenium was added to the nutrient solution, the essential oil content in hyssop decreased sharply. However, foliar treatment of plants with high concentrations of selenium (40 µM) did not lead to a decrease in the content of essential oil in hyssop. The content of essential oil in these plants did not differ significantly from that of the plants receiving foliar treatment with 10 µM and 20 µM of selenium and was significantly higher compared to that of control plants. An increase in the essential oil content in Se-treated plants was previously established for some species of the Lamiaceae family, in particular for sage (Salvia officinalis) [61,62], field mint (Mentha arvensis), balm (Melissa officinalis) [63], and basil (Ocimum basilicum) [23,63], as well as for plants of other families, for example, lemon verbena (Lippia citriodora) [64], St. John’s wort (Hypericum perforatum) [65], and Iranian borage (Echiuma moenum) [66]. Other authors, on the contrary, note that selenium treatment of plants does not significantly affect the essential oil content [48,67]. The authors explain the obtained results by the fact that when plants are treated with selenium, a decrease in the accumulation of some essential oil components and an increase in others are observed and ultimately lead to an unreliable effect of selenium on the total content of essential oil.
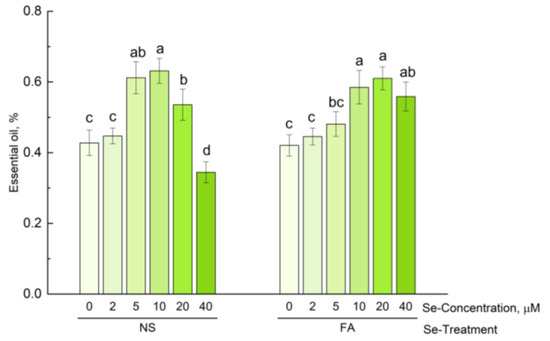
Figure 2.
Effect of type and concentration of Se application on essential oil content in hyssop. Different letters indicate significant differences among plants due to selenium concentration according to Tukey HSD test (p ≤ 0.05, n = 4). NS, nutrient solution; FA, foliar application.
The result obtained by the authors in the present study, on the reduction in the essential oil content when selenium is added to the nutrient solution at a maximum concentration (40 µM), is consistent with the results on the reduction in the essential oil content in the leaves of Iranian borage when using high concentrations of selenium [66]. Thus, the effect of selenium on the qualitative and quantitative composition of essential oil depends both on the type of plant and the concentration used.
3.4. Effect of Se Application on Antioxidant Activity of Hyssop Extracts
The antioxidant activity of plant extracts depends on the presence of a whole complex of phytocomponents in them. In this research, three methods were used to evaluate the antioxidant activity of hyssop extracts. DPPH and ABTS assays are based on the ability of antioxidants to scavenge free radicals. FRAP analysis serves as an indicator of the reducing capacity of plant extracts [23]. To compare the data obtained by all three methods, Trolox was used as a standard. The results of studies of the effect of selenium concentration when using different types of treatment on the antioxidant activity of hyssop extracts are presented in Table 3.

Table 3.
Effect of type and concentration of Se application on the antioxidant activity of hyssop extracts.
The antioxidant activity measured by all three methods was higher compared to control for hyssop grown on a nutrient solution containing 5–40 µM of selenium and sprayed with selenium at concentrations of 10–40 µM (Table 3). At the same time, maximum antioxidant activity measured by the DPPH and FRAP methods was determined for the extract of hyssop receiving foliar treatment with selenium at a concentration of 20 µM. The antioxidant activity according to the ABTS method was maximal in hyssop grown on a nutrient medium containing 10 µM of selenium. These results are consistent with experimental data on an increase in antioxidant activity when selenium is added to a nutrient solution or foliar treatment, obtained in earlier studies by some authors [23,26,46,68]. The increase in the antioxidant activity of plant extracts is probably due to an increase in the biosynthesis of phenolic or other secondary metabolites with antioxidant properties. This assumption is consistent with the data on the increase in the content of phenolic compounds during the treatment of plants with selenium, obtained in this research. Other potential explanations for the increase in antioxidant activity of plant extracts after selenium treatment include the impact of selenium on the redox metabolism of glutathione, as well as the direct antioxidant effects of selenium and its metabolites. The decrease in antioxidant activity observed at a high concentration of selenium in nutrient solution (40 µM), on the contrary, may be associated with its pro-antioxidant effect and the use of antioxidant components to protect cells from reactive oxygen species. Additional research on the oxidative stress parameters in hyssop plants treated with a high concentration of selenium is needed, nevertheless, to verify the latter hypothesis.
4. Conclusions
Selenium treatment of hyssop grown in hydroponics at concentrations of 2–40 µM did not have a significant effect on plant growth and biomass but led to a significant increase in the concentration of this trace element in shoots. The effect of selenium on the content of phenolic compounds, essential oil, and the antioxidant activity of hyssop depended on the applied selenium concentration. The optimal selenium concentrations were 10 µM when selenium was added to the nutrient medium and 20 µM when selenium was added by foliar treatment. When using these concentrations in hyssop plants, a higher total content of hydroxycinnamic acids and higher contents of rosmarinic and chlorogenic acids are found, in addition to a higher total content of phenolic compounds, higher content of essential oil, and higher antioxidant activity compared to other experimental plants. Based on the obtained data, further research on the limits of hyssop tolerance to selenium, as well as the study of potential molecular mechanisms and the role of selenium in the regulation of the secondary metabolism of plants, is promising.
Author Contributions
Conceptualization, L.S., P.F. and P.M.; methodology, L.S.; validation, A.G. and D.K.; investigation, T.S., S.N. and A.G.; resources, L.S.; data curation, P.F. and P.M.; writing—original draft preparation, L.S.; writing—review and editing, P.F.; visualization, A.G.; supervision, L.S.; project administration, L.S.; funding acquisition, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 22-14-00106, https://rscf.ru/en/project/22-14-00106/ (accessed on 5 October 2022).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Borrelli, F.; Pagano, E.; Formisano, C.; Piccolella, S.; Fiorentino, A.; Tenore, G.C.; Izzo, A.A.; Rigano, D.; Pacifico, S. Hyssopus officinalis subsp. aristatus: An unexploited wild-growing crop for new disclosed bioactives. Ind. Crops Prod. 2019, 140, 111594. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Echeverria Guevara, M.P.; Paganetto, G.; Grandini, A.; Maresca, I.; Menghini, L.; Di Martino, L.; Marengo, A.; Tacchini, M. Wild Italian Hyssopus officinalis subsp. aristatus (Godr.) Nyman: From Morphological and Phytochemical Evidences to Biological Activities. Plants 2021, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Khammar, A.A.; Moghaddam, M.; Asgharzade, A.; Sourestani, M.M. Nutritive Composition, Growth, Biochemical Traits, Essential Oil Content and Compositions of Salvia officinalis L. Grown in Different Nitrogen Levels in Soilless Culture. J. Soil Sci. Plant Nutr. 2021, 21, 3320–3332. [Google Scholar] [CrossRef]
- Sgherri, C.; Cecconami, S.; Pinzino, C.; Navari-Izzo, F.; Izzo, R. Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 2010, 123, 416–422. [Google Scholar] [CrossRef]
- Khan, S.; Purohit, A.; Vadsaria, N. Hydroponics: Current and future state of the art in farming. J. Plant Nutr. 2021, 44, 1515–1538. [Google Scholar] [CrossRef]
- Kalinichenko, L.V. Hyssop—A new crop for hydroponics. Gavrish 2013, 5, 40–41. Available online: http://gavrish-journal.ru/images/En/gavrish_2013_5.pdf (accessed on 5 October 2022).
- Maia, J.; Leite, R.S.; Feres, C.I.M.e.A.; Jones, K.M.P. Hydroponic Medicinal Plants: A Review of the Literature. Rev. Bionorte 2014, 3, 31–41. Available online: https://www.revistabionorte.com.br/arquivos_up/artigos/a69.pdf (accessed on 5 October 2022).
- Aghaei, K.; Ghasemi Pirbalouti, A.; Mousavi, A.; Badi, H.N.; Mehnatkesh, A. Effects of foliar spraying of l-phenylalanine and application of bio-fertilizers on growth, yield, and essential oil of hyssop [Hyssopus officinalis L. subsp. Angustifolius (Bieb.)]. Biocatal. Agric. Biotechnol. 2019, 21, 101318. [Google Scholar] [CrossRef]
- Akoumianaki-Ioannidou, A.; Kapama, D.; Mpantouna, A.; Moustakas, N.K. Cadmium Effects on Hyssop (Hyssopus officinalis L.) Morphology and Cd Uptake in Relation to Substrate Acidity/Alkalinity. Not. Bot. Horti Agrobot. 2019, 47, 1394–1399. [Google Scholar] [CrossRef]
- Jman Redzic, S. Wild Edible Plants and Their Traditional Use in the Human Nutrition in Bosnia-Herzegovina. Ecol. Food Nutr. 2006, 45, 189–232. [Google Scholar] [CrossRef]
- Kizil, S.; Güler, V.; Kirici, S.; Turk, M. Some agronomic characteristics and essential oil composition of Hyssop (Hyssopus officinalis L.) under cultivation conditions. Acta Sci. Pol. Hortorum Cultus. Ogrod. 2016, 15, 193–207. [Google Scholar]
- Venditti, A.; Bianco, A.; Frezza, C.; Conti, F.; Bini, L.M.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Damiano, S.; Lupidi, G.; et al. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis subsp. aristatus (Godr.) Nyman from central Italy. Ind. Crops Prod. 2015, 77, 353–363. [Google Scholar] [CrossRef]
- Rashidi, S.; Eikani, M.H.; Ardjmand, M. Extraction of Hyssopus officinalis L. essential oil using instant controlled pressure drop process. J. Chromatogr. A 2018, 1579, 9–19. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus Essential Oil: An Update of Its Phytochemistry, Biological Activities, and Safety Profile. Oxid Med. Cell. Longev. 2022, 2022, 8442734. [Google Scholar] [CrossRef]
- Wesolowska, A.; Jadczak, D. Comparison of the Chemical Composition of Essential Oils Isolated from Hyssop (Hyssopus officinalis L.) with Blue, Pink and White Flowers. J. Essent. Oil Bear. Plants 2018, 21, 938–949. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Hristov, A.N. Lavender and hyssop productivity, oil content, and bioactivity as a function of harvest time and drying. Ind. Crops Prod. 2012, 36, 222–228. [Google Scholar] [CrossRef]
- Özer, H.; Sökmen, M.; Güllüce, M.; Adigüzel, A.; Kilic, H.; Şahin, F.; Sökmen, A.; Bariş, Ö. In-vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of Hyssopus officinalis L. ssp. Angustifolius. Ital. J. Food Sci. 2006, 18, 73–83. [Google Scholar]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Fathiazad, F.; Mazandarani, M.; Hamedeyazdan, S. Phytochemical analysis and antioxidant activity of Hyssopus officinalis L. from Iran. Adv. Pharm. Bull. 2011, 1, 63–67. [Google Scholar] [CrossRef]
- Schiavon, M.; dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium Fertilization Alters the Chemical Composition and Antioxidant Constituents of Tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.-J.; Kang, H.-M.; Lee, Y.-T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Wang, Y.; Ying, Z.; Bian, Z.; Zhu, W.; Liu, W.; Yang, L.; Jiang, D. How Exogenous Selenium Affects Anthocyanin Accumulation and Biosynthesis-Related Gene Expression in Purple Lettuce. Polish J. Environ. Stud. 2017, 26, 717–722. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of Selenium Enrichment and Type of Application on Yield, Functional Quality and Mineral Composition of Curly Endive Grown in a Hydroponic System. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.A.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Kannan, S. Foliar Fertilization for Sustainable Crop Production BT—Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Lichtfouse, E., Ed.; Springer: Dordrecht, Netherlands, 2010; pp. 371–402. [Google Scholar]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Fernandez, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and partitioning of selenium in basil (Ocimum basilicum L.) plants grown in hydroponics. Sci. Hortic. 2017, 225, 271–276. [Google Scholar] [CrossRef]
- Skrypnik, L.; Styran, T.; Savina, T.; Golubkina, N. Effect of Selenium Application and Growth Stage at Harvest on Hydrophilic and Lipophilic Antioxidants in Lamb’s Lettuce (Valerianella locusta L. Laterr.). Plants 2021, 10, 2733. [Google Scholar] [CrossRef]
- Tavakoli, S.; Enteshari, S.; Yousefifard, M. Investigation of the effect of selenium on growth, antioxidant capacity and secondary metabolites in Melissa officinalis. Iran. J. Plant Physiol. 2020, 10, 3125–3134. [Google Scholar]
- Kurkova, T.; Skrypnik, L.; Zalieckienėe, E. Features of plant material pre-treatment for selenium determination by atomic absorption and fluorimetric methods. Chemija 2008, 19, 40–43. [Google Scholar]
- Štefan, M.B.; Rodríguez, J.V.; Blažeković, B.; Kindl, M.; Vladimir-Knežević, S. Total hydroxycinnamic acids assay: Prevalidation and application on Lamiaceae species. Food Anal. Methods 2014, 7, 326–336. [Google Scholar] [CrossRef]
- Sevket, A.L.P.; Ercisli, S.; Jurikova, T.; Cakir, O.; Gozlekci, S. Bioactive content of rose hips of different wildly grown Rosa dumalis genotypes. Not. Bot. Horti Agrobot. 2016, 44, 472–476. [Google Scholar] [CrossRef]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Mohamadpoor, H.; Bajalan, I.; Malekpoor, F. Chemical Compositions and Antioxidant Activity of Essential Oils from Inflorescences of Two Landraces of Hyssop [Hyssopus officinalis L. subsp. angustifolius (Bieb.)] Cultivated in Southwestern, Iran. J. Essent. Oil Bear. Plants 2019, 22, 1074–1081. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta—Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H. Selenium in plants. In Progress in Botany; Luettge, U., Ed.; Springer: Heidelberg, Germany, 2015; pp. 93–107. [Google Scholar]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Habibi, G.; Ghorbanzade, P.; Abedini, M. Effects of selenium application on physiological parameters of Melissa officinalis L. plants. Iran. J. Med. Aromat. Plants 2016, 32, 698–714. [Google Scholar]
- Mezeyová, I.; Hegedusova, A.; Andrejiová, A.; Hegedus, O.; Golian, M. Phytomass and content of essential oils in Ocimum basilicum after foliar treatment with selenium. J. Int. Sci. Publ. 2016, 4, 19–27. [Google Scholar]
- Hawrylak-Nowak, B. Enhanced selenium content in sweet basil (Ocimum basilicum L.) by foliar fertilization. Veg. Crop. Res. Bull. 2008, 69, 63–72. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Deyton, D.E.; Kopsell, D.E. Selenization of Basil and Cilantro Through Foliar Applications of Selenate-selenium and Selenite-selenium. HortSci. Horts 2009, 44, 438–442. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Skrypnik, L.; Golovin, A.; Savina, T. Effect of salicylic acid on phenolic compounds, antioxidant and antihyperglycemic activity of Lamiaceae plants grown in a temperate climate. Front. Biosci. 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Groth, S.; Budke, C.; Neugart, S.; Ackermann, S.; Kappenstein, F.-S.; Daum, D.; Rohn, S. Influence of a Selenium Biofortification on Antioxidant Properties and Phenolic Compounds of Apples (Malus domestica). Antioxidants 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium Biofortification in Radish Enhances Nutritional Quality via Accumulation of Methyl-Selenocysteine and Promotion of Transcripts and Metabolites Related to Glucosinolates, Phenolics, and Amino Acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Põldma, P.; Moor, U.; Tõnutare, T.; Herodes, K.; Rebane, R. Selenium treatment under field conditions affects mineral nutrition, yield and antioxidant properties of bulb onion (Allium cepa L.). Acta Sci. Pol. Hortorum Cultus 2013, 12, 167–181. [Google Scholar]
- Linling, L.I.; Jie, Y.U.; Honghui, Y.; Sanxing, Z.H.A.; Kun, D.; Xian, X.; Yanyan, L.U.O.; Cheng, S.; Cheng, H. High-density kinetic analysis of the metabolomic and transcriptomic response of Ginkgo biloba flavonoids biosynthesis to selenium treatments. Not. Bot. Horti Agrobot. 2019, 47, 792–803. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef]
- Ghasemian, S.; Masoudian, N.; Saeid Nematpour, F.; Safipour Afshar, A. Selenium nanoparticles stimulate growth, physiology, and gene expression to alleviate salt stress in Melissa officinalis. Biologia 2021, 76, 2879–2888. [Google Scholar] [CrossRef]
- Khalid, K.A. Evaluation of Salvia officinalis L. Essential Oil under Selenium Treatments. J. Essent. Oil Res. 2011, 23, 57–60. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M. Selenium and salt interactions in sage (Salvia officinalis L.): Growth and yield, chemical content, ion uptake. Ind. Crops Prod. 2021, 171, 113855. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, G.P.; Park, K.W. Status of selenium contents and effect of selenium treatment on essential oil contents in several Korean herbs. Hortic. Sci. Technol. 2001, 19, 384–388. [Google Scholar]
- Jalali, M.; Abdollahi Moghaddam, H.; Sohrabi, F. Effect of concentration and type of selenium application on the quantity and quality of essential oil of Lemon verbena (Lippia citriodora L.). J. Plant Res. (Iran. J. Biol.) 2021, 35, 496–510. [Google Scholar]
- Nazari, M.R.; Abdossi, V.; Hargalani, F.Z.; Larijani, K. Antioxidant potential and essential oil properties of Hypericum perforatum L. assessed by application of selenite and nano-selenium. Sci. Rep. 2022, 12, 6156. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh Rostam Kalaei, M.; Abdossi, V.; Danaee, E. Evaluation of foliar application of selenium and flowering stages on selected properties of Iranian Borage as a medicinal plant. Sci. Rep. 2022, 12, 12568. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, M.; Antić-Mladenović, S.; Ristić, M.; Maksimović, S.; Blagojević, S. Influence of selenium on the yield and quality of chamomile (Chamomilla recutita (L.) Rausch.). Rostl. Výroba 2000, 46, 123–126. [Google Scholar]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of Sodium Selenate Improves Dietary Mineral Contents and Antioxidant Capacity of Culinary Herb Microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).