Estimation of Seed Longevity in Onion Seed Lots by a Vigor Test of Radicle Emergence Test in Artificial Ageing Conditions
Abstract
1. Introduction
2. Materials and Methods
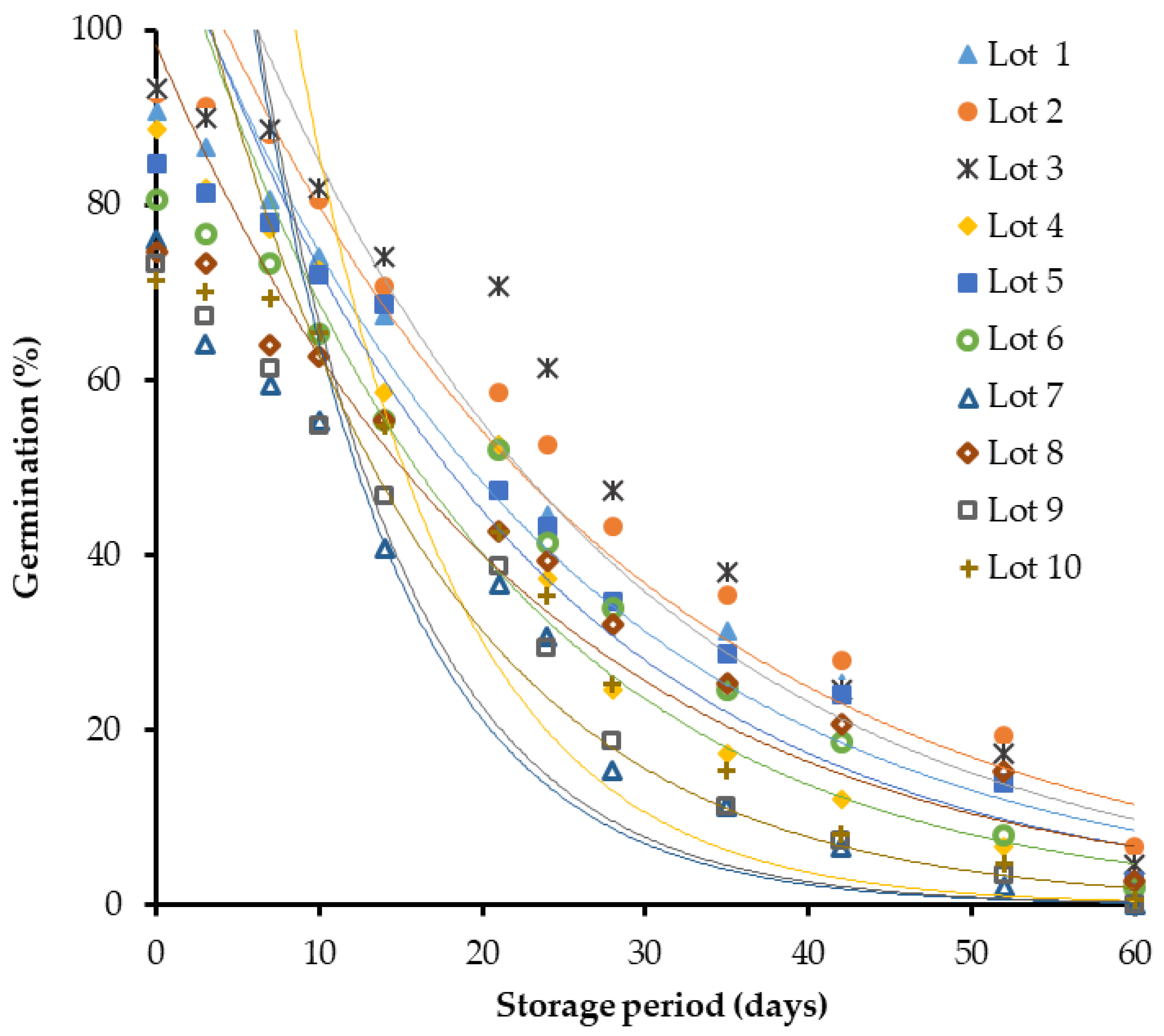
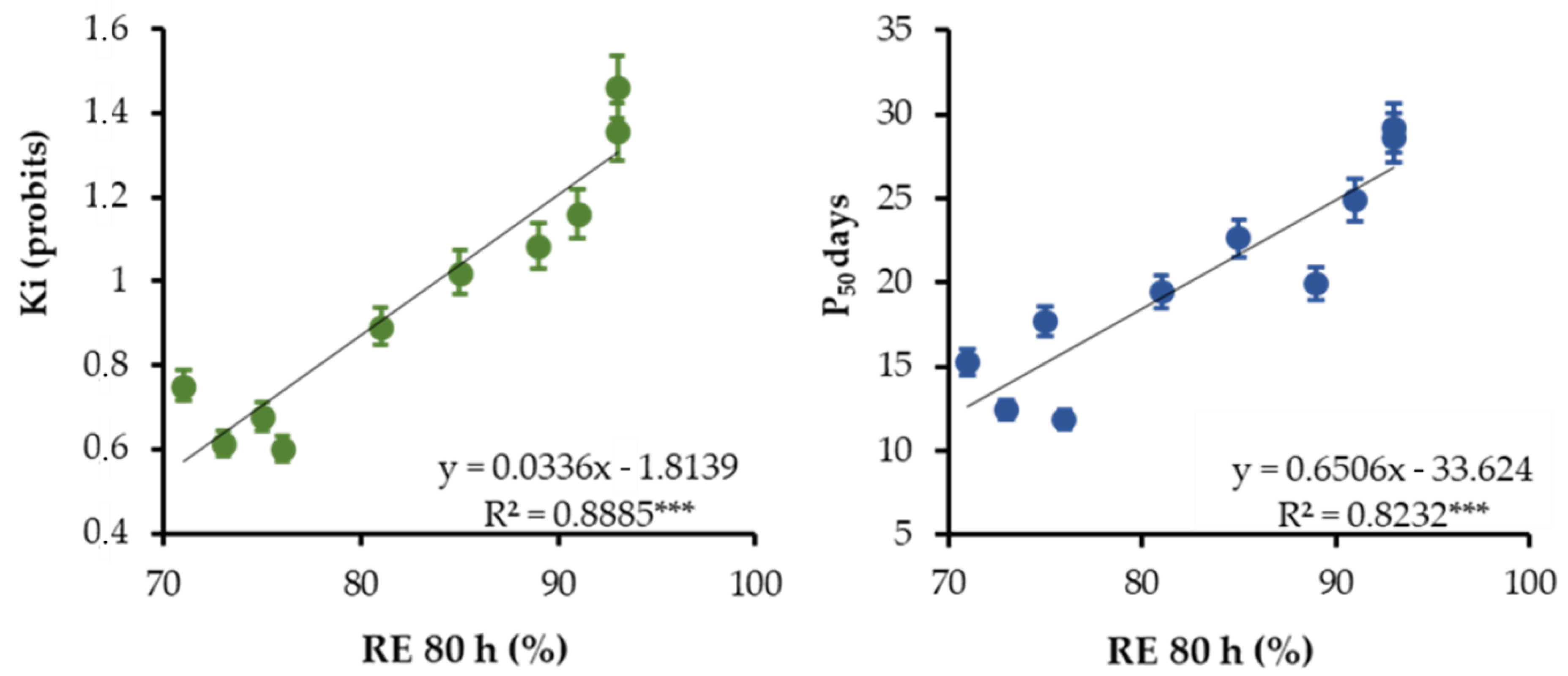
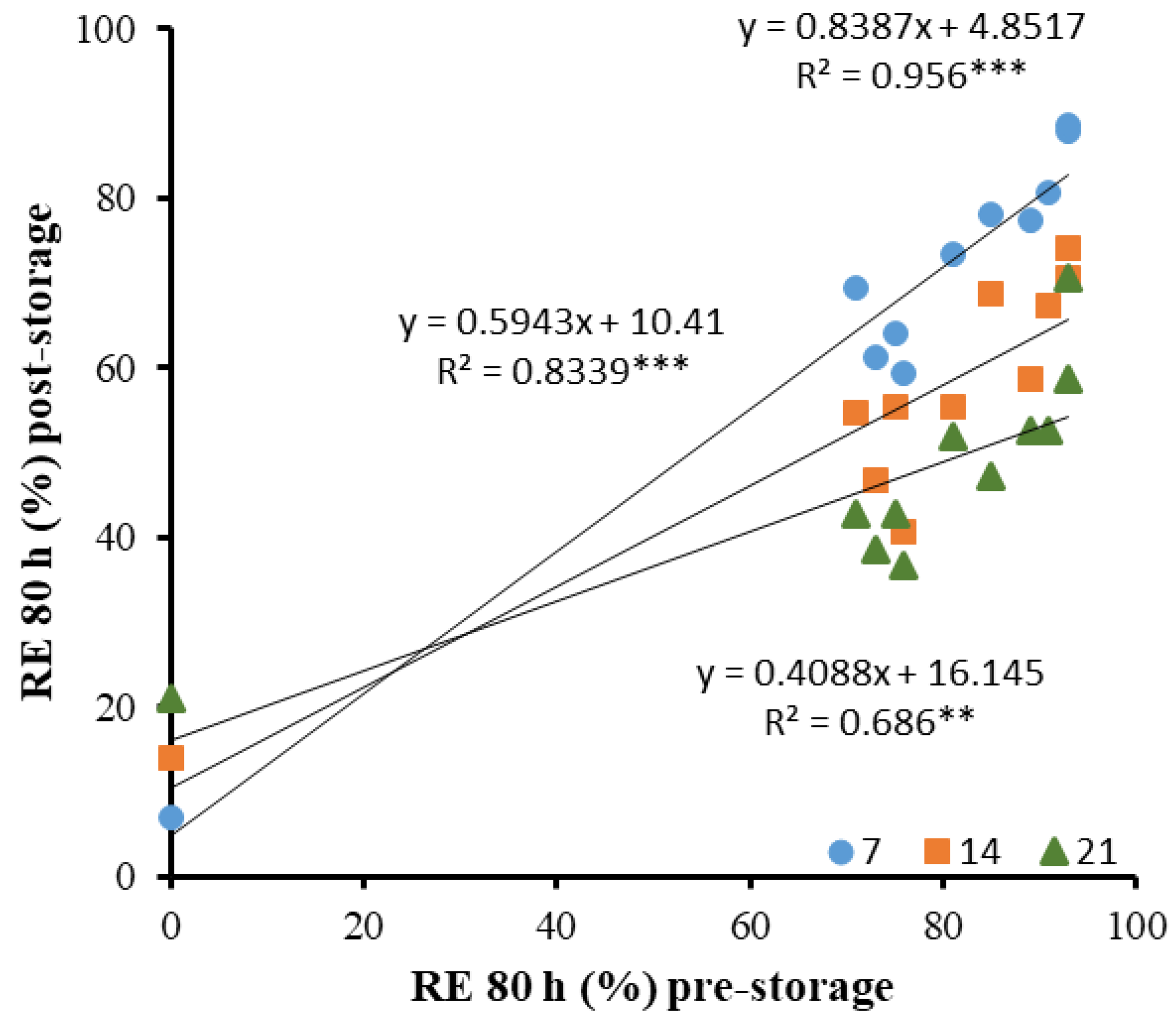
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, A.A.; Matthews, S. Seed ageing/repair hypothesis leads to new testing methods. Seed Technol. 2012, 34, 15–25. [Google Scholar]
- Finch-Savage, W.E.; Basse, G.W. Seed vigor and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.A. Seed Vigor and Its Assessment. In Handbook of Seed Science and Technology; Basra, J.S., Ed.; The Haworth Press: Binghamton, NY, USA, 2006; pp. 603–648. [Google Scholar]
- Eksi, C.; Demir, I. The use of a shortened controlled deterioration vigor test in predicting field emergence and longevity of onion seed lots. Seed Sci. Technol. 2011, 39, 190–198. [Google Scholar] [CrossRef]
- Demir, I.; Celikkol, T.; Sarıkamıs, G.; Eksi, C. Vigor tests to estimate seedling emergence potential and longevity in Viola seed lots. HortScience 2011, 46, 402–405. [Google Scholar] [CrossRef]
- Demir, I.; Kenanoglu, B.B.; Ozden, E. Seed vigor tests to estimate seedling emergence in cress (Lepidium sativum L.) seed lots. Not. Bot. Horti Agrobot. 2019, 47, 881–886. [Google Scholar] [CrossRef]
- Demir, I.; Ozden, E.; Gökdas, Z.; Njie, S.E.; Aydin, M. Radicle emergence test predicts normal germination percentages of onion seed lots with different cultivars and genotypes. Mustafa Kemal Univ. J. Agric. Sci. 2020, 25, 434–442. [Google Scholar] [CrossRef]
- Powell, A.A. Seed vigor in the 21st century. Seed Sci. Technol. 2022, 50, 45–73. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing, 2021 ed.; International Seed Testing Association: Bassersdorf, Switzerland, 2021. [Google Scholar]
- Matthews, S.; Khajeh Hosseini, M. Length of the lag period of germination and metabolic repair explain vigor differences in seed lots of maize (Zea mays). Seed Sci. Technol. 2007, 35, 200–212. [Google Scholar] [CrossRef]
- Matthews, S.; Powell, A.A. Towards automated single counts of radicle emergence to predict seed and seedling vigor. Seed Test. Int. 2011, 142, 44–48. [Google Scholar]
- Guloksuz, T.; Demir, I. Vigor tests in Geranium, Salvia, Gazania, and Impatiens seed lots to estimate seedling emergence potential in modules. Propag. Ornam. Plants 2012, 12, 133–138. [Google Scholar]
- Ermis, S.; Karslıoglu, M.; Ozden, E.; Demir, I. Use of a single radicle emergence count as a vigor test in prediction of seedling emergence potential of leek seed lots. Seed Sci. Technol. 2015, 43, 308–312. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Wang, Y.R.; Powell, A.A. Frequent individual counts of radicle emergence and mean just germination time predict seed vigor of Avena sativa and Elymus nutans. Seed Sci. Technol. 2016, 44, 189–198. [Google Scholar] [CrossRef]
- Mavi, K.; Powell, A.A.; Matthews, S. Rate of radicle emergence and leakage of electrolytes provide quick predictions of percentage normal seedlings in standard germination tests of radish (Raphanus sativus). Seed Sci. Technol. 2016, 44, 393–409. [Google Scholar] [CrossRef]
- Ilbi, H.; Powell, A.A.; Alan, O. Single radicle emergence counts for predicting vigor of marigold (Tagetes spp.) seed lots. Seed Sci. Technol. 2020, 48, 381–389. [Google Scholar] [CrossRef]
- Ozden, E.; Ozdamar, C.; Demir, I. Radicle emergence test estimates predictions of percentage normal seedlings in standard germination tests of aubergine (Solanum melongena L.) seed lots. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 177–182. [Google Scholar] [CrossRef]
- Ermis, S.; Oktem, G.; Mavi, K.; Hay, F.R.; Demir, I. The radicle emergence test and storage longevity of cucurbit rootstock seed lots. Seed Sci. Technol. 2022, 50, 1–10. [Google Scholar] [CrossRef]
- Thirusendura, S.D.; Saraswathy, S. Seed viability, seed deterioration and seed quality improvements in stored onion seeds: A review. J. Hortic. Sci. Biotechnol. 2017, 93, 1–7. [Google Scholar] [CrossRef]
- Demir, I.; Ozcoban, M. Dry and Ultra-dry storage of pepper, aubergine, winter squash, summer squash, bean, cowpea, okra, onion, leek, cabbage, radish, lettuce and melon seeds at −20 °C and 20 °C over five years. Seed Sci. Technol. 2007, 35, 165–175. [Google Scholar] [CrossRef]
- Roberts, E.H.; Ellis, R.H. Water and seed survival. Ann. Bot. 1989, 63, 289–297. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Hay, F.R.; Valdez, R.; Lee, J.S.; Cruz, P.C.S. Seed longevity phenotyping: Recommendations on research methodology. J. Exp. Bot. 2019, 70, 425–434. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. Restor. Ecol. 2020, 28, 249–255. [Google Scholar] [CrossRef]
- Powell, A.A.; Matthews, S. Prediction of the storage potential of onion seed under commercial storage conditions. Seed Sci. Technol. 1984, 12, 641–647. [Google Scholar]
- Matthews, S.; Khajeh-Hosseini, M. Mean germination time as an indicator of emergence performance in soil of seed lots of maize (Zea mays). Seed Sci. Technol. 2006, 34, 339–347. [Google Scholar] [CrossRef]
- Demir, I.; Ermis, S.; Mavi, K.; Matthews, S. Mean germination time of pepper seed lots (Capsicum annuum L.) predicts size and uniformity of seedlings in germination tests and transplant modules. Seed Sci. Technol. 2008, 36, 21–30. [Google Scholar] [CrossRef]
- Matthews, S.; Wagner, M.-H.; Ratzenboeck, A.; Khajeh Hosseini, M.; Casarini, E.; El Khadem, R.; Yakhlifi, M.; Powell, A.A. Early counts of radicle emergence during germination as a repeatable and reproducible vigor test for maize. Seed Test. Int. 2011, 141, 39–45. [Google Scholar]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex-situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Surki, A.A.; de Vos, R.C.H.; Kodde, J. Seed storage at elevated partial pressure of oxygen, a fast method for analyzing seed ageing under dry conditions. Ann. Bot. 2012, 110, 1149–1159. [Google Scholar] [CrossRef]
- Whitehouse, K.J.; Hay, F.R.; Ellis, R.H. Improvement in rice seed storage longevity from high-temperature drying is a consistent positive function of harvest moisture content above a critical value. Seed Sci. Res. 2018, 28, 332–339. [Google Scholar] [CrossRef]
- Lee, J.S.; Velasco-Punzalan, M.; Pacleb, M.; Valdez, R.; Kretzschmar, T.; McNally, K.L.; Ismail, A.M.; Cruz, P.C.S.; Sackville Hamilton, N.R.; Hay, F.R. Variation in seed longevity among diverse Indica rice varieties. Ann. Bot. 2019, 124, 447–460. [Google Scholar] [CrossRef]
- Shinohara, T.; Ducournau, S.; Matthews, S.; Wagner, M.-H.; Powell, A.A. Early counts of radicle emergence, counted manually and by image analysis, can reveal differences in the production of normal seedlings and the vigor of seed lots of cauliflower. Seed Sci. Technol. 2021, 49, 219–235. [Google Scholar] [CrossRef]
- Tekrony, D.M.; Nelson, C.; Egli, D.B.; White, G.M. Predicting soybean seed germination during warehouse storage. Seed Sci. Technol. 1993, 21, 127–137. [Google Scholar]
- Matthews, S.; El-Khadem, R.; Casarini, E.; Khajeh Hosseini, M.; Nasehzadeh, M.; Wagner, M.-H. Rate of physiological germination compared with the cold test and accelerated ageing as a repeatable vigor test for maize (Zea mays). Seed Sci. Technol. 2010, 38, 379–389. [Google Scholar] [CrossRef]
- Demilly, D.; Ducournau, S.; Wagner, M.H.; Dürr, D. Digital imaging of seed germination. In Plant Image Analysis: Fundamentals and Applications; Gupta, S.D., Ibaraki, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 147–162. [Google Scholar] [CrossRef]
- Matthews, S.; Wagner, M.H.; Kerr, L.; McLaren, G.; Powell, A.A. Automated determination of germination time courses by image capture and early counts of radicle emergence lead to a new vigor test for winter oilseed rape (Brassica napus). Seed Sci. Technol. 2012, 40, 413–424. [Google Scholar] [CrossRef]
- Wagner, M.H.; Ducournau, S.; Luciani, A.; Léchappé, J. From knowledge-based research towards accurate and rapid testing of seed quality in winter rape. Seed Sci. Res. 2012, 22, 80–85. [Google Scholar] [CrossRef]
- Hay, F.R.; Davies, R.M.; Dickie, J.B.; Merritt, D.J.; Wolkis, D.M. More on seed longevity phenotyping. Seed Sci. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Merritt, D.J.; Martyn, A.J.; Ainsley, P.; Young, R.E.; Seed, L.U.; Thorpe, M.; Hay, F.R.; Commander, L.E.; Shackelford, N.; Offord, C.A.; et al. A continental-scale study of seed lifespan in experimental storage examining seed, plant, and environmental traits associated with longevity. Biodivers. Conserv. 2014, 23, 1081–1104. [Google Scholar] [CrossRef]


| Seed Lot | TG (%) | NG (%) | Seed mc (%) | RE 80 h |
|---|---|---|---|---|
| 1 | 97 a | 89 ab | 11.9 | 91 |
| 2 | 97 a | 85 bc | 11.9 | 93 |
| 3 | 99 a | 94 a | 11.8 | 93 |
| 4 | 98 a | 88 bc | 11.8 | 89 |
| 5 | 99 a | 89 ab | 11.7 | 85 |
| 6 | 92 b | 82 cd | 11.7 | 81 |
| 7 | 95 ab | 77 de | 12.1 | 76 |
| 8 | 97 a | 78 de | 12.1 | 75 |
| 9 | 85 c | 74 e | 11.9 | 73 |
| 10 | 91 b | 78 de | 12.1 | 71 |
| Seed Lots | Ki (SEM) (Probits) | P50 (SEM) (Days) | Slope, σ (SEM) (Probits Day−1) |
|---|---|---|---|
| 1 | 1.16 (0.05) | 24.9 (0.16) | −0.0466 (0.0008) |
| 2 | 1.36 (0.03) | 28.6 (0.46) | −0.0474 (0.0004) |
| 3 | 1.46 (0.06) | 29.2 (0.40) | −0.0501 (0.0006) |
| 4 | 1.08 (0.03) | 19.9 (0.20) | −0.0539 (0.0013) |
| 5 | 1.02 (0.04) | 22.6 (0.07) | −0.0451 (0.0020) |
| 6 | 0.89 (0.04) | 19.5 (0.14) | −0.0458 (0.0017) |
| 7 | 0.60 (0.03) | 11.8 (0.07) | −0.0511 (0.0016) |
| 8 | 0.68 (0.02) | 17.7 (0.11) | −0.0383 (0.0004) |
| 9 | 0.61 (0.01) | 12.4 (0.41) | −0.0494 (0.0012) |
| 10 | 0.75 (0.03) | 15.2 (0.32) | −0.0493 (0.0015) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir, I.; Kuzucu, C.O.; Ermis, S.; Memis, N.; Kadioglu, N. Estimation of Seed Longevity in Onion Seed Lots by a Vigor Test of Radicle Emergence Test in Artificial Ageing Conditions. Horticulturae 2022, 8, 1063. https://doi.org/10.3390/horticulturae8111063
Demir I, Kuzucu CO, Ermis S, Memis N, Kadioglu N. Estimation of Seed Longevity in Onion Seed Lots by a Vigor Test of Radicle Emergence Test in Artificial Ageing Conditions. Horticulturae. 2022; 8(11):1063. https://doi.org/10.3390/horticulturae8111063
Chicago/Turabian StyleDemir, Ibrahim, Canan Oztokat Kuzucu, Sıtkı Ermis, Nurcan Memis, and Neslihan Kadioglu. 2022. "Estimation of Seed Longevity in Onion Seed Lots by a Vigor Test of Radicle Emergence Test in Artificial Ageing Conditions" Horticulturae 8, no. 11: 1063. https://doi.org/10.3390/horticulturae8111063
APA StyleDemir, I., Kuzucu, C. O., Ermis, S., Memis, N., & Kadioglu, N. (2022). Estimation of Seed Longevity in Onion Seed Lots by a Vigor Test of Radicle Emergence Test in Artificial Ageing Conditions. Horticulturae, 8(11), 1063. https://doi.org/10.3390/horticulturae8111063






