Alleviating Effects of Linalool Fumigation on Botrytis cinerea Infections in Postharvest Tomato Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials
2.2. Pathogen Preparation
2.3. In Vitro Effects of Linalool on B. cinerea Growth
2.4. In Vivo Effects of Linalool on B. cinerea Growth
2.5. Determination of the Disease Index of Tomato Fruits Gray Mold
2.6. Determination of Antioxidant Enzymes, Phenylalanine Ammonia-Lyase (PAL) and Polyphenol Oxidase Enzyme (PPO) Activity
2.7. Determination of AsA and MDA Content
2.8. Determination of Cell Wall-Related Enzymes
2.9. Preparation of Paraffin Slices of Tomato Pericarp
2.10. Statistical Analysis
3. Results
3.1. Inhibition of B. cinerea by Linalool
3.1.1. Linalool Was Selected as the Substance for B. cinerea Inhibition
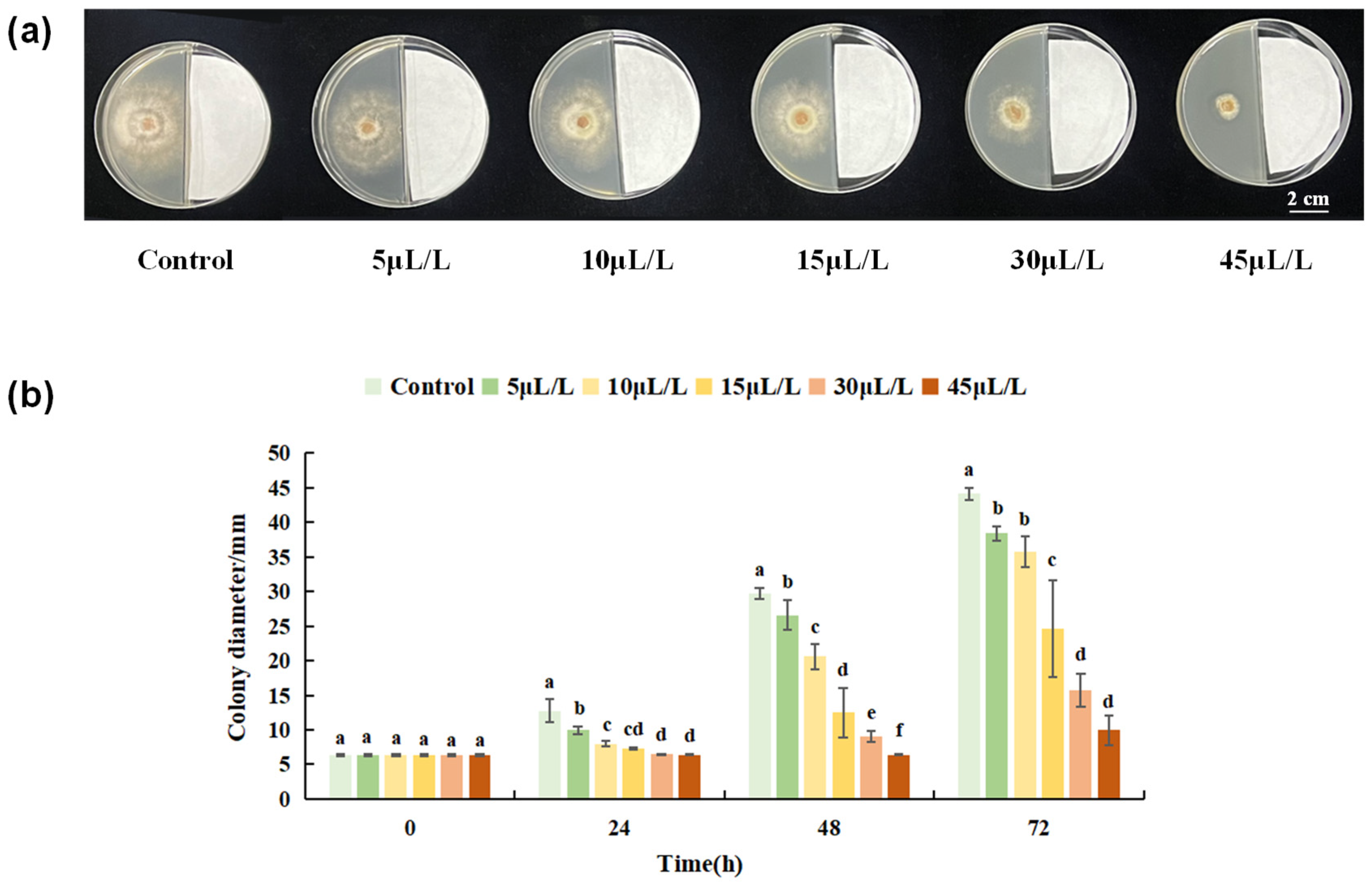
3.1.2. Inhibitory Effect of Linalool on B. cinerea Colonies
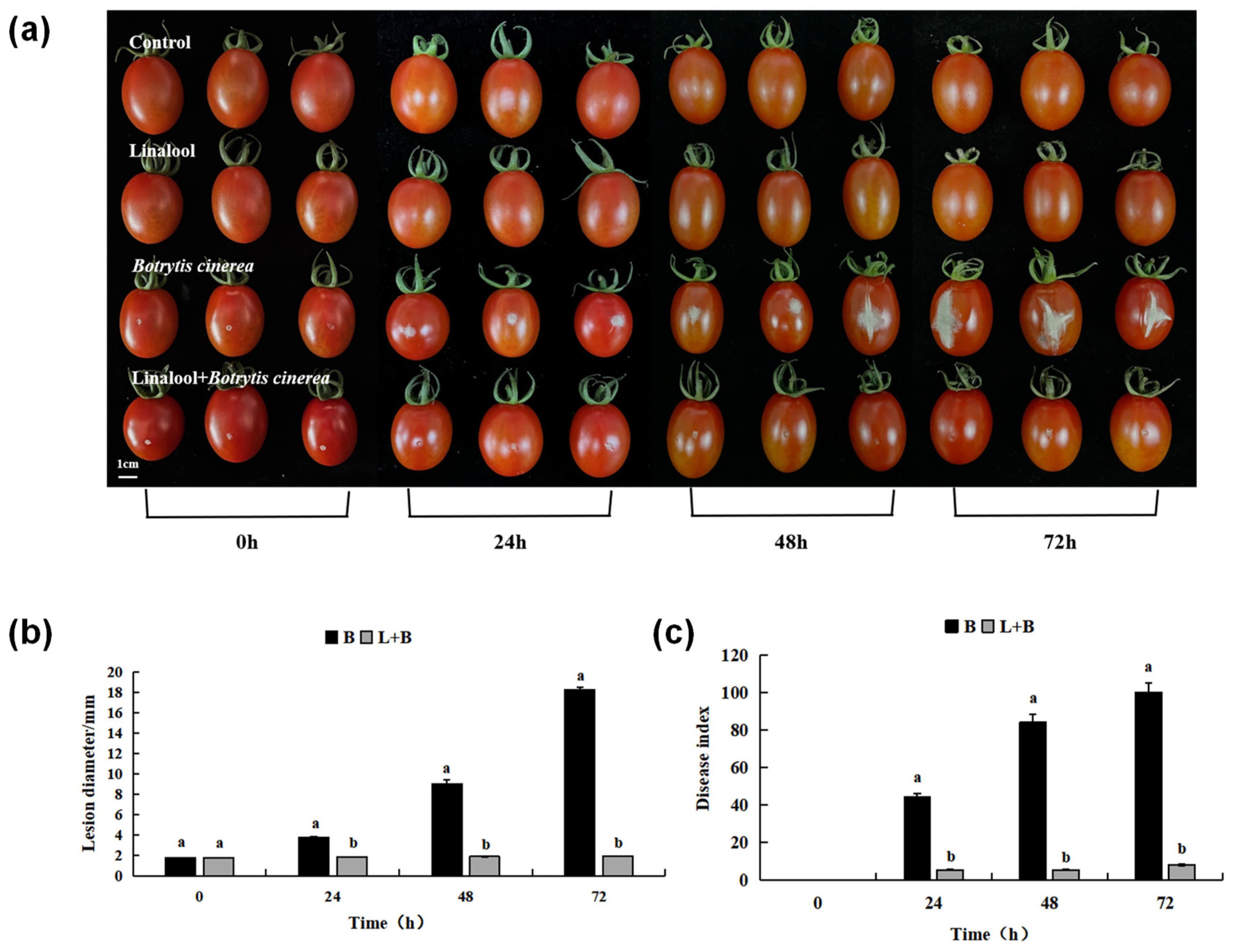
3.2. Effects of Linalool Fumigation on the Gray Mold Control of Tomato Fruits
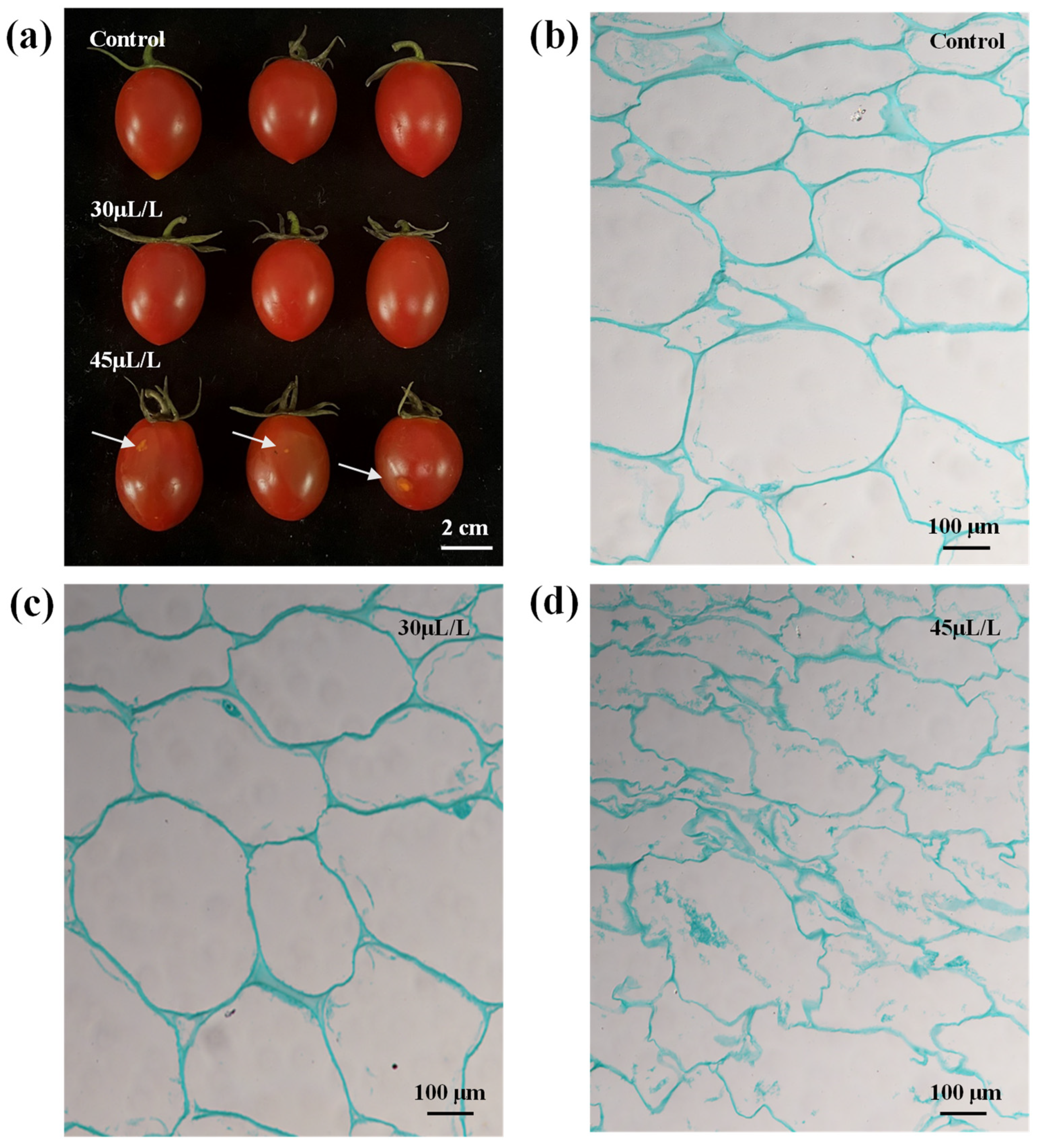
3.2.1. Screening of Linalool Fumigation Concentration in Tomato Fruits
3.2.2. Effects of Linalool Fumigation on the Expansion of Tomato Fruit Disease Spots
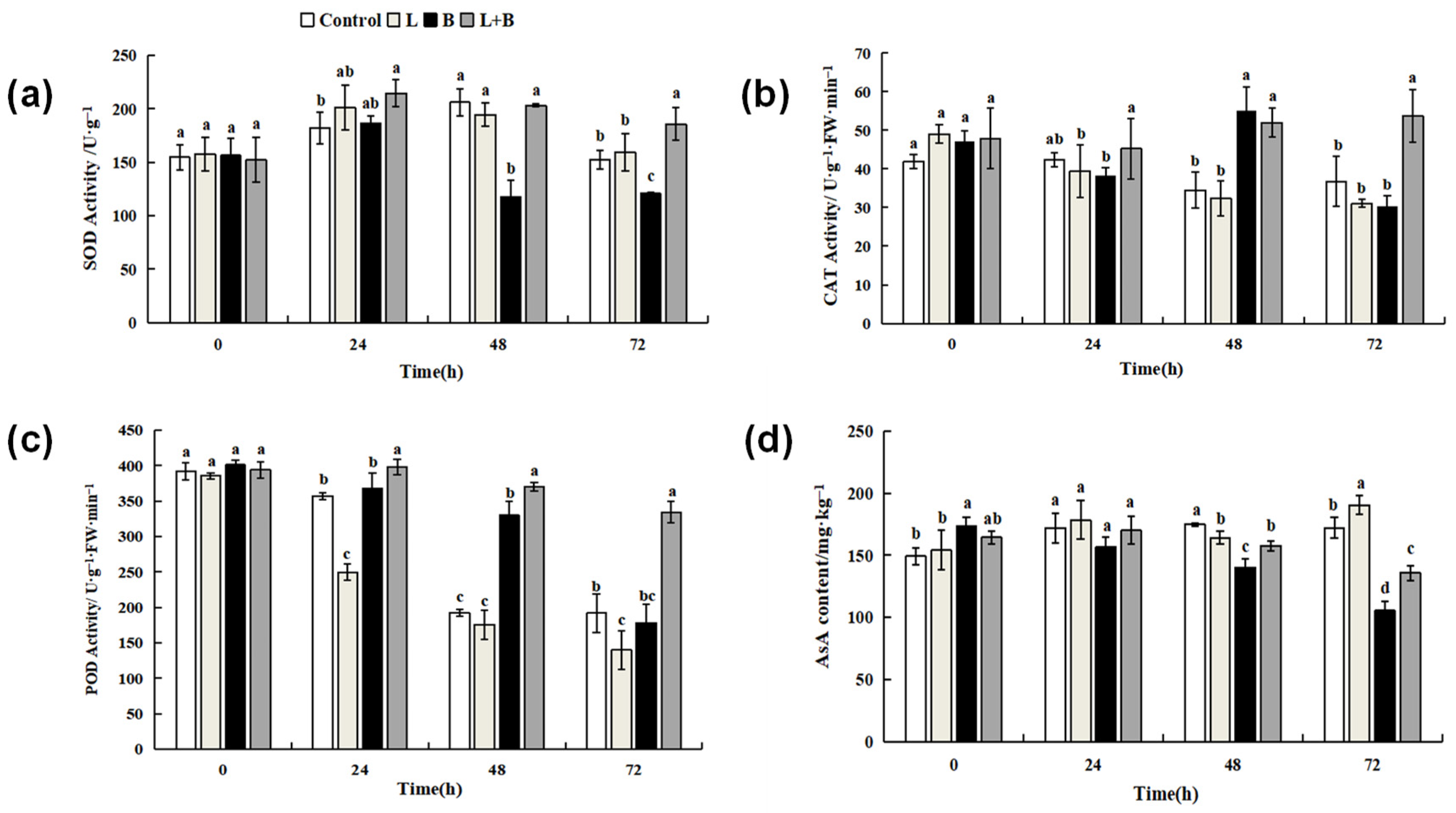
3.3. Effects of Linalool Fumigation on Antioxidant Systems
3.3.1. Effects of Linalool Fumigation on Defense Enzymes Activities and AsA Content in Tomato Fruits
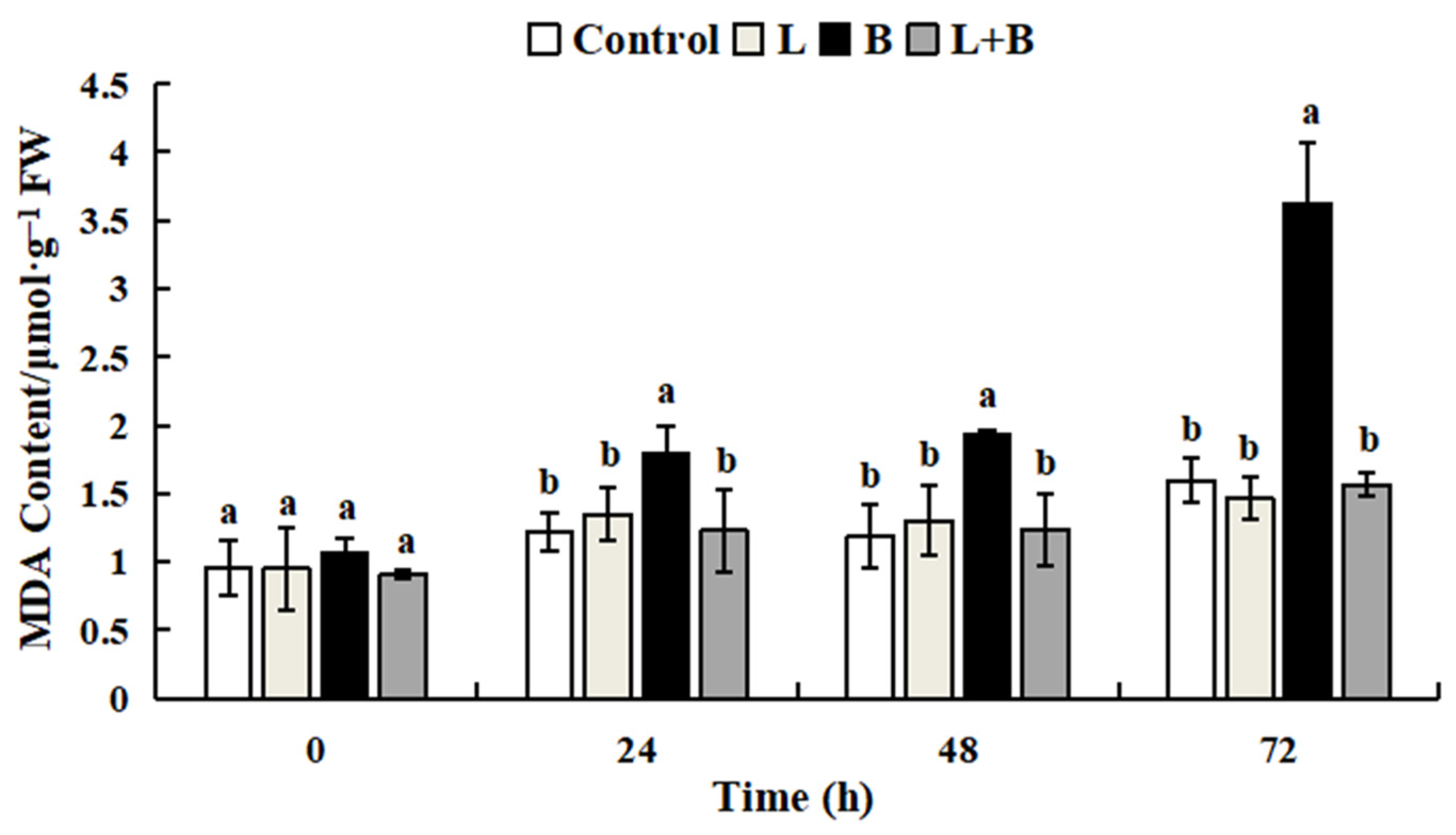
3.3.2. Effect of Linalool Fumigation on Tomato Fruit MDA Content
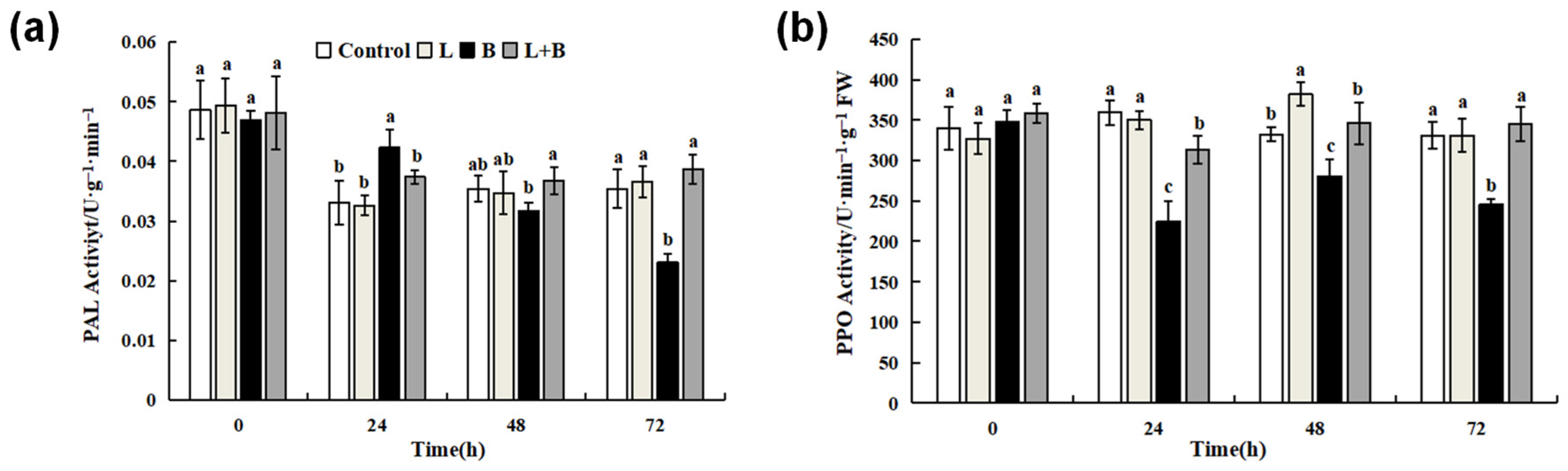
3.4. Effects of Linalool Fumigation on PAL and PPO Activity
3.5. Effects of Linalool Fumigation on the Activity of Softening Related Enzymes in Tomato Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.Q.; Zhang, Y.X.; Gao, Z.P.; Yang, W.C. Breeding for Resistance to Tomato Bacterial Diseases in China: Challenges and Prospects. Hortic. Plant J. 2018, 4, 193–207. [Google Scholar] [CrossRef]
- Kavitha, P.; Shivashankara, K.S.; Rao, V.K.; Sadashiva, A.T.; Ravishankar, K.V.; Sathish, G.J. Genotypic variability for antioxidant and quality parameters among tomato cultivars, hybrids, cherry tomatoes and wild species. J. Sci. Food Agric. 2014, 94, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.W.; Tuteja, N. Post-harvest quality risks by stress/ethylene: Management to mitigate. Protoplasma 2015, 252, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, A.I.; Izzati, M.Z.; Kalsom, Y.U. Diversity of Fusarium Species Associated with Post-harvest Fruit Rot Disease of Tomato. Sains Malays. 2013, 42, 911–920. [Google Scholar]
- Wang, A.Y.; Lou, B.G.; Xu, T.; Lin, C. Defense responses in tomato fruit induced by oligandrin against Botrytis cinerea. Afr. J. Biotechnol. 2011, 10, 4596–4601. [Google Scholar]
- Ahmed, F.A.; Arif, M.; Alvarez, A.M. Antibacterial Effect of Potassium Tetraborate Tetrahydrate against Soft Rot Disease Agent Pectobacterium carotovorum in Tomato. Front. Microbiol. 2017, 8, 1728. [Google Scholar] [CrossRef]
- Shao, T.Y.; Zhao, J.J.; Zhu, T.S.; Chen, M.X.; Wu, Y.W.; Long, X.H.; Gao, X.M. Relationship between rhizosphere soil properties and blossom-end rot of tomatoes in coastal saline-alkali land. Appl. Soil Ecol. 2018, 127, 96–101. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Techn. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Choudhury, D.; Dobhal, P.; Srivastava, S.; Saha, S.; Kundu, S. Role of botanical plant extracts to control plant pathogens—A review. Indian J. Agric. Res. 2018, 52, 341–346. [Google Scholar]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.Y.; Sun, H.L. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Parasuraman, V.; Sharmin, A.M.; Anand, M.A.V.; Sivakumar, A.S.; Surendhiran, D.; Sharesh, G.; Kim, S. Fabrication and bacterial inhibitory activity of essential oil linalool loaded biocapsules against Escherichia coli. J. Drug Deliv. Sci. Technol. 2022, 74, 103495. [Google Scholar] [CrossRef]
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.E.; Miron, A. Coriander essential oil and linalool—interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2019, 68, 156–164. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lai, W.L.; Chuang, K.C.; Lee, M.H.; Tsai, Y.C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 2013, 51, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Evaluation of Antifungal Activity of Antimicrobial Agents on Cheddar Cheese. Packag. Technol. Sci. 2014, 27, 49–58. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.X.; Chen, H.M.; Zhong, Q.P.; Hou, Y.Q.; Chen, W.J.; Chen, W.X. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Li, Y.S.; Ren, F.; Chen, D.; Chen, H.M.; Chen, W.X. Antibacterial Mechanism of Linalool against Pseudomonas fragi: A Transcriptomic Study. Foods 2022, 11, 14. [Google Scholar] [CrossRef]
- Piesik, D.; Pańka, D.; Delaney, K.J.; Skoczek, A.; Lamparski, R.; Weaver, D.K. Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.). J. Plant Physiol. 2011, 168, 878–886. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Tong, Z.C.; Zhang, X.; Wang, Y.Y.; Fang, W.G.; Li, L.; Luo, Z.S. Unveiling the Mechanisms for the Plant Volatile Organic Compound Linalool To Control Gray Mold on Strawberry Fruits. J. Agric. Food Chem. 2019, 67, 9265–9276. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Kim, J.H.; Choi, H.W.; Keum, Y.S.; Chun, S.C. Effect of Thymol and Linalool Fumigation on Postharvest Diseases of Table Grapes. Mycobiology 2015, 42, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, J.J.; Han, H.Y.; Zhao, J.H.; Li, C.Y. In-vitro and in-planta Botrytis cinerea Inoculation Assays for Tomato. Bio-Protocol 2018, 8, e2810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, R.L.; Fang, X.J.; Wu, W.J.; Han, Y.C.; Chen, H.J.; Xu, F.; Gao, H.Y. Botrytis cinerea infection affects wax composition, content and gene expression in blueberry fruit. Postharvest Biol. Technol. 2022, 192, 112020. [Google Scholar] [CrossRef]
- Hou, H.Y.; Zhang, X.Y.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. Peerj 2020, 8, e9626. [Google Scholar] [CrossRef]
- Yu, W.Q.; Zhao, R.R.; Sheng, J.P.; Shen, L. SIERF2 Is Associated with Methyl Jasmonate-Mediated Defense Response against Botrytis cinerea in Tomato Fruit. J. Agric. Food Chem. 2018, 66, 9923–9932. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.J.; Wang, X.Y.; Yao, L.; Sheng, J.P.; Shen, L. Exogenous ferulic acid treatment increases resistance against Botrytis cinerea in tomato fruit by regulating nitric oxide signaling pathway. Postharvest Biol. Technol. 2021, 182, 111678. [Google Scholar] [CrossRef]
- Brito, C.; Hansen, H.; Espinoza, L.; Faundez, M.; Olea, A.F.; Pino, S.; Diaz, K. Assessing the Control of Postharvest Gray Mold Disease on Tomato Fruit Using Mixtures of Essential Oils and Their Respective Hydrolates. Plants 2021, 10, 1719. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Afiyanti, M.; Chen, H.J. Catalase activity is modulated by calcium and calmodulin in detached mature leaves of sweet potato. J. Plant Physiol. 2014, 171, 35–47. [Google Scholar] [CrossRef]
- Minaeva, O.M.; Akimova, E.E.; Tereshchenko, N.N.; Zyubanova, T.I.; Apenysheva, M.V.; Kravets, A.V. Effect of Pseudomonas Bacteria on Peroxidase Activity in Wheat Plants when Infected with Bipolaris sorokiniana. Russ. J. Plant Physiol. 2018, 65, 717–725. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.I.; Merodio, C. Effect of high carbon dioxide concentration on pal activity phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar]
- Arakawa, N.; Tsutsumi, K.; Sanceda, N.G. A rapid and sensitive method for the determination of ascorbic acid using 4,7-diphenyl-l, 10-phenanthroline. Agric. Biol. Chem. 1981, 45, 1289–1290. [Google Scholar] [CrossRef]
- Yang, G.Q.; Zhao, S.; Gong, J.; Huang, M.; Yu, W.Q.; Zhang, K.K.; Hu, D.Y. Dissipation and the effects of thidiazuron on antioxidant enzyme activity and malondialdehyde content in strawberry. J. Sci. Food Agric. 2019, 99, 4331–4337. [Google Scholar] [CrossRef]
- Wang, Y.S.; Ding, M.D.; Pang, Y.L.; Gu, X.G.; Gao, L.P.; Xia, T. Analysis of Interfering Substances in the Measurement of Malondialdehyde Content in Plant Leaves. Asian J. Chem. 2013, 25, 6293–6297. [Google Scholar] [CrossRef]
- Li, X.M.; Zhao, W.C.; Zhou, X.X.; Feng, J.P.; Gao, Y.J.; Yao, X.H.; Liu, Y.; Liu, J.; Yang, R.; Zhao, F.K.; et al. The use of toluidine blue staining combined with paraffin sectioning and the optimization of freeze-thaw counting methods for analysing root-knot nematodes in tomato. Hortic. Environ. Biotechnol. 2017, 58, 620–626. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- He, R.R.; Chen, W.J.; Chen, H.M.; Zhong, Q.P.; Zhang, H.L.; Zhang, M.; Chen, W.X. Antibacterial mechanism of linalool against L. monocytogenes, a metabolomic study. Food Control 2021, 132, 108533. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Schalechet, F.; Wilkinson, J.; Matsui, K.; Tadmor, Y.; Nam, K.H.; Amar, O.; Lastochkin, E.; Larkov, O.; Ravid, U.; et al. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 2001, 127, 1256–1265. [Google Scholar] [CrossRef]
- Wu, B.; Cao, X.; Liu, H.; Zhu, C.; Klee, H.; Zhang, B.; Chen, K. UDP-glucosyltransferase PpUGT85A2 controls volatile glycosylation in peach. J. Exp. Bot. 2019, 70, 925–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Ma, D.; Liu, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. Magnolol inhibits gray mold on postharvest fruit by inducing autophagic activity of Botrytis cinerea. Postharvest Biol. Technol. 2021, 180, 111596. [Google Scholar] [CrossRef]
- Rahman, M.U.; Hanif, M.; Wan, R.; Hou, X.; Ahmad, B.; Wang, X. Screening Vitis genotypes for responses to Botrytis cinerea and evaluation of antioxidant enzymes, reactive oxygen species and jasmonic acid in resistant and susceptible hosts. Molecules 2019, 24, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for enhancing plant resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, H.; Gong, P.; Sadeghnezhad, E.; Pang, Q.; Dong, T.; Li, T.; Jin, H.; Fang, J. Chitosan induces jasmonic acid production leading to resistance of ripened fruit against B. cinerea infection. Food Chem. 2021, 337, 127772. [Google Scholar]
- Riseh, R.S.; Dashti, H.; Vazvani, M.G. Changes in the activity of enzymes pheylalanine ammonia-lyase, polyphenol oxidase, and peroxidase in some wheat genotypes against take-all disease. J. Agric. Sci. Technol. 2021, 23, 929–942. [Google Scholar]
- Zhang, X.B.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Vanitha, S.C.; Niranjana, S.R.; Umesha, S. Role of phenylalanine ammonia-lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. J. Phytopathol. 2009, 157, 552–557. [Google Scholar] [CrossRef]
- Jiang, L.L.; Jin, P.; Wang, L.; Yu, X.; Wang, H.Y.; Zheng, Y.H. Methyl jasmonate primes defense responses against Botrytis cinerea and reduces disease development in harvested table grapes. Sci. Hortic. 2015, 192, 218–223. [Google Scholar] [CrossRef]
- Sun, C.C.; Huang, Y.; Lian, S.; Saleem, M.; Li, B.H.; Wang, C.X. Improving the biocontrol efficacy of Meyerozyma guilliermondii Y-1 with melatonin against postharvest gray mold in apple fruit. Postharvest Biol. Technol. 2020, 171, 111351. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Zhu, Z.; Xu, L.; Wu, B.; Li, J. Botrytis cinerea mediated cell wall degradation accelerates spike stalk browning in Munage grape. J. Food Biochem. 2022, 46, e14271. [Google Scholar] [CrossRef]
- Rasoul, M.A.A.; Marei, G.I.K.; Abdelgalei, S.A.M. Evaluation of antibacterial properties and biochemical effects of monoterpenes on plant pathogenic bacteria. Afr. J. Microbiol. Res. 2012, 6, 3667–3672. [Google Scholar]
- Ranjbar, A.; Ramezanian, A.; Shekarforoush, S.; Niakousari, M.; Eshghi, S. Antifungal activity of thymol against the main fungi causing pomegranate fruit rot by suppressing the activity of cell wall degrading enzymes. Food Sci. Technol. 2022, 161, 113303. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; Li, H.; Wang, Q.; Wang, J.; Ge, J.; Yang, X.; Wang, X.; Li, X.; Zhang, Y.; Zhang, R.; et al. Alleviating Effects of Linalool Fumigation on Botrytis cinerea Infections in Postharvest Tomato Fruits. Horticulturae 2022, 8, 1074. https://doi.org/10.3390/horticulturae8111074
Shen Q, Li H, Wang Q, Wang J, Ge J, Yang X, Wang X, Li X, Zhang Y, Zhang R, et al. Alleviating Effects of Linalool Fumigation on Botrytis cinerea Infections in Postharvest Tomato Fruits. Horticulturae. 2022; 8(11):1074. https://doi.org/10.3390/horticulturae8111074
Chicago/Turabian StyleShen, Qing, Haosen Li, Qifang Wang, Jianquan Wang, Jiarui Ge, Xiaoyu Yang, Xiaoyun Wang, Xiuming Li, Yan Zhang, Ruimin Zhang, and et al. 2022. "Alleviating Effects of Linalool Fumigation on Botrytis cinerea Infections in Postharvest Tomato Fruits" Horticulturae 8, no. 11: 1074. https://doi.org/10.3390/horticulturae8111074
APA StyleShen, Q., Li, H., Wang, Q., Wang, J., Ge, J., Yang, X., Wang, X., Li, X., Zhang, Y., Zhang, R., & Shi, Q. (2022). Alleviating Effects of Linalool Fumigation on Botrytis cinerea Infections in Postharvest Tomato Fruits. Horticulturae, 8(11), 1074. https://doi.org/10.3390/horticulturae8111074





