Effect of Combination of KMnO4 Oxidation and UV-C Radiation on Postharvest Quality of Refrigerated Pears cv. ‘Ercolini’
Abstract
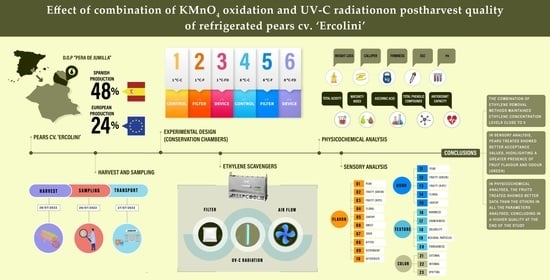
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Physicochemical Variables
- -
- Acid solution: 30 g of metaphosphoric acid (Acrós Organics, Geel, Belgium) and 80 mL of acetic acid (Panreac, Castellar del Vallés, Barcelona, Spain) were added in a 1 L flask and levelled.
- -
- Dichlorophenol solution: 250 mg 2,6-dichlorophenol indophenol (Scharlab S.L., Barcelona, Spain), 210 mg sodium hydrogen carbonate (Panreac, Castellar del Vallés, Barcelona, Spain) were weighed, dissolved and levelled in a 1 L flask.
2.4. Descriptive Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Ethylene
3.2. Physico-Chemical Variables Analysed
3.3. Sensory Analysis
3.4. Correlation Matrix and Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bower, J.; Biasi, W.; Mitcham, E. Effect of Ethylene in the Storage Environment on Quality of ‘Bartlett Pears’. Postharvest Biol. Technol. 2003, 28, 371–379. [Google Scholar] [CrossRef]
- Hiwasa, K.; Kinugasa, Y.; Amano, S.; Hashimoto, A.; Nakano, R.; Inaba, A.; Kubo, Y. Ethylene Is Required for Both the Initiation and Progression of Softening in Pear (Pyrus communis L.) Fruit. J. Exp. Bot. 2003, 54, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Golias, J.; Balik, J.; Nemcova, A.; Snurkovic, P.; Koziskova, J. Changes in Asian and European Pear Cultivars Fruit after Harvest in Various Storage Conditions. III Balk. Symp. Fruit Grow. Acta Hortic. 2016, 1139, 609–615. [Google Scholar] [CrossRef]
- Nguyen, L.L.; Pham, T.T.; Syium, Z.H.; Zsom-Muha, V.; Baranyai, L.; Zsom, T.; Hitka, G. Delay of 1-MCP Treatment on Post-Harvest Quality of ‘Bosc Kobak’ Pear. Horticulturae 2022, 8, 89. [Google Scholar] [CrossRef]
- Jesús, G.B.; de Murcia, R. Informe sobre los parámetros de la calidad, contenido en Sólidos solubles (azucares) y Firmeza, 495 de los frutos de peral variedad ‘Ercolini’, cultivados en las condiciones medio ambientales de la comarca de Jumilla ‘Altiplano’ 496 (Murcia) en los años 2006, 2007 y 2008. Región Murcia Cons. Agric. Y Agua 2011, 1–6. [Google Scholar]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Miranda-Molina, F.D.; Artés-Hernández, F. Postharvest Quality Retention of Apricots by Using a Novel Sepiolite–Loaded Potassium Permanganate Ethylene Scavenger. Postharvest Biol. Technol. 2020, 160, 111061. [Google Scholar] [CrossRef]
- Mansourbahmani, S.; Ghareyazie, B.; Zarinnia, V.; Kalatejari, S.; Mohammadi, R.S. Study on the Efficiency of Ethylene Scavengers on the Maintenance of Postharvest Quality of Tomato Fruit. J. Food Meas. Charact. 2018, 12, 691–701. [Google Scholar] [CrossRef]
- Charoenchongsuk, N.; Matsumoto, D.; Itai, A.; Murayama, H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae 2018, 4, 22. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; Acosta-Motos, J.R.; Núñez-Delicado, E.; Gabaldón, J.A.; López-Miranda, S. Combined Effect of Potassium Permanganate and Ultraviolet Light as Ethylene Scavengers on Post-Harvest Quality of Peach at Optimal and Stressful Temperatures. Agronomy 2022, 12, 616. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Núñez-Delicado, E.; Acosta-Motos, J.R. Novel Combination of Ethylene Oxidisers to Delay Losses on Postharvest Quality, Volatile Compounds and Sensorial Analysis of Tomato Fruit. LWT 2022, 170, 114054. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, G.H.; Kim, S.-W. Ethylene Gas Decomposition Using ZSM-5/WO3-Pt-Nanorod Composites for Fruit Freshness. ACS Sustain. Chem. Eng. 2019, 7, 11250–11257. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Suppakul, P. Active and Intelligent Packaging: The Indication of Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Esturk, O.; Ayhan, Z.; Gokkurt, T. Production and Application of Active Packaging Film with Ethylene Adsorber to Increase the Shelf Life of Broccoli (Brassica oleracea L. Var. Italica). Packag. Technol. Sci. 2014, 27, 179–191. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active Packaging from Chitosan-Titanium Dioxide Nanocomposite Film for Prolonging Storage Life of Tomato Fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Geyer, M.; Herppich, W.B.; Rauh, C.; Mahajan, P. V Photocatalytic and Photochemical Oxidation of Ethylene: Potential for Storage of Fresh Produce—A Review. Food Bioprocess Technol. 2017, 10, 982–1001. [Google Scholar] [CrossRef]
- Pathak, N. Photocatalysis and Vacuum Ultraviolet Light Photolysis as Ethylene Removal Techniques for Potential Application in Fruit Storage; Technische Universität Berlin: Berlin, Germany, 2019. [Google Scholar]
- Tytgat, T.; Hauchecorne, B.; Abakumov, A.; Smits, M.; Vinken, K.; Verbruggen, S.; Lenaerts, S. Photocatalytic Process Optimisation for Ethylene Oxidation. Chem. Eng. J. 2011, 209, 494–500. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Melatonin Inhibits Ethylene Synthesis via Nitric Oxide Regulation To Delay Postharvest Senescence in Pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Artés-Hernández, F.; Ávalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Ventura-Sobrevilla, J.M.; Martínez-Hernández, G.B. Current Scenario of Adsorbent Materials Used in Ethylene Scavenging Systems to Extend Fruit and Vegetable Postharvest Life. Food Bioprocess Technol. 2018, 11, 511–525. [Google Scholar] [CrossRef]
- Smith, A.; Poulston, S.; Rowsell, L.; Terry, L.; Anderson, J. A New Palladium-Based Ethylene Scavenger to Control Ethylene-Induced Ripening of Climacteric Fruit. Platin. Met. Rev. 2009, 53, 112–122. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Makkumrai, W.; Sivertsen, H.; Sugar, D.; Ebeler, S.E.; Negre-Zakharov, F.; Mitcham, E.J. Effect of Ethylene and Temperature Conditioning on Sensory Attributes and Chemical Composition of “Comice” Pears. J. Agric. Food Chem. 2014, 62, 4988–5004. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernández, A.; Paredes-López, O. Fruit Quality: New Insights for Biotechnology. Crit. Rev. Food Sci. Nutr. 2012, 52, 272–289. [Google Scholar] [CrossRef]
- Gamrasni, D.; Ben-Arie, R.; Goldway, M. 1-Methylcyclopropene (1-MCP) Application to Spadona Pears at Different Stages of Ripening to Maximize Fruit Quality after Storage. Postharvest Biol. Technol. 2010, 58, 104–112. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, B.; Zhang, C.; Song, Z.; Ma, R. Determination of Fruit Maturity and Its Prediction Model Based on the Pericarp Index of Absorbance Difference (IAD) for Peaches. PLoS ONE 2017, 12, e0177511. [Google Scholar] [CrossRef]
- Nielsen, S.S. Vitamin C Determination by Indophenol Method BT—Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 143–146. ISBN 978-3-319-44127-6. [Google Scholar]
- Kidron, M.; Harel, E.; Mayer, A.M. Catechol Oxidase Activity in Grapes and Wine. Am. J. Enol. Vitic. 1978, 29, 30–35. [Google Scholar]
- López-Miranda, S.; Serrano-Martínez, A.; Hernández-Sánchez, P.; Guardiola, L.; Pérez-Sánchez, H.; Fortea, I.; Gabaldón, J.A.; Núñez-Delicado, E. Use of Cyclodextrins to Recover Catechin and Epicatechin from Red Grape Pomace. Food Chem. 2016, 203, 379–385. [Google Scholar] [CrossRef]
- Noguera-Artiaga, L.; Salvador, M.; Fregapane, G.; Collado, J.; Wojdyło, A.; López Lluch, D.; Carbonell-Barrachina, A. Functional and Sensory Properties of Pistachio Nuts as Affected by Cultivar. J. Sci. Food Agric. 2019, 99, 6696–6705. [Google Scholar] [CrossRef]
- Gittins, C.; Calvo, P.; Miranda, M.J.; Barda, N. Developing a Sensory Descriptive Panel for Pear Quality Evaluation. Acta Hortic. 2011, 909, 617–624. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Recent Advances in Detecting and Regulating Ethylene Concentrations for Shelf-Life Extension and Maturity Control of Fruit: A Review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene Scavengers for Active Packaging of Fresh Food Produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Sammi, S.; Masud, T. Effect of Different Packaging Systems on the Quality of Tomato (Lycopersicon esculentum Var. Rio Grande) Fruits during Storage. Int. J. Food Sci. Technol. 2009, 44, 918–926. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Hao, S.; Kojima, M.; Sakakibara, H.; Ozeki-Iida, Y.; Zheng, Y.; Fei, Z.; Zhong, S.; Giovannoni, J.J.; Rose, J.K.C.; et al. Ethylene Suppresses Tomato (Solanum lycopersicum L.) Fruit Set through Modification of Gibberellin Metabolism. Plant J. 2015, 83, 237–251. [Google Scholar] [CrossRef]
- Le Nguyen, L.P.; Zsom, T.; Dam, M.S.; Baranyai, L.; Hitka, G. Evaluation of the 1-MCP Microbubbles Treatment for Shelf-Life Extension for Melons. Postharvest Biol. Technol. 2019, 150, 89–94. [Google Scholar] [CrossRef]
- Fan, X.; Shu, C.; Zhao, K.; Wang, X.; Cao, J.; Jiang, W. Regulation of Apricot Ripening and Softening Process during Shelf Life by Post-Storage Treatments of Exogenous Ethylene and 1-Methylcyclopropene. Sci. Hortic. 2018, 232, 63–70. [Google Scholar] [CrossRef]
- Emadpour, M.; Ghareyazie, B.; Kalaj, Y.R.; Entesari, M.; Bouzari, N. Effect of the Potassium Permanganate Coated Zeolite Nanoparticles on the Quality Characteristic and Shelf Life of Peach and Nectarine. Int. J. Agric. Technol. 2015, 11, 1411–1421. [Google Scholar]
- Crisosto, C.H.; Gugliuzza, G.; Garner, D.; Palou, L. Understanding the Role of Ethylene in Peach Cold Storage Life. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS), Leuven, Belgium, 30 June 2001; pp. 287–288. [Google Scholar]
- Hu, Q.; Fang, Y.; Yang, Y.; Ma, N.; Zhao, L. Effect of Nanocomposite-Based Packaging on Postharvest Quality of Ethylene-Treated Kiwifruit (Actinidia deliciosa) during Cold Storage. Food Res. Int. 2011, 44, 1589–1596. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Gunaseelan, K.; Wang, M.Y.; Luo, L.; Wang, T.; Norling, C.L.; Johnston, S.L.; Maddumage, R.; Schröder, R.; Schaffer, R.J. Dissecting the Role of Climacteric Ethylene in Kiwifruit (Actinidia chinensis) Ripening Using a 1-Aminocyclopropane-1-Carboxylic Acid Oxidase Knockdown Line. J. Exp. Bot. 2011, 62, 3821–3835. [Google Scholar] [CrossRef]
- Argenta, L.C.; Mattheis, J.P.; Fan, X.T.; Amarante, C.V.T. Managing “Bartlett” Pear Fruit Ripening with 1-Methylcyclopropene Reapplication during Cold Storage. Postharvest Biol. Technol. 2016, 113, 125–130. [Google Scholar] [CrossRef]
- Escribano, S.; Lopez, A.; Sivertsen, H.; Biasi, W.V.; Macnish, A.J.; Mitcham, E. Impact of 1-Methylcyclopropene Treatment on the Sensory Quality of ‘Bartlett’ Pear Fruit. Postharvest Biol. Technol. 2016, 111, 305–313. [Google Scholar] [CrossRef]
- Chiriboga, M.A.; Bordonaba, J.G.; Schotsmans, W.C.; Larrigaudiere, C.; Recasens, I. Antioxidant Potential of “Conference” Pears during Cold Storage and Shelf Life in Response to 1-Methylcyclopropene. LWT 2013, 51, 170–176. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium Permanganate-Based Ethylene Scavengers for Fresh Horticultural Produce as an Active Packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- Salamanca, F.A.; Balaguera-López, H.; Herrera, A. Effect of Potassium Permanganate on Some Postharvest Characteristics of Tomato “Chonto” Fruits (Solanum lycopersicum L.). Acta Hortic. 2014, 1016, 171–176. [Google Scholar] [CrossRef]
- Willner, B.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Bartlett Pear Brandies by Means of the Sensomics Concept. J. Agric. Food Chem. 2013, 61, 9583–9593. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, X.; Zhou, Q.; Shi, F.; Wei, B.; Cheng, S.; Tan, Z.; Ji, S. Low Temperature Conditioning Alleviates Loss of Aroma-Related Esters of “Nanguo” Pears by Regulation of Ethylene Signal Transduction. Food Chem. 2018, 264, 263–269. [Google Scholar] [CrossRef]
- Chai, J.; Liao, B.; Li, R.; Liu, Z. Changes in Taste and Volatile Compounds and Ethylene Production Determined the Eating Window of ‘Xuxiang’ and ‘Cuixiang’ Kiwifruit Cultivars. Postharvest Biol. Technol. 2022, 194, 112093. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, X.; Ji, Y.; Luo, M.; Sun, Y.; Zhou, Q.; Ji, S. Potential of Ethylene in Alleviating Cold-Induced Volatile Esters Loss of ‘Nanguo’ Pears by Regulating the Lipoxygenase Pathway. Environ. Exp. Bot. 2022, 194, 104723. [Google Scholar] [CrossRef]
- Li, X.; Gao, S.; Yang, L.; Yin, M.; Li, J.; Zhang, H.; Ji, S. Ethylene Promotes Ester Biosynthesis through PuERF13/PuDof2.5 Synergically Activated PuAAT1 during Ripening of Cold-Stored ‘Nanguo’ Pear. Postharvest Biol. Technol. 2023, 195, 112108. [Google Scholar] [CrossRef]
- Serra, S.; Anthony, B.; Masia, A.; Giovannini, D.; Musacchi, S. Determination of Biochemical Composition in Peach (Prunus persica L. Batsch) Accessions Characterized by Different Flesh Color and Textural Typologies. Foods 2020, 9, 1452. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Rodríguez-Hernández, A.M.; Castillo-Campohermoso, M.A.; Artés-Hernández, F. An Innovative Ethylene Scrubber Made of Potassium Permanganate Loaded on a Protonated Montmorillonite: A Case Study on Blueberries. Food Bioprocess Technol. 2019, 12, 524–538. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of Lycopene, Antioxidant Activity, Total Soluble Solids and Weight Loss of Tomato during Postharvest Storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]
- Rai, G.; Kumar, R.; Singh, A.; Rai, P.; Rai, M.; Chaturvedi, A.K.; Rai, A. Changes in Antioxidant and Phytochemical Properties of Tomato (Lycopersicon Esculentum Mill.) under Ambient Condition. Pak. J. Bot. 2012, 44, 667–670. [Google Scholar]



| Parameters | Weight (g) | Calliper (mm) | Firmness (N) | Soluble Solid Content (SSC) (%) | Total Acidity TA (%) | Colour |
|---|---|---|---|---|---|---|
| Data | 116 ± 10 | 54.3 ± 2.5 | 52.6 ± 4.5 | 11.8 ± 0.9 | 0.35 ± 0.06 | a *: −10.9 ± 2.8 b *: 40.3 ± 4.8 L *: 70.1 ± 3.6 |
| Method | Navigator Balance, Ohaus Europe Gmbh (Nänikon, Switzerland). | Mitutoyo 530-122, Mitutoyo Spain (Guipúzcoa, Spain). | CT3 texturometer, AMETEK Brookfield (Middleboro, MA, USA). | Pocket Brix-Acidity meter, Atago (Tokyo, Japan). | Pocket Brix-Acidity meter, Atago (Tokyo, Japan). | Colourpin II, Natural Color System (Stockholm, Sweden). |
| Treatments | 1 °C-C | 1 °C-F | 1 °C-FD | 8 °C-C | 8 °C-F | 8 °C-FD |
|---|---|---|---|---|---|---|
| Temperature | 1 °C | 1 °C | 1 °C | 8 °C | 8 °C | 8 °C |
| Relative humidity | 90% | 90% | 90% | 90% | 90% | 90% |
| Ethylene scavenger | None | Filter | Filter + Device | None | Filter | Filter + Device |
| Treatments | Weight (%) | Calliper (%) | Firmness (N) | |||
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| 1 °C-C | 100 | 82.9 ± 3.5 b | 100 | 82.5 ± 1.5 b | 48.9 ± 1.7 | 34.4 ± 1.6 c |
| 1 °C-F | 91.7 ± 6.3 a | 91.6 ± 1.5 a | 43.4 ± 1.6 ab | |||
| 1 °C-FD | 97.1 ± 8.3 a | 92.5 ± 0.7 a | 44.9 ± 2.1 a | |||
| 8 °C-C | 89.9 ± 4.4 a | 83.3 ± 2.0 b | 26.1 ± 2.6 d | |||
| 8 °C-F | 91.1 ± 2.8 a | 89.9 ± 1.7 a | 33.0 ± 1.8 c | |||
| 8 °C-FD | 93.2 ± 1.6 a | 95.4 ± 2.7 a | 37.4 ± 2.1 bc | |||
| Ethylene (E) | - | ** | - | *** | - | *** |
| Temperature (T) | - | n.s. | - | ** | - | *** |
| E × T | - | ** | - | n.s. | - | n.s. |
| Treatments | SSC (%) | TA (g L−1) | MI | |||
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| 1 °C-C | 12.5 ± 0.42 | 15.5 ± 0.65 ab | 3.69 ± 0.47 | 1.76 ± 0.29 c | 34.4 ± 2.44 | 95.9 ± 16.2 ab |
| 1 °C-F | 12.5 ± 0.29 c | 2.93 ± 0.29 ab | 43.7 ± 3.7 c | |||
| 1 °C-FD | 12.0 ± 0.41 c | 3.27 ± 0.46 a | 39.4 ± 6.3 c | |||
| 8 °C-C | 17.5 ± 0.65 a | 1.51 ± 0.22 c | 125.5 ± 22.0 a | |||
| 8 °C-F | 15.3 ± 1.10 b | 1.93 ± 0.29 bc | 84.1 ± 12.1 abc | |||
| 8 °C-FD | 15.0 ± 0.41 b | 2.09 ± 0.29 bc | 75.7 ± 10.1 bc | |||
| Ethylene (E) | - | *** | - | *** | - | *** |
| Temperature (T) | - | *** | - | *** | - | *** |
| E × T | - | n.s. | - | n.s. | - | n.s. |
| Treatments | Ascorbic Acid (mg 100mL−1) | TPC (g kg−1) | Antioxidant capacity (μmol kg−1) | |||
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| 1 °C-C | 5.0 ± 0.47 | 2.9 ± 0.37 bcd | 0.49 ± 0.03 | 0.26 ± 0.02 cd | 4.17 ± 0.08 | 2.18 ± 0.14 c |
| 1 °C-F | 3.8 ± 0.28 ab | 0.35 ± 0.04 ab | 2.83 ± 0.06 ab | |||
| 1 °C-FD | 4.4 ± 0.21 a | 0.39 ± 0.03 a | 3.14 ± 0.22 a | |||
| 8 °C-C | 1.7 ± 0.22 d | 0.21 ± 0.04 d | 1.78 ± 0.14 d | |||
| 8 °C-F | 2.0 ± 0.39 cd | 0.29 ± 0.05 bc | 2.21 ± 0.05 c | |||
| 8 °C-FD | 3.1 ± 0.48 abc | 0.36 ± 0.05 ab | 2.56 ± 0.19 bc | |||
| Ethylene (E) | - | *** | - | *** | - | *** |
| Temperature (T) | - | *** | - | ** | - | *** |
| E × T | - | n.s. | - | n.s. | - | n.s. |
| Sensory Descriptor | ANOVA | Day 0 | 1 °C (Day 28) | 8 °C (Day 28) | ||||
|---|---|---|---|---|---|---|---|---|
| C | F | FD | C | F | FD | |||
| COLOUR | ||||||||
| External | ** | 9.0 a | 6.0 c | 7.0 bc | 8.5 b | 2.0 e | 3.0 de | 4.0 d |
| Internal | *** | 8.0 a | 6.0 b | 6.0 b | 7.5 a | 2.0 c | 2.0 c | 5.0 b |
| Spotting | * | 1.0 c | 8.0 a | 5.5 b | 3.0 c | 8.5 a | 9.0 a | 6.0 b |
| ODOUR | ||||||||
| Pear | *** | 7.0 a | 4.0 c | 5.5 b | 7.0 a | 1.5 d | 2.0 d | 2.0 d |
| Fruity (Green) | *** | 8.0 a | 4.0 bc | 5.0 b | 8.0 a | 1.0 c | 1.0 c | 3.0 c |
| Fruity (ripe) | *** | 4.0 d | 8.0 bc | 8.0 bc | 5.0 d | 9.0 a | 9.0 a | 7.0 b |
| Floral | ** | 3.0 a | 1.0 b | 1.0 b | 2.5 a | 1.0 b | 1.0 b | 2.5 a |
| Earthy | n.s. | 1.5 | 3.0 | 3.0 | 1.0 | 3.5 | 4.0 | 3.0 |
| FLAVOUR | ||||||||
| Pear | ** | 8.5 a | 4.0 b | 4.5 b | 7.0 a | 1.5 d | 1.5 d | 2.5 c |
| Fruity (Green) | *** | 8.5 a | 5.0 b | 6.0 b | 7.0 a | 0.5 d | 1.0 d | 3.0 c |
| Fruity (ripe) | *** | 2.0 d | 4.0 c | 4.0 c | 2.5 d | 9.5 a | 9.5 a | 7.0 b |
| Floral | * | 3.0 a | 1.5 b | 2.0 b | 3.0 a | 1.0 b | 1.0 b | 2.0 b |
| Earthy | * | 3.0 a | 4.5 a | 4.0 a | 2.5 bc | 1.0 b | 2.0 b | 2.0 b |
| Sweet | * | 4.0 b | 3.0 c | 3.5 c | 5.0 a | 4.5 b | 5.5 a | 5.0 a |
| Sour | n.s. | 1.0 | 1.0 | 1.0 | 1.5 | 2.0 | 2.5 | 2.0 |
| Bitter | * | 0.5 c | 0.5 c | 0.5 c | 0.5 c | 3.0 a | 2.0 b | 2.0 b |
| Astringent | n.s. | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 1.5 | 1.5 |
| Aftertaste | ** | 4.0 a | 2.0 b | 2.5 b | 4.5 a | 1.0 b | 1.5 b | 2.5 b |
| TEXTURE | ||||||||
| Hardness | *** | 9.0 a | 6.0 c | 6.0 c | 7.5 b | 2.0 e | 2.5 e | 4.5 d |
| Crunchiness | *** | 9.0 a | 6.0 c | 5.5 c | 7.5 b | 1.0 d | 1.5 d | 3.0 d |
| Solubility | *** | 9.0 a | 7.0 bc | 6.5 bc | 8.0 b | 6.0 c | 7.5 b | 8.0 b |
| Residual particles | n.s. | 3.0 | 5.0 | 4.5 | 2.5 | 3.5 | 4.0 | 3.5 |
| Fibrousness | n.s. | 1.0 | 3.0 | 3.0 | 1.0 | 3.0 | 3.0 | 3.0 |
| W | C | F | SSC | pH | TA | MI | AA | TPC | |
|---|---|---|---|---|---|---|---|---|---|
| C | 0.5976 ** | - | - | - | - | - | - | - | - |
| F | 0.1449 n.s. | 0.2914 n.s. | - | - | - | - | - | - | - |
| SSC | −0.1510 n.s. | −0.3793 * | −0.8344 *** | - | - | - | - | - | - |
| pH | −0.0620 n.s. | −0.2884 n.s. | −0.7391 *** | 0.8209 *** | - | - | - | - | - |
| TA | 0.2226 n.s. | 0.2732 n.s. | 0.7369 *** | −0.6806 ** | −0.6353 ** | - | - | - | - |
| MI | −0.2499 n.s. | −0.3314 n.s. | −0.8158 *** | 0.7661 *** | 0.7083 *** | −0.8862 *** | - | - | - |
| AA | 0.1693 n.s. | 0.3262 n.s. | 0.7097 *** | −0.6896 ** | −0.6577 ** | 0.6204 ** | −0.6240 ** | - | - |
| TPC | 0.2170 n.s. | 0.5682 * | 0.7509 *** | −0.6930 ** | −0.7238 *** | 0.6594 ** | −0.7129 *** | 0.6207 ** | - |
| ORAC | 0.1422 n.s. | 0.4062 * | 0.8681 *** | −0.8010 *** | −0.6596 ** | 0.6980 ** | −0.7054 *** | 0.8058 *** | 0.7003 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Salinas, R.; Acosta-Motos, J.R.; Pérez-López, A.J.; Noguera-Artiaga, L.; Núñez-Delicado, E.; Burló, F.; López-Miranda, S. Effect of Combination of KMnO4 Oxidation and UV-C Radiation on Postharvest Quality of Refrigerated Pears cv. ‘Ercolini’. Horticulturae 2022, 8, 1078. https://doi.org/10.3390/horticulturae8111078
Alonso-Salinas R, Acosta-Motos JR, Pérez-López AJ, Noguera-Artiaga L, Núñez-Delicado E, Burló F, López-Miranda S. Effect of Combination of KMnO4 Oxidation and UV-C Radiation on Postharvest Quality of Refrigerated Pears cv. ‘Ercolini’. Horticulturae. 2022; 8(11):1078. https://doi.org/10.3390/horticulturae8111078
Chicago/Turabian StyleAlonso-Salinas, Ramiro, José Ramón Acosta-Motos, Antonio J. Pérez-López, Luis Noguera-Artiaga, Estrella Núñez-Delicado, Francisco Burló, and Santiago López-Miranda. 2022. "Effect of Combination of KMnO4 Oxidation and UV-C Radiation on Postharvest Quality of Refrigerated Pears cv. ‘Ercolini’" Horticulturae 8, no. 11: 1078. https://doi.org/10.3390/horticulturae8111078
APA StyleAlonso-Salinas, R., Acosta-Motos, J. R., Pérez-López, A. J., Noguera-Artiaga, L., Núñez-Delicado, E., Burló, F., & López-Miranda, S. (2022). Effect of Combination of KMnO4 Oxidation and UV-C Radiation on Postharvest Quality of Refrigerated Pears cv. ‘Ercolini’. Horticulturae, 8(11), 1078. https://doi.org/10.3390/horticulturae8111078