Evaluation of the Effects of Process Conditions on the Extraction of Glucosinolates from Broccoli Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Material
2.2. Experimental Design
2.3. Extraction and Desulfation of Glucosinolates (GSL)
2.4. Identification and Quantification of Desulfoglucosinolates
2.5. Statistical Analysis
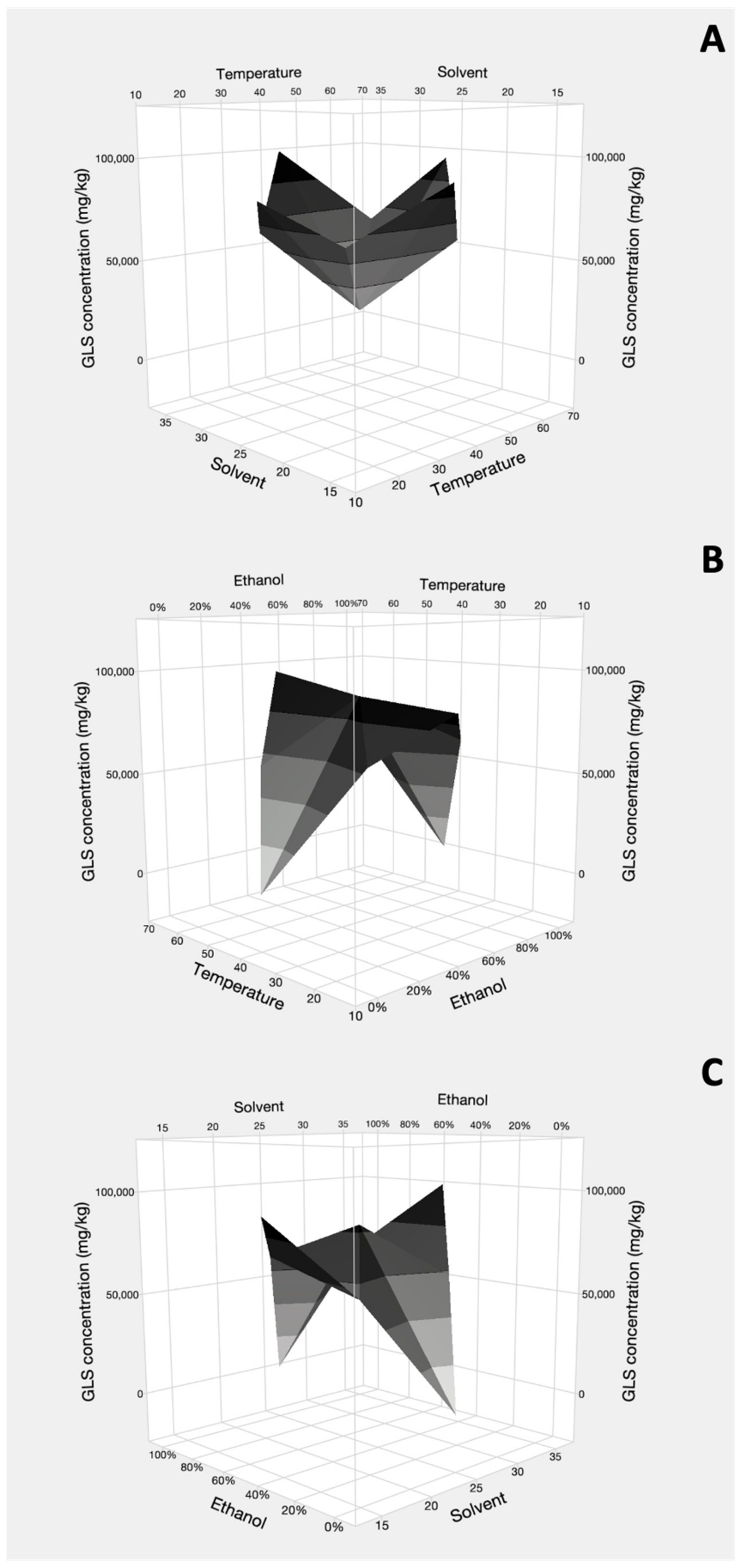
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre-and post-harvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Q.; Mo, X.-F.; Lin, F.-Y.; Zhan, X.-X.; Feng, X.-L.; Zhang, X.; Lou, H.; Zhang, C.X. Intake of total cruciferous vegetable and its contents of glucosinolates and isothiocyanates, glutathione S-transferases polymorphisms and breast cancer risk: A case–control study in China. Brit. J. Nutr. 2020, 124, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-J.; Ji, Q.-Q.; Wang, Z.; Shen, L.-H.; He, B. Moringa oleifera seeds mitigate myocardial injury and prevent ventricular failure induced by myocardial infarction. Am. J. Transl. Res. 2020, 12, 4511. [Google Scholar] [PubMed]
- Su, X.; Wang, S.; Zhang, H.; Yang, G.; Bai, Y.; Liu, P.; Meng, L.; Jiang, X.; Xin, Y. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 through epigenetic modification. J. Cell. Mol. Med. 2021, 25, 4408–4419. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, K.V.; Andrade, D.C.; Toledo, C.; Schwarz, K.G.; Uribe-Ojeda, A.; Ríos-Gallardo, A.P.; Quintanilla, R.A.; Contreras, S.; Mahn, A.; Del Rio, R. Dietary supplementation of a sulforaphane-enriched broccoli extract protects the heart from acute cardiac stress. J. Funct. Foods 2020, 75, 104267. [Google Scholar] [CrossRef]
- Peng, N.; Jin, L.; He, A.; Deng, C.; Wang, X. Effect of sulphoraphane on newborn mouse cardiomyocytes undergoing ischaemia/reperfusion injury. Pharm. Biol. 2019, 57, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Sánchez-Garibay, C.; Rojas-Morales, P.; Galván-Arzate, S.; Buelna-Chontal, M.; Pavón, N.; Pedraza-Chaverrí, J.; Königsberg, M.; Zazueta, C. Sulforaphane protects from myocardial ischemia-reperfusion damage through the balanced activation of Nrf2/AhR. Free Radic. Biol. Med. 2019, 143, 331–340. [Google Scholar] [CrossRef]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [Green Version]
- Kamal, R.M.; Razis, A.F.A.; Sukri, N.S.M.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial health effects of glucosinolates-derived isothiocyanates on cardiovascular and neurodegenerative diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Saha, S.; Hollands, W.; Teucher, B.; Needs, P.W.; Narbad, A.; Ortori, C.A.; Barrett, D.A.; Rossiter, J.T.; Mithen, R.F.; Kroon, P.A. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol. Nutr. Food Res. 2012, 56, 1906–1916. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Synergistic combinations of curcumin, sulforaphane, and dihydrocaffeic acid against human colon cancer cells. Int. J. Mol. Sci. 2020, 21, 3108. [Google Scholar] [CrossRef]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxidative Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [Green Version]
- Bose, C.; Alves, I.; Singh, P.; Palade, P.T.; Carvalho, E.; Børsheim, E.; Jun, S.R.; Cheema, A.; Boerma, M.; Awasthi, S.; et al. Sulforaphane prevents age-associated cardiac and muscular dysfunction through Nrf2 signaling. Aging Cell 2020, 19, e13261. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, D.; Chen, D.; Zhang, Q.; Dong, H.; Wu, W.; Lu, H.; Wu, G. Sulforaphane, a natural isothiocyanate compound, improves cardiac function and remodeling by inhibiting oxidative stress and inflammation in a rabbit model of chronic heart failure. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 1473. [Google Scholar] [CrossRef] [Green Version]
- Ares, A.M.; Bernal, J.; Martín, M.T.; Bernal, J.L.; Nozal, M.J. Optimized formation, extraction, and determination of sulforaphane in broccoli by liquid chromatography with diode array detection. Food Anal. Methods 2014, 7, 730–740. [Google Scholar] [CrossRef]
- Tian, Q.; Rosselot, R.A.; Schwartz, S.J. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal. Biochem. 2005, 343, 93–99. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Maldini, M.; Baima, S.; Morelli, G.; Scaccini, C.; Natella, F. A liquid chromatography-mass spectrometry approach to study “glucosinoloma” in broccoli sprouts. J. Mass Spectrom. 2012, 47, 1198–1206. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, J.K.; Park, S.; Lee, S.W.; Li, X.; Kim, Y.B.; Uddin, M.R.; Park, N.I.; Kim, S.J.; Park, S.U. Metabolic profiling of glucosinolates, anthocyanins, carotenoids, and other secondary metabolites in kohlrabi (Brassica oleracea var. gongylodes). J. Agric. Food Chem. 2012, 60, 8111–8116. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Zou, L.; Sun, J.; Song, X.; Mao, J.; Wu, Y. Simultaneous hydrolysis and extraction increased erucin yield from broccoli seeds. ACS Omega 2021, 6, 6385–6392. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnham, M.W.; Stephenson, K.K.; Fahey, J.W. Glucoraphanin level in broccoli seed is largely determined by genotype. HortScience 2005, 40, 50–53. [Google Scholar] [CrossRef]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; Gonzalez-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food. Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- Kushad, M.M.; Cloyd, R.; Babadoost, M. Distribution of glucosinolates in ornamental cabbage and kale cultivars. Sci. Hortic. 2004, 101, 215–221. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate–myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: Extraction, degradation, and applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Rohn, S.; Mewis, I.; Schreiner, M.; Kroh, L.W. Influence of the chemical structure on the thermal degradation of the glucosinolates in broccoli sprouts. Food Chem. 2012, 130, 1–8. [Google Scholar] [CrossRef]
- Krumbein, A.; Schonhof, I.; Schreiner, M. Composition and contents of phytochemicals (glucosinolates, carotenoids and chlorophylls) and ascorbic acid in selected Brassica species (B. juncea, B. rapa subsp. nipposinica var. chinoleifera, B. rapa subsp. chinensis and B. rapa subsp. rapa). J. Appl. Bot. Food Qual. 2005, 79, 168–174. [Google Scholar]
- Rebello, N.L. In vitro screening of the sunscreen potential of hydroalcoholic Erythrina variegata bark extract. Int. J. Green Pharm. 2016, 10, S131–S135. [Google Scholar]
- Carro, R.T.; Isla, M.I.; Ríos, J.L.; Giner, R.M.; Alberto, M.R. Anti-inflammatory properties of hydroalcoholic extracts of Argentine Puna plants. Food Res. 2015, 67, 230–237. [Google Scholar] [CrossRef]
- Tao, C.; He, B.B. Isolation of intact glucosinolates from mustard seed meal to increase the sustainability of biodiesel utilization. In Proceedings of the ASAE/CSAE Annual International Meeting, Ottawa, ON, Canada, 1–4 August 2004; pp. 6703–6713. [Google Scholar]
- Powell, E.E.; Hill, G.A.; Juurlink, B.H.J.; Carrier, D.J. Glucoraphanin extraction from Cardaria draba: Part 1. Optimization of batch extraction. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2005, 80, 985–991. [Google Scholar]
- Campos, D.; Chirinos, R.; Barreto, O.; Noratto, G.; Pedreschi, R. Optimized methodology for the simultaneous extraction of glucosinolates, phenolic compounds and antioxidant capacity from maca (Lepidium meyenii). Ind. Crops Prod. 2013, 49, 747–754. [Google Scholar] [CrossRef]
- Sharma, H.K.; Ingle, S.; Singh, C.; Sarkar, B.C.; Upadhyay, A. Effect of various process treatment conditions on the allyl isothiocyanate extraction rate from mustard meal. J. Food Sci. Technol. 2012, 49, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.N.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Ultrasound-assisted extraction of Catharanthus roseus (L.) G. Don (Patricia White cultivar) stem for maximizing saponin yield and antioxidant capacity. J. Food Process. Preserv. 2018, 42, e13597. [Google Scholar] [CrossRef]
- Matuszek, K.; Pankalla, E.; Grymel, A.; Latos, P.; Chrobok, A. Studies on the solubility of terephthalic acid in ionic liquids. Molecules 2019, 25, 80. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Villalobos, V.V.; Padilla, C.R.; Naccha, J.R.; Bracamonte, O.H.; Angulo, J.V.; Alama, O.B. Optimization of maca (Lepidium meyenii) glucosinolates extraction by genetic algorithms and response surface. Sci. Agropecu. 2016, 7, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F. Microwave-assisted extraction of saponin, phenolic and flavonoid compounds from Trigonella foenum-graecum seed based on two level factorial design. J. Appl. Res. Med. Aromat. Plants 2019, 14, 100212. [Google Scholar] [CrossRef]
| Independent Variables | Coded Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| x1 Temperature (°C) | 15 | 25 | 40 | 55 | 65 |
| x2 Sample:Solvent | 1:15 | 1:20 | 1:25 | 1:30 | 1:35 |
| x3 Ethanol (%) | 0 | 25 | 50 | 75 | 100 |
| Treatment | Temperature (°C) | Sample: Solvent | Ethanol (%) |
|---|---|---|---|
| 1 | 25 | 1:20 | 25 |
| 2 | 25 | 1:30 | 25 |
| 3 | 25 | 1:20 | 75 |
| 4 | 25 | 1:30 | 75 |
| 5 | 15 | 1:25 | 50 |
| 6 | 55 | 1:20 | 50 |
| 7 | 55 | 1:30 | 25 |
| 8 | 55 | 1:20 | 75 |
| 9 | 55 | 1:30 | 75 |
| 10 | 65 | 1:25 | 50 |
| 11 | 40 | 1:15 | 50 |
| 12 | 40 | 1:35 | 50 |
| 13 | 40 | 1:25 | 0 |
| 14 | 40 | 1:25 | 100 |
| Central Points | 40 | 1:25 | 50 |
| Treatment | Glucosinolate Concentration (mg/Kg DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucoiberin | Progoitrin | Glucoraphanin | 1-Hydroxy-3-indoylmethyl | 4-Hydroxy-glucobrassicin | Glucobrassicanapin | Glucoeurocin | Gluconasturtiin | 4-Methoxy-glucobrassicin | Neoglucobrassicin | Total | |
| 1 | 3113 ± 43 d,e | 18,366 ± 450 b,c,d | 7015 ± 86 e | 8489 ± 528 b,c | 16,024 ± 760 c,d | 1117 ± 75 e,f | 4141 ± 232 f | n.d. | 429 ± 63 c,d,e | 19 ± 14 e | 58,696 ± 1833 d,e |
| 2 | 3746 ± 134 b,c,d,e | 21,482 ± 1264 a,b | 7434 ± 322 d,e | 8475 ± 535 b,c | 17,004 ± 1098 c,d | 1280 ± 150 e | 4443 ± 305 e,f | n.d. | 346 ± 42 d,e,f | n.d. | 64,009 ± 3873 d,e |
| 3 | 4040 ± 253 b,c,d | 16,834 ± 783 b,c,d | 11,350 ± 884 b,c | 8970 ± 495 b,c | 20,028 ± 1190 b,c | 1658 ± 98 e | 6088 ± 362 c,d,e,f | 325 ± 27 c,d | 257 ± 8 e,f,g | 222 ± 31 b,c | 69,773 ± 4024 c,d,e |
| 4 | 3955 ± 359 b,c,d | 16,274 ± 1714 c,d | 10,285 ± 1123 b,c,d | 8339 ± 926 c | 18,561 ± 1982 c,d | 1406 ± 129 e | 5365 ± 556 c,d,e,f | 201 ± 52 d,e | 113 ± 33 g,h | 57 ± 34 e | 64,555 ± 6789 c,d,e |
| 5 | 4361 ± 171 a,b,c | 21,363 ± 184 a,b,c | 11,421 ± 701 b,c | 11480 ± 175 a,b | 24,573 ± 293 a,b | 3061 ± 217 c | 7261 ± 115 a,b,c | 467 ± 25 b,c | 434 ± 17 c,d,e | 262 ± 14 a,b | 84,682 ± 260 a,b,c |
| 6 | 2973 ± 288 de | 15,048 ± 618 d | 7040 ± 637 e | 6643 ± 462 c | 13,319 ± 910 d | 1023 ± 29 e,f | 4632 ± 309 d,e,f | 87 ± 19 e,f | 639 ± 14 b,c | 94 ± 15 d,e | 51,498 ± 2990 e |
| 7 | 3748 ± 317 b,c,d,e | 15,885 ± 1182 d | 7521 ± 1024 d,e | 6879 ± 461c | 14,661 ± 1028 c,d | 891 ± 98 e,f | 4271 ± 310 e,f | n.d. | 620 ± 44 b,c | n.d. | 54,441 ± 4320 d,e |
| 8 | 3529 ± 673 bcde | 15,650 ± 3670 d | 9648 ± 1945 c,d,e | 8428 ± 2106c | 18,814 ± 4615 c,d | 3109 ± 414 c | 6193 ± 1574 c,d,e | 553 ± 157 b | 518 ± 144 b,c,d | 229 ± 114 b,c | 66,670 ± 15221 c,d,e |
| 9 | 4343 ± 231 a,b,c | 17,853 ± 515 b,c,d | 11,734 ± 777 b,c | 9162 ± 296 b,c | 19,632 ± 650 b,c | 2980 ± 130 c,d | 6466 ± 197 b,c,d | 454 ± 24 b,c | 468 ± 14 b,c,d,e | 113 ± 30 c,d,e | 73,204 ± 2821 b,c,d |
| 10 | 4711 ± 566 a,b | 24,688 ± 2580 a | 13,352 ± 1566 a,b | 13615 ± 2028 a | 26,975 ± 3839 a | 1831 ± 330 d,e | 8813 ± 1353 a | 943 ± 125 a | 962 ± 124 a | 360 ± 86 a | 96,249 ± 12,550 a |
| 11 | 2635 ± 284 e | 23,692 ± 3443 a | 7114 ± 927 e | 12,537 ± 1902 a | 28,481 ± 2023 a | 7611 ± 930 a | 8902 ± 902 a | 622 ± 166 b | 677 ± 163 b | 208 ± 36 b,c,d | 92,480 ± 10271 a,b |
| 12 | 5505 ± 952 a | 24,320 ± 1815 a | 14,986 ± 1862 a | 12287 ± 935 a | 27,888 ± 1953 a | 7069 ± 804 a,b | 8229 ± 609 a,b | n.d. | 147 ± 94 f,g | n.d. | 100,094 ± 9016 a |
| 13 | 1374 ± 198 f | 329 ± 84 e | 74 ± 40 f | 442 ± 62 d | 317 ± 40 e | n.d. | n.d. | n.d. | 58 ± 20 g,h | n.d. | 2131 ± 430 f |
| 14 | 264 ± 46 f | 57 ± 19 e | 788 ± 134 f | 431 ± 79 d | 936 ± 92 e | n.d. | 173 ± 43 g | n.d. | n.d. | n.d. | 2269 ± 307 f |
| Central Points | 3201 ± 200 c,d,e | 16,232 ± 499 d | 10,056 ± 599 c,d,e | 9070 ± 86 b,c | 18,328 ± 701 c,d | 6392 ± 701 b | 6190 ± 164 c,d,e | 519 ± 45 b,c | 625 ± 35 b,c | 242 ± 18 a,b | 70,853 ± 1738 c,d,e |
| Total GLS Yield | Glucoraphanin Yield | |||
|---|---|---|---|---|
| Extraction 1 | 116,687 ± 5594 a | 85.20% | 29,724 ± 993 a | 83.90% |
| Extraction 2 | 14,228 ± 626 b | 8.10% | 2840 ± 201 b | 10.20% |
| Extraction 3 | 3926 ± 268 c | 2.70% | 956 ± 129 c | 2.80% |
| Extraction 4 | 2216 ± 145 c | 2.30% | 822 ± 35 c | 1.60% |
| Extraction 5 | 1959 ± 28 c | 1.50% | 532 ± 51 c | 1.40% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojorquez-Rodríguez, E.M.; Guajardo-Flores, D.; Jacobo-Velázquez, D.A.; Serna-Saldívar, S.O. Evaluation of the Effects of Process Conditions on the Extraction of Glucosinolates from Broccoli Sprouts. Horticulturae 2022, 8, 1090. https://doi.org/10.3390/horticulturae8111090
Bojorquez-Rodríguez EM, Guajardo-Flores D, Jacobo-Velázquez DA, Serna-Saldívar SO. Evaluation of the Effects of Process Conditions on the Extraction of Glucosinolates from Broccoli Sprouts. Horticulturae. 2022; 8(11):1090. https://doi.org/10.3390/horticulturae8111090
Chicago/Turabian StyleBojorquez-Rodríguez, Erika Melissa, Daniel Guajardo-Flores, Daniel A. Jacobo-Velázquez, and Sergio O. Serna-Saldívar. 2022. "Evaluation of the Effects of Process Conditions on the Extraction of Glucosinolates from Broccoli Sprouts" Horticulturae 8, no. 11: 1090. https://doi.org/10.3390/horticulturae8111090
APA StyleBojorquez-Rodríguez, E. M., Guajardo-Flores, D., Jacobo-Velázquez, D. A., & Serna-Saldívar, S. O. (2022). Evaluation of the Effects of Process Conditions on the Extraction of Glucosinolates from Broccoli Sprouts. Horticulturae, 8(11), 1090. https://doi.org/10.3390/horticulturae8111090








