Gibberellic Acid Concentrations and Storage of Caryocar brasiliense (Caryocaraceae) Seeds Propagated in Tubes
Abstract
1. Introduction
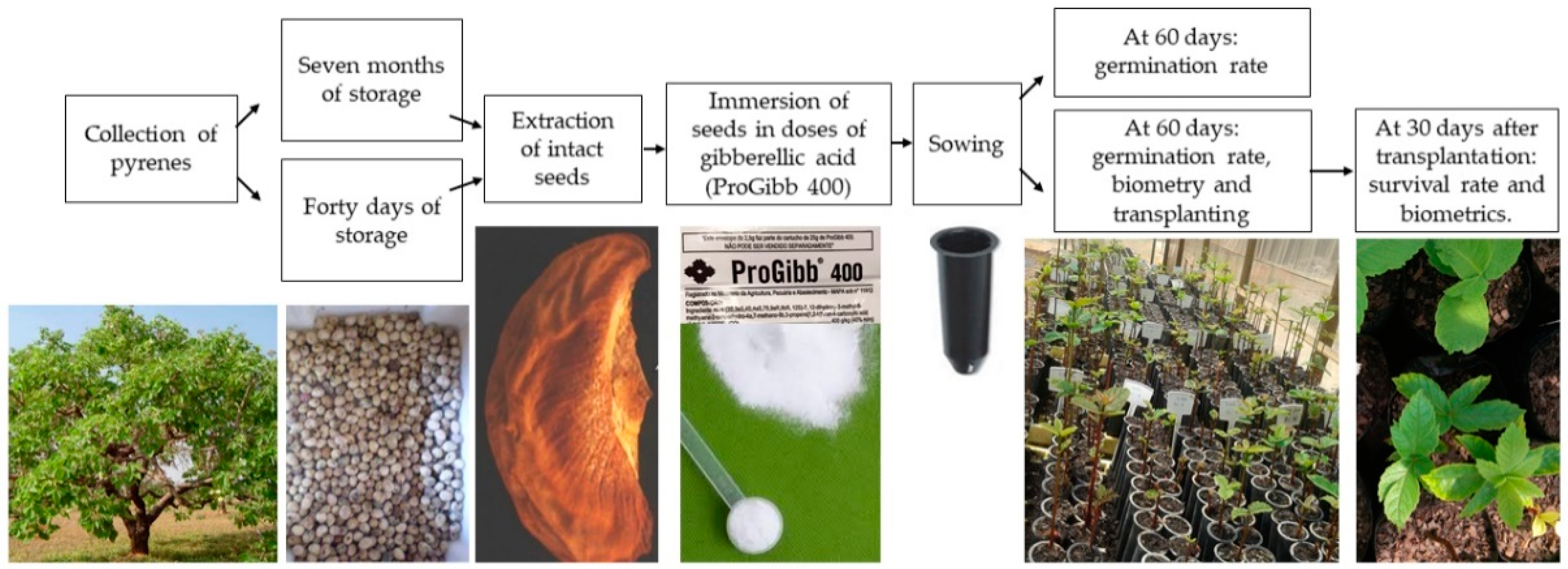
2. Materials and Methods
3. Results and Discussion
3.1. Seeds Stored for Seven Months
3.2. Seeds Stored for 40 Days
3.3. Analysis of Principal Components of the Parameters Evaluated in Seeds Stored for 40 Days
3.4. Parameters Evaluated after Transplanting to Bags
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, L.C.L.; Morais, L.M.O.; Guimarães, A.Q.; Almada, E.D.; Barbosa, P.M.; Drumond, M.A. Traditional knowledge and uses of the Caryocar brasiliense Cambess. (Pequi) by “quilombolas” of Minas Gerais, Brazil: Subsidies for sustainable management. Braz. J. Biol. 2016, 76, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.M.M.; Antoniassi, R.; De Faria-Machado, A.F. Pequi: A Brazilian fruit with potential uses for the fat industry. OCL 2017, 24, 507. [Google Scholar] [CrossRef]
- Lucillia, R.D.O.; Santana, F.C.; Torres-Leal, F.L.; Melo, I.L.; Yoshime, L.T.; Matos-Neto, E.M.; Mancini-Filho, J. Pequi (Caryocar brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: Antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef]
- Giroldo, A.B.; Scariot, A. Land use and management affects the demography and conservation of an intensively harvested Cerrado fruit tree species. Biol. Conserv. 2015, 191, 150–158. [Google Scholar] [CrossRef]
- Dombroski, J.L.D.; Paiva, R.; Alves, J.M.C.; Santos, B.R.; Nogueira, R.C.; de Oliveira Paiva, P.D.; Barbosa, S. Métodos para a superação da dormência fisiológica de Caryocar brasiliense Camb. Cerne 2010, 16, 131–135. [Google Scholar] [CrossRef][Green Version]
- Silva, R.S.; Ribeiro, L.M.; Mercadante-Simões, M.O.; Nunes, Y.R.F.; Lopes, P.S.N. Seed structure and germination in buriti (Mauritia flexuosa)—The swamp palm. Flora 2014, 209, 674–685. [Google Scholar] [CrossRef]
- Sousa, A.M.S.; Lopes, P.S.N.; Ribeiro, L.M.; Andrade, M.S.; Mercadante-Simões, M.O. Structural aspects of germination control in pyrenes of Caryocar brasiliense (Caryocaraceae). Trees 2017, 31, 1–16. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; p. 1600. [Google Scholar]
- Sousa, A.M.S.; Lopes, P.S.N.; Ribeiro, L.M.; Santiago, T.A.; Lacerda, V.R.; Martins, C.P.S. Germination and storage of Caryocar brasiliense seeds. Seed Sci. Technol. 2017, 45, 557–569. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia E Desenvolvimento Vegetal; Artmed Editora: Porto Alegre, Brazil, 2017. [Google Scholar]
- Dias, I.M.; Barreto, I.D.C.; Ferreira, R.A. Efeito de dosagens de fertilizantes fosfatado na determinação de volume ótimo de produção de mudas de espécies florestais nativas. Sci. Agr. Paran. 2016, 15, 471–475. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Regras Para Análise de Sementes; Mapa/ACS: Brasília, Brazil, 2009; p. 399.
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Oliveira, S.S. Efeito de Giberelina, Fungicida, Tratamentos Mecânicos e Período de Armazenamento Sobre a Germinação de Sementes de Pequizeiro. Master’s Thesis, Federal University of Mato Grosso, Cuiabá, Brazil, 2002. [Google Scholar]
- Barreto, L.C.; Garcia, Q.S.; Morales, M.; Müller, M.; Munné-Bosch, S. Vitamin E and defense-related phytohormones are reliable markers of embryo growth in macaw palm fruits exposed to various storage conditions. Plant Cell Tissue Organ Cult. 2014, 118, 203–213. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Bray, C.M.; West, C.E. The importance of safeguarding genome integrity in germination and seed longevity. J. Exp. Bot. 2015, 66, 3549–3558. [Google Scholar] [CrossRef]
- da Silva, G.C.; de-la-Cruz-Chacón, I.; Honório, A.B.M.; Martin, B.C.; Sousa, M.C.; Campos, F.G.; Boaro, C.S.F.; Ferreira, G. Temperature and GA3 as Modulating Factors in the Biosynthesis of Alkaloids during Imbibition and Early Development of Annona x atemoya Mabb. cv. ‘Gefner’ Seedlings. Horticulturae 2022, 8, 766. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Giordani, S.C.O.; dos Santos, P.H.R.; Titon, M.; Fernandes, J.E.S.C. Environmental and genetic concerns on genetic gains via selection in Pequi mother tree for seeds emergence. Afr. J. Agric. 2017, 12, 2140–2146. [Google Scholar] [CrossRef]
- Moura, N.F.; Chaves, L.Z.; Naves, R.V.; Aguiar, A.V.; Sobierajski, G.R. Variabilidade entre procedências e progênies de Pequizeiro (Caryocar brasiliense Camb.). Sci. For. For. Sci. 2013, 41, 103–112. Available online: https://www.alice.cnptia.embrapa.br/alice/bitstream/doc/964426/1/ANANDA.pdf (accessed on 29 September 2022).
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, 25504. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Claeys, H.; De Bodt, S.; Inzé, D. Gibberellins and DELLAs: Central nodes in growth regulatory networks. Trends Plant Sci. 2014, 19, 231–239. [Google Scholar] [CrossRef]
- Ferrera, T.S.; Pelissaro, T.M.; Eisinger, S.M.; Righi, E.Z.; Buriol, G.A. Fenologia de espécies nativas arbóreas na região central do estado do Rio Grande do Sul. Ciência Florest. 2017, 27, 753–766. [Google Scholar] [CrossRef]
- Gasparin, E.; de Avila, A.L.; Araujo, M.M.; Filho, A.C.; Dorneles, D.U.; Foltz, D.R.B. Influência do substrato e do volume de recipiente na qualidade das mudas de Cabralea canjerana (Vell.) Mart. em viveiro e no campo. Ciência Florest. 2014, 24, 553–563. [Google Scholar] [CrossRef]


| GA3 (mg L−1) | GSI | Germination (%) | Dead Seeds (%) |
|---|---|---|---|
| Dry seeds | 0.01 a* | 2.71 abc | 97.29 a |
| 0 | 0.01 a | 0.84 c | 99.16 a |
| 50 | 0.02 a | 4.17 ab | 95.83 a |
| 100 | 0.03 a | 6.04 a | 93.96 a |
| 500 | 0.02 a | 6.25 a | 93.75 a |
| 1000 | 0.01 a | 2.50 abc | 97.50 a |
| 2000 | 0.01 a | 1.67 bc | 98.33 a |
| Overall average | 0.01 | 3.45 | 96.54 |
| CV (%) | 1.17 | 26.95 | 3.73 |
| MSD | 0.01 | 0.97 | 6.44 |
| GA3 (mg L−1) | GSI | Germination (%) | Dead Seeds (%) | Number of Leaves | Leaf Area (cm²) | Stem Diameter (cm) |
|---|---|---|---|---|---|---|
| Dry seeds | 0.59 a* | 67.00 ab | 23.00 cd | 3.09 b | 8.03 b | 3.02 a |
| 0 | 0.73 a | 74.00 a | 20.00 d | 4.17 b | 9.90 ab | 3.10 a |
| 50 | 0.72 a | 64.00 ab | 27.00 bcd | 4.55 a | 11.20 ab | 3.10 a |
| 100 | 0.55 a | 47.00 b | 52.00 a | 4.52 a | 9.54 ab | 3.19 a |
| 500 | 0.82 a | 69.00 ab | 28.00 bcd | 4.80 a | 11.08 ab | 3.36 a |
| 1000 | 0.75 a | 60.00 ab | 38.00 abc | 4.70 a | 11.21 ab | 3.02 a |
| 2000 | 0.61 a | 55.00 ab | 41.00 ab | 4.50 a | 12.34 a | 2.93 a |
| Overall average | 0.68 | 62.28 | 32.71 | 4.33 | 10.35 | 3.10 |
| CV (%) | 19.06 | 10.29 | 10.92 | 14.02 | 14.83 | 11.51 |
| MSD | 0.03 | 1.64 | 1.26 | 1.50 | 3.84 | 0.56 |
| GA3 (mg L−1) | Number of Leaves | Leaf Area (cm²) | Stem Diameter (cm) | Stem Length (cm) | Survival Rate (%) |
|---|---|---|---|---|---|
| Dry seeds | 3.40 a* | 10.40 a | 3.60 a | 11.20 b | 78.57 a |
| 0 | 3.80 a | 10.40 a | 4.00 a | 14. 40 ab | 62.50 a |
| 50 | 3.80 a | 12.40 a | 3.80 a | 15.60 ab | 79.10 a |
| 100 | 4.20 a | 12.40 a | 3.80 a | 16.40 ab | 91.07 a |
| 500 | 3.80 a | 11.60 a | 3.60 a | 16.20 ab | 72.30 a |
| 1000 | 3.40 a | 10.20 a | 3.60 a | 17.60 ab | 63.15 a |
| 2000 | 3.40 a | 12.80 a | 3.40 a | 18.40 a | 63.04 a |
| CV (%) | 21.27 | 25.00 | 5.14 | 12.26 | 18.01 |
| MSD | 0.08 | 0.17 | 0.01 | 0.09 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerda, V.R.; Pagehú, L.F.; Gonçalves, A.P.; Vieites, R.L.; Lopes, P.S.N. Gibberellic Acid Concentrations and Storage of Caryocar brasiliense (Caryocaraceae) Seeds Propagated in Tubes. Horticulturae 2022, 8, 1094. https://doi.org/10.3390/horticulturae8111094
Lacerda VR, Pagehú LF, Gonçalves AP, Vieites RL, Lopes PSN. Gibberellic Acid Concentrations and Storage of Caryocar brasiliense (Caryocaraceae) Seeds Propagated in Tubes. Horticulturae. 2022; 8(11):1094. https://doi.org/10.3390/horticulturae8111094
Chicago/Turabian StyleLacerda, Vander Rocha, Levi Fraga Pagehú, Armando Pego Gonçalves, Rogério Lopes Vieites, and Paulo Sérgio Nascimento Lopes. 2022. "Gibberellic Acid Concentrations and Storage of Caryocar brasiliense (Caryocaraceae) Seeds Propagated in Tubes" Horticulturae 8, no. 11: 1094. https://doi.org/10.3390/horticulturae8111094
APA StyleLacerda, V. R., Pagehú, L. F., Gonçalves, A. P., Vieites, R. L., & Lopes, P. S. N. (2022). Gibberellic Acid Concentrations and Storage of Caryocar brasiliense (Caryocaraceae) Seeds Propagated in Tubes. Horticulturae, 8(11), 1094. https://doi.org/10.3390/horticulturae8111094







