Effect of Flower Development Stages on the Dynamics of Volatile Compounds in Ylang-Ylang (Cananga odorata) Essential Oil
Abstract
:1. Introduction
2. Materials and methods
2.1. Plant Material
2.2. Essential Oil Extraction
2.3. Density and Yield Measurements
2.4. GC-MS Analysis
2.5. Statistical Analysis
3. Results and discussion
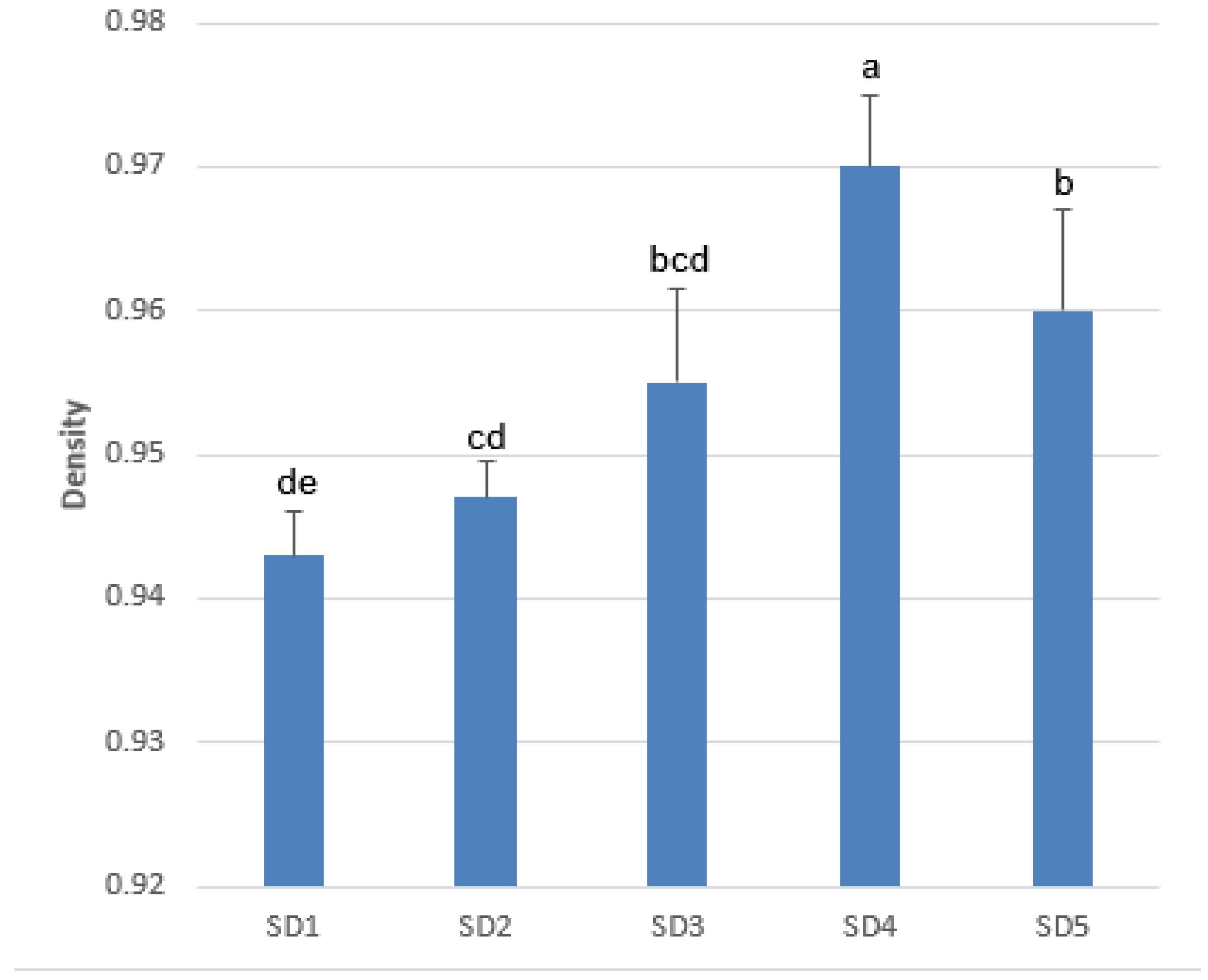
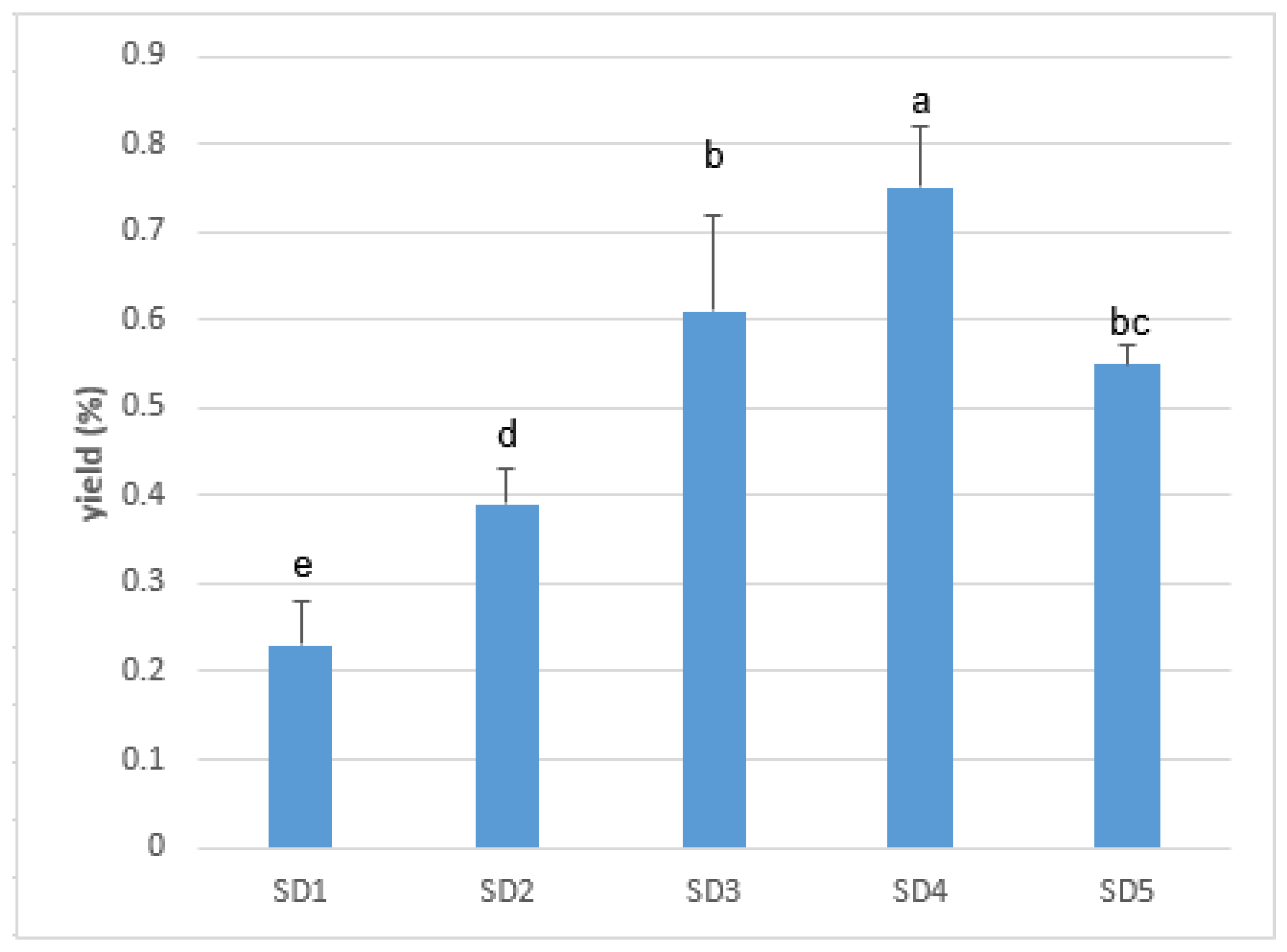
3.1. Density and Ylang-Ylang Essential Oil Extraction Yield
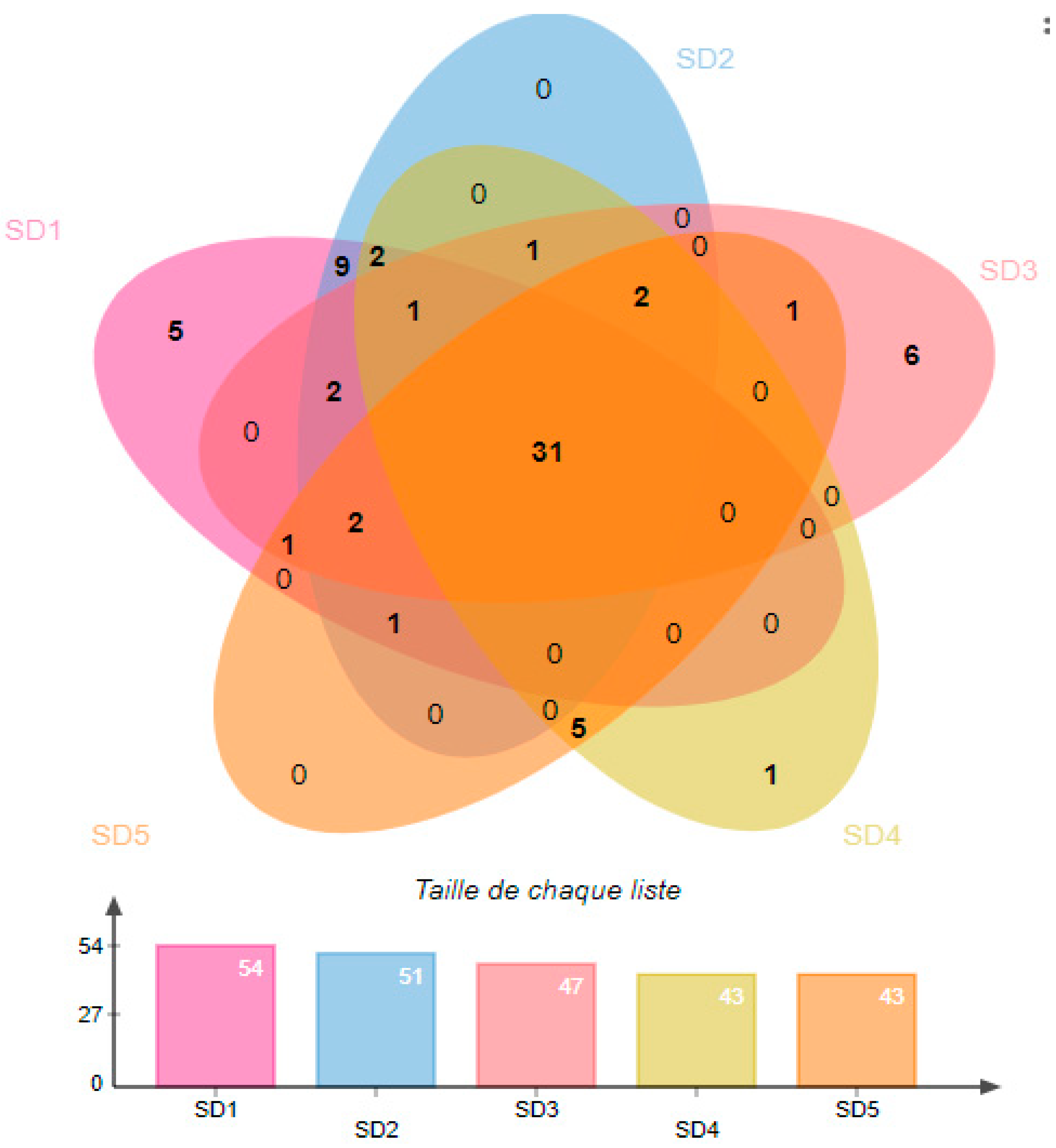
3.2. Diversity of Volatile Compounds in Ylang-Ylang Flowers
3.3. Evolution of Groups, Subgroups, and Oxygenated Compounds between Stages of Development
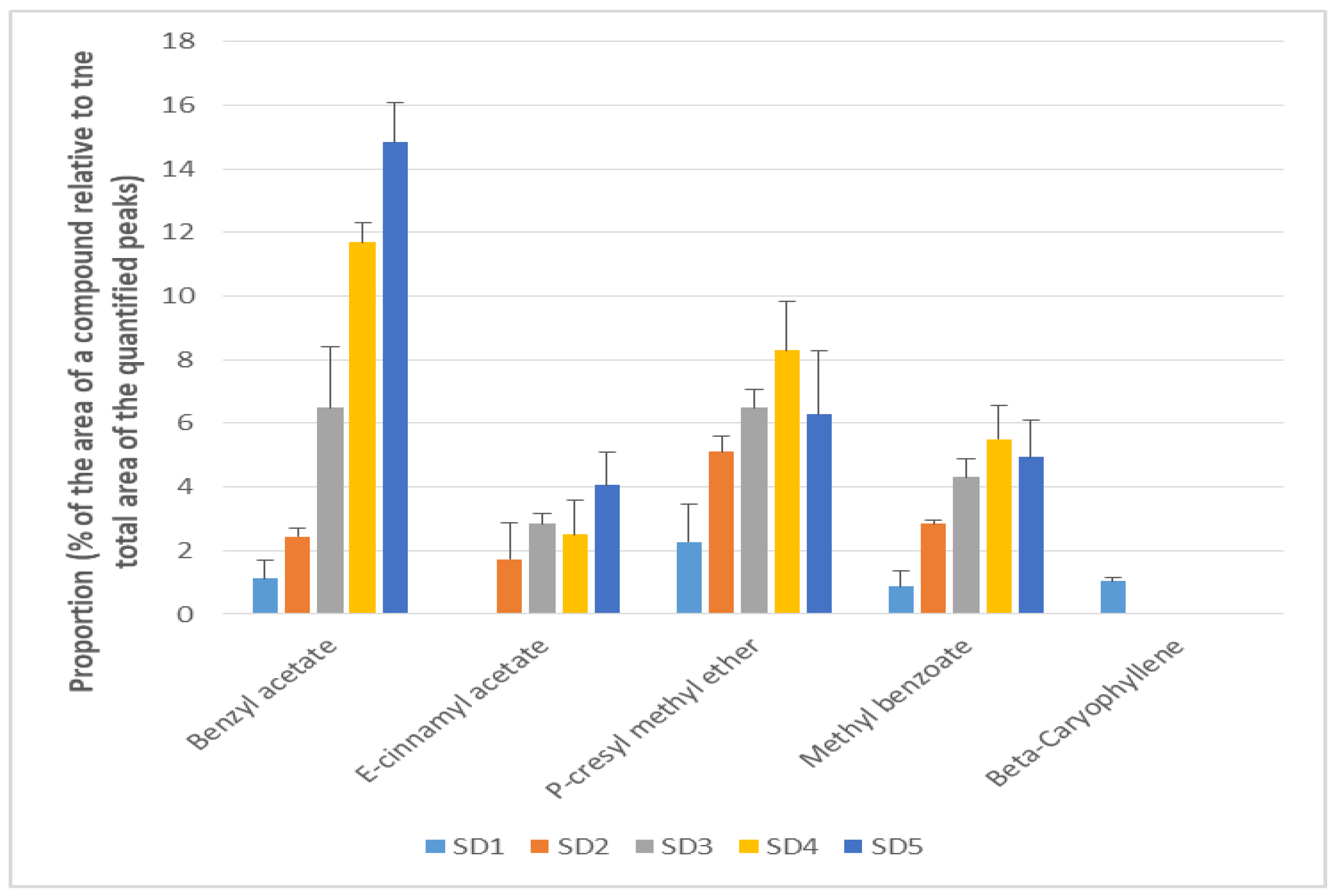
3.4. Evolution of Individual Volatile Compounds
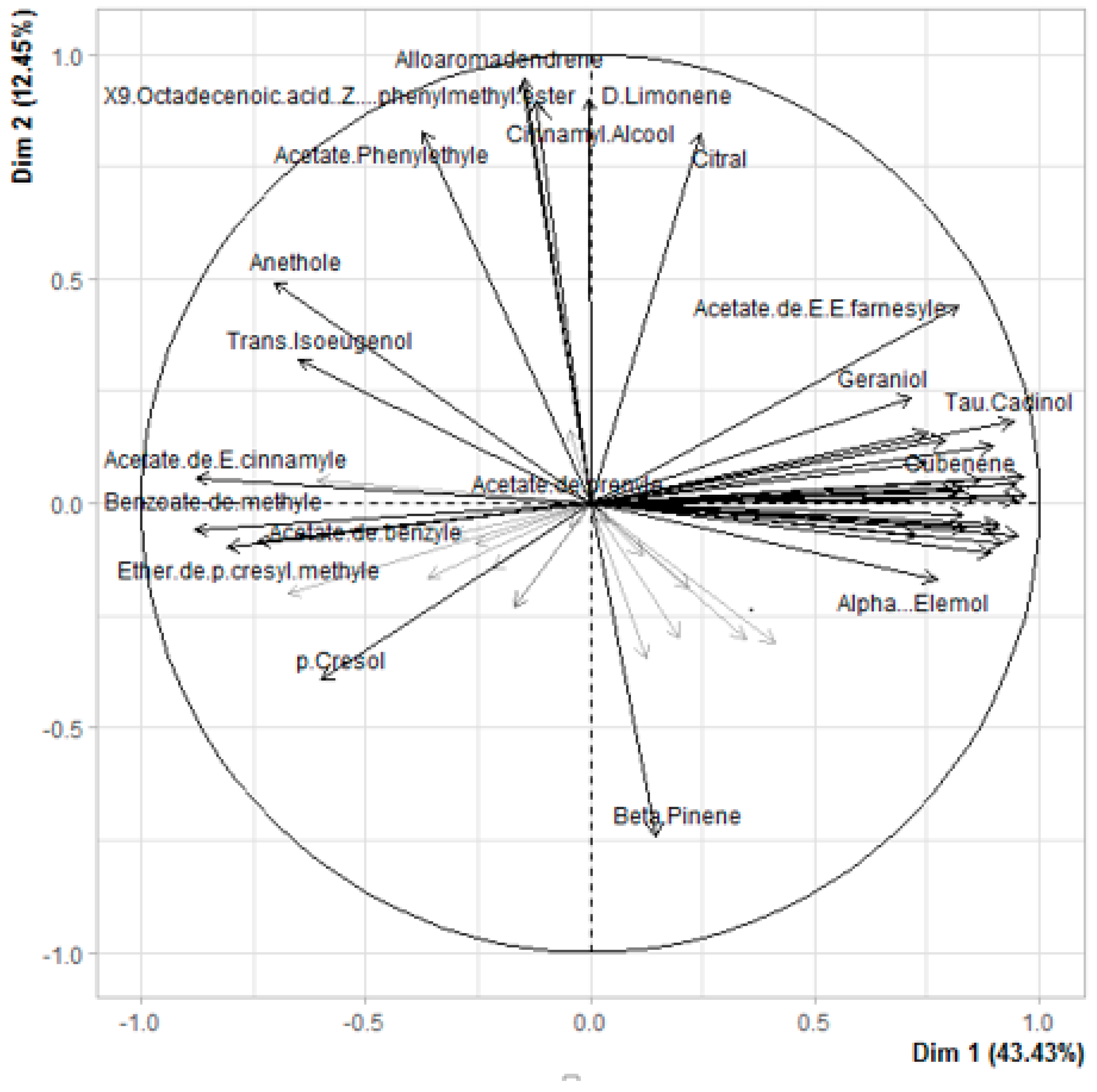
3.5. Principal Component Analysis of Maturity of Ylang-Ylang Flowers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Compounds | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Methyl benzoate | - | 0.81 *** | −0.75 *** | −0.95 *** | −0.21 | 0.52 * | −0.77 ***. | −0.71 ** | −0.83 *** | −0.94 *** | 0.67 ** | −0.71 ** | 0.92 *** | 0.77 *** |
| 2 | Benzyl acetate | 0.81 *** | - | −0.56 | −0.82 *** | 0.06 | 0.37 | −0.57 * | −0.66 ** | −0.75 *** | −0.78 *** | 0.30 | −0.89 | 0.62 * | 0.81 *** |
| 3 | Farnesyl acetate | −0.75 *** | −0.56 | - | 0.72 ** | 0.30 | −0.71 ** | 0.59 ** | 0.63 ** | 0.83 *** | 0.85 *** | −0.68 ** | 0.59 * | −0.78 *** | −0.56 * |
| 4 | Benzyl benzoate | −0.95 *** | −0.82 *** | 0.72 ** | - | 0.34 | −0.53 * | 0.64 ** | 0.63 ** | 0.79 *** | 0.95 *** | −0.70 ** | 0.65 ** | −0.83 *** | −0.68 ** |
| 5 | Benzyl salicylate | −0.21 | 0.06 | 0.30 | 0.34 | - | −0.24 | −0.01 | −0.08 | 0.04 | 0.24 | −0.60 ** | 0.18 | −0.34 | 0.34 |
| 6 | Geranyl acetate | 0.52 * | 0.37 | −0.71 ** | −0.53 * | −0.01 | - | −0.59 * | −0.27 | −0.61 * | −0.68 ** | 0.27 | −0.34 | 0.38 | 0.57 * |
| 7 | Beta-Caryophyllene | −0.77 *** | −0.57 * | 0.59 * | 0.64 ** | −0.24 | −0.59 * | - | 0.69 ** | 0.73 *** | 0.73 *** | −0.41 | 0.54* | −0.69 ** | −0.87 *** |
| 8 | D-Germacrene | −0.71 ** | −0.66 ** | 0.63 ** | 0.63 ** | −0.08 | −0.27 | 0.69 ** | - | 0.82 *** | 0.71 ** | −0.55 * | 0.64 ** | −0.66 ** | −0.69 ** |
| 9 | Apha-Farnesene | −0.83 *** | −0.75 *** | 0.83 *** | 0.79 *** | 0.04 | −0.61 ** | 0.73 *** | 0.82 *** | - | 0.86 *** | −0.63 ** | 0.72 ** | −0.77 *** | −0.78 *** |
| 10 | Farnesol | −0.94 *** | −0.78 *** | 0.85 *** | 0.95 *** | 0.24 | −0.68 ** | 0.73 *** | 0.71 ** | 0.86 *** | - | −0.70 ** | 0.66 ** | −0.83 *** | −0.73 *** |
| 11 | Linalool | 0.67 ** | 0.30 | −0.68 ** | −0.70 ** | −0.60 * | 0.27 | −0.41 | −0.55 * | −0.63 ** | −0.70 ** | - | −0.21 | 0.81 *** | 0.23 |
| 12 | Geraniol | −0.71 ** | −0.89 *** | 0.59 * | 0.65 ** | −0.18 | −0.34 | 0.54 * | 0.64 ** | 0.72 ** | 0.66 ** | −0.21 | - | −0.62 ** | −0.82 *** |
| 13 | P-cresyl methyl ether | 0.92 *** | 0.62 *** | −0.78 *** | −0.83 *** | −0.34 | 0.38 | −0.69 ** | −0.66 ** | −0.77 *** | 0.83 *** | −0.81 *** | −0.62 ** | - | 0.62 ** |
| 14 | E-cinnamyl acetate | 0.77 *** | 0.81 *** | −0.56 * | −0.68 ** | 0.34 | 0.57 * | −0.87 *** | −0.69 ** | −0.78 *** | −0.73 *** | 0.23 | −0.82 *** | 0.62 ** | - |
References
- Guenther, E. The Essential Oils; Van Nostrand Company, Inc.: New York, NY, USA, 1952; p. 5. [Google Scholar]
- Bocquel, B.O. Etudes des effets d’un traitement thermique sur la qualité (flaveur et couleur) de quelques plantes aromatiques: Basilic, Ocimum basilicum L.; Menthe, Mentha piperita, Persil, Petroselinum sativum Hoffm, Estragon, Artemesia, Dracunculus L. Ph.D. Dissertation, Université de Paris, Paris, France, July 1991. [Google Scholar]
- Stashenko, E.E.; Tores, W.; Morales, R.M. A study of the compositional variation of the essential oil of ylang-ylang (Cananga odorata HoK Fil. and Thomson, forma genuina) during flower development. J. High Resolut. Chromatogr. 1995, 18, 101–104. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, D.S. Headspace solid-phase microextraction for characterization of fragrances of lemon verbena (Aloysia triphylla) by gas chromatographye–mass spectrometry. J. Sep. Sci. 2004, 27, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Baroso, J.G.; Pedro, L.G.; Schefer, J.C. Factors affecting secondary metabolite production in plants: Volatiles components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Wang, L.M.; Li, M.T.; Jin, W.W.; Li, S.; Zhang, S.Q.; Yu, L.J. Variations in the components of Osmanthus fragrans Lour. essential oil at different stages of flowering. Food Chem. 2009, 114, 233–236. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Hamaidi, M.S.; Saidi, F.; Hakim, Y. Extraction, composition et propriétés physico-chimiques de l’huile essentielle du Géranium Rosat (Pelargonium graveolens L.) cultivé dans la plaine de Mitidja (Algérie). Nat. Tech. J. 2010, 3, 37–45. [Google Scholar]
- Benini, C.; Ringuet, M.; Wathelet, J.P.; Lognay, G.; du Jardin, P.; Fauconnier, M.L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2011, 27, 356–366. [Google Scholar] [CrossRef]
- Rodriguez-Solana, R.; Daferera, D.J.; Mitsi, C.; Trigas, P.; Polissiou, M.; Tarantili, P.A. Comparative chemotype determination of lamiaceae plants by means of GC-MS, FTIR, and dispersive-Raman spectroscopic techniques and GC-FID quantification. Ind. Crops Prod. 2014, 62, 22–33. [Google Scholar] [CrossRef]
- Baranauskaitė, J.; Jakštas, V.; Ivanauskas, L.; Kopustinskienė, D.M.; Drakšienė, G.; Masteikova, R.; Bernatonienė, J. Optimization of carvacrol, rosmarinic, oleanolic and ursolic acid extraction from oregano herbs (Origanum onites L. Origanum vulgare spp. hirtum and Origanum vulgare L.). Nat. Prod. Res. 2016, 30, 672–674. [Google Scholar] [CrossRef]
- Fyad, K.; Cheriti, A.; Bourmita, Y.; Belboukhari, N. Variabilité de la composition chimique des huiles essentielles de Coriandrum sativum L. et Pimpinella anisum L. au cours de développement végétatif. PhytoChem BioSub J. 2013, 7, 35–39. [Google Scholar]
- Gaspar, F.; Leeke, G. Essential oil from Origanum vulgare L. ssp. Virens (Hoffm. et Link) letswaart: Content, composition and distribution within the bracts. J. Essent. Oil Res. 2004, 16, 82–84. [Google Scholar] [CrossRef]
- Benini, C.; Danflous, J.P.; Wathelet, J.P.; du Jardin, P.; Fauconnier, M.L. L’ylang-ylang [Cananga odorata (Lam.) Hook.f. & Thomson]: Une plante à huile essentielle méconnue dans une filière en danger. Biotechnol. Agron. Soc. Environ. 2010, 14, 693–705. [Google Scholar]
- Dodeur, G. Biologie Florale et Gestion des Plantations D’ylang-ylang (Cananga odorata (Lamarck) J.D. Hoker & Thomson, Variété Genuina) à Mayotte; Faculté Universitaire des Sciences Agronomiques de Gembloux: Gembloux, Belgium, 2009. [Google Scholar]
- McGaw, D.R.; Watson, M.; Paltoo, V.; ChangYen, I.; Grannum, J. A comparison of methods for the extraction of the fragrance from ylang-ylang. In Proceedings of the Conference Paper: 6th International Symposium on Supercritical Fluids, University of the West Indies, St. Augustine, Trinidad and Tobago, 28–30 April 2003. [Google Scholar]
- Kowalski, R.; Sugier, D.; Sugier, P.; Kołodziej, B. Evaluation of the chemical composition of essential oils with respect to the maturity of flower heads of Arnica montana L. and Arnica chamissonis Less. cultivated for industry. Ind. Crop. Prod. 2015, 76, 857–865. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M. Variation in essential oil composition, anatomical, and antioxidant characteristics of Achillea filipendulina Lam. as affected by different phenological stages. J. Essent. Oil Res. 2021, 33, 283–298. [Google Scholar] [CrossRef]
- Olivero, J.; Gracia, T.; Payares, P.; Vivas, R.; Diaz, D.; Daza, E.; Paul, G. Molecular structure and gas chromatographic retention behavior of the components of ylang-ylang oil. J. Pharm. Sci. 1997, 86, 625–630. [Google Scholar] [CrossRef]
- Gaydou, E.M.; Randriamiharisoa, R.; Bianchini, J.P. Composition of the essential oil of Ylang-Ylang (Cananga odorata Hook Fil. & Thomson forma genuina) from Madagascar. J. Agric. Food Chem. 1986, 34, 481–487. [Google Scholar] [CrossRef]
- Benini, C.; Mahy, G.; Bizoux, J.P.; Wathelet, J.P.; du Jardin, P.; Brostaux, Y.; Fauconnier, M.L. Comparative chemical and molecular variability of Cananga odorata (Lam.) Hook. f. & Thomson forma genuina (Ylang-Ylang) in the Western Indian Ocean islands: Implication for valorization. Chem. Biodivers. 2012, 9, 1389–1402. [Google Scholar] [CrossRef]
- Brokl, M.; Fauconnier, M.L.; Benini, C.; Lognay, G.; du Jardin, P.; Focant, J.F. Improvement of Ylang-Ylang essential oil characterization by GC-GC-TOFMS. Molecules 2013, 18, 1783–1797. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.W.; Hao, C.-Y.; Lui, S.Z.; Wu, G.; Tan, L.H.; Xu, F.; Hu, R.S. Volatile Organic Compound Emissions from Different Stages of Cananga odorata Flower Development. Molecules 2014, 19, 8965–8980. [Google Scholar] [CrossRef] [Green Version]
- AFNOR (Association Française de Normalisation). Norme Française NF ISO 3063: Huile Essentielle d’ylang-ylang [Cananga Odorata (Lamarck) J.D. Hooker et Thomson Forma Genuina]; AFNOR: Paris, France, 2005. [Google Scholar]
- Schade, F.; Legge, R.L.; Thomson, J.E. Fragrance volatiles of developing and senescing carnation flowers. Phytochemistry 2001, 56, 703–710. [Google Scholar] [CrossRef]
- Watanabe, N.; Watanabe, S.; Nakajima, R.; Moon, J.-H.; Shimokihara, K.; Inagaki, J.; Etoh, H.; Asai, T.; Sakata, K.; Ina, K. Formation of flower fragrance compounds from their precursors by enzymatic action during flower opening. Biosc. Biotech. Biochem. 1993, 57, 1101–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loughrin, J.; Hamilton-Kemp, T.R.; Burton, H.R.; Anderson, R.A.; Hildebrand, D.F. Glycosidically bound volatile components of Nicotiana sylvestris and Nicotiana suaveolens flowers. Phytochemistry 1992, 31, 1537–1540. [Google Scholar] [CrossRef]
- Ackermann, I.E.; Banthorpe, V.; Fordham, W.D.; Kinder, J.P.; Poots, I. Β-Glucosides of aroma components from petals of Rosa species: Assay, occurrence, and biosynthetic implications. J. Plant Physiol. 1989, 134, 567–572. [Google Scholar] [CrossRef]
- Bischof-Deichnik, C.; Holtuijzen, J.; Stahl-Biskup, E. Multivariate statistical analysis of the essential oil composition of Thymus praecox Opizssppolytrichus (Kern. ex Borb,) Ron. colected in the Tyrolean Alps. Flavour Fragr. J. 2000, 15, 1–6. [Google Scholar] [CrossRef]
- Ali, I.B.; Zaouali, Y.; Bejaoui, A.; Boussaid, M. Variation of the Chemical Composition of Essential Oils in Tunisian Populations of Thymus algeriensis BOISS. et REUT. (Lamiaceae) and Implication for Conservation. Chem. Biodivers. 2010, 7, 1276–1289. [Google Scholar] [CrossRef]
- Honarvar, M.; Khosh-Khui, M.; Javidnia, K. Factors affecting essential oil quantity and quality of Damask rose in two regions of Southern Iran. Acta Horticult. 2010, 870, 241–248. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crop. Prod. 2013, 46, 311–316. [Google Scholar] [CrossRef]
- Munoz-Bertomeu, J.; Arrillaga, I.; Segura, J. Essential oil variation within and among natural populations of Lavandula latifolia and its relation to their ecological areas. Biochem. Syst. Ecol. 2007, 35, 479–488. [Google Scholar] [CrossRef]
- Temraz, A.; Cioni, P.L.; Flamini, G.; Braca, A. Chemical composition of the essential oil from Jasminum pubescens leaves and flowers. Nat. Prod. Commun. 2009, 4, 1729–1732. [Google Scholar] [CrossRef] [Green Version]
- Paolini, J.; Barboni, T.; Desjobert, J.M.; Djabou, N.; Muselli, A.; Costa, J. Chemical composition, intraspecies variation and seasonal variation in essential oils of Calendula arvensis L. Biochem. Syst. Ecol. 2010, 38, 865–874. [Google Scholar] [CrossRef]
- Dugo, G.; Bonaccorsi, I.; Sciarrone, D.; Costa, R.; Dugo, P.; Mondello, L.; Santi, L.; Fakhry, H.A. Charaterization of oils from the fruits leaves and flowers of the bitter orande tree. J. Essent. Oil Res. 2011, 23, 45–59. [Google Scholar] [CrossRef]
- Verdoonk, J.C.; Ric De Vos, C.H.; Verhoeven, H.A.; Haring, M.A.; van Tunen, A.J.; Schuurink, R.C. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 2003, 62, 997–1008. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Wan, Y.; Li, T.; Liu, X.; Sun, Z.; Li, Z. Volatile Organic Compounds Emissions from Luculia pinceana Flower and its Changes at Different Stages of Flower Development. Molecules 2016, 21, 531. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.F.; Brahim, Y.M.; Jacqueline, S.; Farid, C. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A 2006, 1112, 121–126. [Google Scholar] [CrossRef]
- Barman, M.; Mitra, A. Floral maturation and changing air temperatures influence scent volatiles biosynthesis and emission in Jasminum auriculatum Vahl. Environ. Exp. Bot. 2021, 181, 104296. [Google Scholar] [CrossRef]
- Barman, M.; Mitra, A. Temporal relationship between emitted and endogenous floral scent volatiles in summer-and winter-blooming Jasminum species. Physiol. Plant. 2019, 166, 946–959. [Google Scholar] [CrossRef]
- Sanimah, S.; Suri, R.; Azizun, R.N.; Hazniza, A.; Radzali, M.; Rusli, I.; Hassan, M.D. Volatile compounds of essential oil from different stages of Michelia alba (cempaka putih) flower development. J. Trop. Agric. Fd. Sc. 2008, 36, 109–119. [Google Scholar]
- Mohd-Hairul, A.R.; Namasivayam, P.; Lian, G.E.C.; Abdullah, J.O. Terpenoid, benzenoid, and phenylpropanoid compounds in the floral scent of Vanda Mimi Palmer. J. Plant Biol. 2010, 53, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Brulé, C.; Pecout, W. L’ylang-Ylang: Un Parfum Subtil; Arco-Charbot: Grasse, France; V.F. aromatique: Paris, France, 1995. [Google Scholar]
- Stashenko, E.E.; Prada, N.Q.; Martinez, J.R. HRGC/ FID/NPD and HRGC/MSD study of Colombian ylang-ylang (Cananga odorata) oils obtained by different extraction techniques. J. High Resolut. Chromatogr. 1996, 19, 353–358. [Google Scholar] [CrossRef]
- PAFR (Projet d’Appui aux Filières de Rentes). Perspectives D’avenir de L’Ylang-Ylang Aux Comores Selon Les Applications Dans la Parfumerie: Rapport Final; PAFR: Moroni, Comoros, 1998. [Google Scholar]
- Lago, S.; Rodríguez, H.; Soto, A. Arce, A. Deterpenation of citrus essential oil by liquid-liquid extraction with 1-alkyl-3-methylimidazolium bis (trifluoromethylsulfonyl) amide ionic liquids. J. Chem. Eng. Data 2011, 56, 1273–1281. [Google Scholar] [CrossRef]
- Salha, G.B.; Díaz, R.H.; Labidi, J.; Abderrabba, M. Deterpenation of Origanum majorana L. essential oil by reduced pressure steam distillation. Ind. Crop. Prod. 2017, 109, 116–122. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Agostini, F.; Muniz, L.A.R.; Pauletti, G.F. Fractioning of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016, 178, 90–94. [Google Scholar] [CrossRef]
- Perini, J.F.; Silvestre, W.P.; Agostini, F.; Toss, D.; Pauletti, G.F. Fractioning of Orange (Citrus sinensis L.) essential oil using vacuum fractional distillation. Sep. Sci. Technol. 2017, 52, 1397–1403. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Tobias, G.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Benini, C. Variabilité Morphologique, Génétique Et Chimique de Cananga Odorata (Lam.) Hok F. & Thomson Forma Genuina en Vue de L’Amélioration Qualitative de la Production de L’Huile Essentielle D’Ylang-Ylang Dans Les îles de l’ocean Indien (Thèse de Doctorat); Université de Liège-Gembloux Agro-Bio Tech: Gembloux, Belgium, 2013; p. 172. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2009. [Google Scholar]
- Dudareva, N.; Pichersky, E. Floral scent metabolic pathways: Their regulation and evolution. Biol. Flor. Scent. 2006, 55–78. [Google Scholar] [CrossRef]
- Oyama-Okubo, N.; Ando, T.; Watanabe, N.; Marchesi, E.; Uchida, K.; Nakayama, M. Emission mechanism of floral scent in Petunia axillaris. Biosci. Biotechnol. Biochem. 2005, 69, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Reuveni, M.; Sagi, Z.; Evnor, D.; Hetzroni, A. β-Glucosidase activity is involved in scent production in Narcissus flowers. Plant Sci. 1999, 147, 19–24. [Google Scholar] [CrossRef]

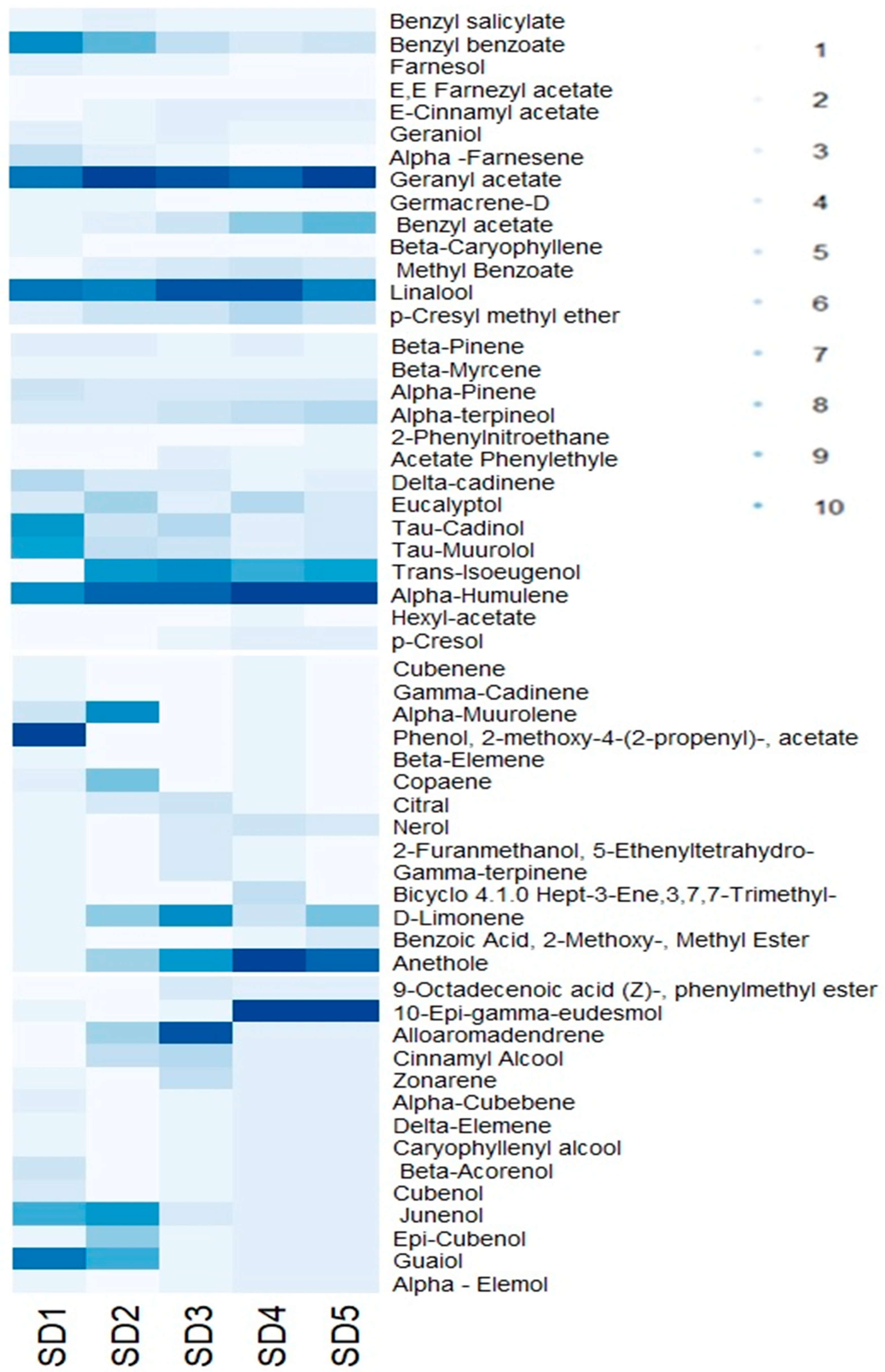

| Chemical Groups | Stage of Development | ||||
|---|---|---|---|---|---|
| SD1 | SD2 | SD3 | SD4 | SD5 | |
| Esters | 43.80 ± 6.87 abcd | 51.89 ± 8.53 bcde | 48.74± 7.62 cde | 52.81 ± 7.81 de | 59.14 ± 8.39 e |
| Alcohols | 33.98 ± 4.52 abcd | 28.22 ± 4.93 bcde | 32.52± 6.17 cde | 30.11 ± 6.25 de | 25.50 ± 5.03 e |
| Terpenes | 18.44 ± 1.58 a | 10.43 ± 1.18 bc | 8.43 ± 0.80 cde | 6.06 ± 0.67 de | 5.38 ± 0.66 e |
| Ether oxides | 2.33 ± 1.42 ab | 5.24 ± 2.74 bcde | 6.80 ± 3.39 cde | 8.57± 4.54 de | 6.56± 3.75 e |
| Phenols | 1.74 ± 2.94 a | 2.64 ± 0.55 bcde | 2.4 ± 1.0 cde | 2.09 ± 0.94 de | 2.14 ± 0.72 e |
| Oxides | 0.83 ± 0.37 a | 1.25 ± 0.23 a | 0.38 ± 0.62 a | 0.81 ± 0.44 a | 0.54 ± 0.42 a |
| Nitrogeneted compounds | - | - | - | 0.06 ± 0.06 a | 0.14 ± 0.09 a |
| Alcenes | - | - | 0.02 ± 0.03 a | 0.32 ± 0.39 d | 0.03 ± 0.05 a |
| Aldehydes | 0.10 ± 0.02 a | 0.82 ± 0.37 a | 0.13 ± 0.07 a | 0.09 ± 0.16 a | - |
| Chemical Subgroups | Stage of Development | ||||
|---|---|---|---|---|---|
| SD1 | SD2 | SD3 | SD4 | SD5 | |
| Sesquiterpenes alcohol | 33.08 ± 5.08 abcd | 27.51 ± 5.6 bcde | 31.53 ± 7.04 cde | 29.38 ± 7.13 de | 24.52 ± 5.74 e |
| Sesquiterpenes | 16.65 ± 1.76 a | 9.04 ± 1.34 bcde | 7.06 ± 0.91 cde | 4.42 ± 0.76 de | 4.44 ± 0.75 e |
| phenylpropanoïde | - | 2.34 ± 0.51 bcde | 2.18 ± 0.72 cde | 1.44 ± 0.20 de | 1.82 ± 0.54 e |
| Non-terpene ester | 25.23 ± 4.85 a | 24.81 ± 4.07 a | 22.89 ± 2.63 a | 27.39 ± 3.68 a | 32.64 ± 4.52 a |
| Monoterpenic Ester | 18.57 ± 5.71 a | 27.08 ± 1.87 a | 25.85 ± 6.29 a | 25.42 ± 4.86 a | 26.50 ± 2 a |
| Monoterpenes | 1.79 ± 0.34 a | 1.40 ± 0.18 a | 1.37 ± 0.13 a | 1.10 ± 0.17 a | 1.12 ± 0.12 a |
| Monoterpenic alcohols | 0.90 ± 0.44 a | 0.65 ± 0.38 a | 0.88 ± 0.39 a | 0.73 ± 0.38 a | 0.98 ± 0.50 a |
| Oxygenated Compounds | Stage of Development | ||||
|---|---|---|---|---|---|
| SD1 | SD2 | SD3 | SD4 | SD5 | |
| Light | 54.47 ± 5.31 a | 70.15 ±6.76 b | 80.39 ± 7.3 cde | 86.83 ± 7.52 d | 84.76 ±7.19 e |
| Heavy | 26.60 ± 4.24 a | 19.02 ±3.66 b | 10.70 ± 1.77 cde | 7.46 ± 1.28 d | 9.53 ± 1.60 e |
| Group | Subgroup | Compound | Stage of Development | ||||
|---|---|---|---|---|---|---|---|
| SD1 | SD2 | SD3 | SD4 | SD5 | |||
| Ester | Monoterpenic ester | Geranyl acetate * | 18.57 ± 5.71 a | 27.08 ± 1.87 a | 25.85 ± 6.29 a | 25.42 ± 4.86 a | 26.5 ± 2 a |
| Ester | Non-terpene ester | Benzyl acetate * | 1.12 ± 0.56 ab | 2.44 ± 0.28 b | 6.49 ± 1.92 c | 11.69 ± 0.61 d | 14.84 ± 1.26 e |
| Ester | Non-terpene ester | Benzyl benzoate * | 16.36 ± 1.75 ab | 13.92 ± 1.47 b | 6.72 ± 3.93 cde | 4.71 ± 2.45 de | 5.83 ± 3.14 e |
| Ester | Non-terpene ester | Methyl benzoate * | 0.87 ± 0.49 a | 2.85 ± 0.12 bc | 4.31 ± 0.59 cde | 5.5 ± 1.07 de | 4.94 ± 1.17 e |
| Ester | Non-terpene ester | E-cinnamyl acetate * | - | 1.73 ± 1.43 bcd | 2.85 ± 0.33 cde | 2.51 ± 1.07 de | 4.06 ± 1.03 e |
| Ester | Non-terpene ester | Benzyl salicylate * | 1.51 ± 0.42 a | 2.58 ± 0.81 a | 1.66 ± 0.95 a | 1.79 ± 1.22 a | 2.38 ± 1.75 a |
| Ester | Non-terpene ester | E,E, Farnezyl acetate * | 0.83 ± 0.46 a | 0.44 ± 0.12 a | 0.46 ± 0.31 a | 0.22 ± 0.1 a | 0.31 ± 0.23 a |
| Ester | Non-terpene ester | Benzoic acid, 2-methoxy-, methyl ester | - | - | - | 0.04 ± 0.06 de | 0.03 ± 0.05 e |
| Ester | Non-terpene ester | Phenol, 2-methoxy-4-(2-propenyl)-, acetate | 4.53 ± 1.23 a | - | - | - | - |
| Ester | Non-terpene ester | Acetate phenyl ethyle | 1.27 ± 2.19 a | 1.07 ± 1.85 a | 0.34 ± 0.47 a | 0.09 ± 0.09 a | 0.18 ± 0.07 a |
| Ester | Non-terpene ester | Acetic acid, hexyle ester | - | - | - | 0.11 ± 0.19 a | 0.11 ± 0.2 a |
| Ester | Non-terpene ester | Acetate Neryle | - | - | 0.02 ± 0.03 a | - | 0.03 ± 0.05 a |
| Ester | Non-terpene ester | Methyl-Anisoate | - | - | - | 0.04 ± 0.06 a | 0.05 ± 0.05 a |
| Ester | Non-terpene ester | 3-Hexen-1-ol, benzoate, (Z)- | 0.02 ± 0.03 a | 0.02 ± 0.01 a | - | - | 0.02 ± 0.03 a |
| Ester | Non-terpene ester | 9-Octadecenoic acid (Z)-, phenylmethyl ester | - | - | 0.06 ± 0.1 | - | - |
| Ester | Non-terpene ester | Geranyl benzoate | 0.03 ± 0.05 a | 0.02 ± 0.01 a | - | - | - |
| Alcohol | Sequiterpene alcohol | Geraniol * | 2.24 ± 1.84 abc | 2.07 ± 1.76 bc | 2.97 ± 0.15 c | 1.46 ± 1.19 de | 1.93 ± 0.8 e |
| Alcohol | Sequiterpene alcohol | Farnesol * | 2.84 ± 0.63 ab | 1.16 ± 0.97 bce | 1.09 ± 0.66 cde | 0.32 ± 0.29 d | 0.83 ± 0.5 ed |
| Alcohol | Sequiterpene alcohol | Linalool * | 18.88 ± 4.12 a | 20.5 ± 3.04 a | 25.69 ± 1.18 a | 25.92 ± 2.11 a | 20.9 ± 5.64 a |
| Alcohol | Sequiterpene alcohol | Tau-Cadinol | 2.96 ± 1.64 a | 0.59 ± 0.32 bcde | 0.96 ± 0.61 cde | 0.34 ± 0.07 de | 0.42 ± 0.19 e |
| Alcohol | Sequiterpene alcohol | Tau-Muurolol | 2.76 ± 1.35 a | 0.84 ± 0.06 bcde | 0.74 ± 0.61 cde | 0.19 ± 0.17 de | 0.43 ± 0.2 e |
| Alcohol | Sequiterpene alcohol | Guaiol | 0.84 ± 0.21 a | 0.07 ± 0.02 bcde | 0.03 ± 0.03 cde | 0.02 ± 0.0 de | 0.03 ± 0.01 e |
| Alcohol | Sequiterpene alcohol | Alpha-terpineol | 0.53 ± 0.47 a | 0.45 ± 0.41 a | 0.74 ± 0.13 a | 0.51 ± 0.48 a | 0.91 ± 0.25 a |
| Alcohol | Sequiterpene alcohol | Junenol | 0.58 ± 0.33 a | 0.05 ± 0.09 bcde | 0.05 ± 0.09 cde | 0.12 ± 0.21 de | 0.04 ± 0.04 e |
| Alcohol | Monoterpenic alcohol | Nerol | 0.33 ± 0.43 a | 0.21 ± 0.36 a | 0.07 ± 0.06 a | 0.71 ± 1.1 a | 0.06 ± 0.09 a |
| Alcohol | Sequiterpene alcohol | Alpha-Elemol | 0.08 ± 0.03 a | 0.01 ± 0.01 bcde | - | - | - |
| Alcohol | Sequiterpene alcohol | 10-Epi-γ-eudesmol | 0.09 ± 0.1 a | 0.03 ± 0.02 a | 0.02 ± 0.01 a | - | 0.02 ± 0.03 a |
| Alcohol | Sequiterpene alcohol | Cubenol | 0.17 ± 0.17 a | 0.06 ± 0.1 a | - | - | - |
| Alcohol | Sequiterpene alcohol | Epi-Cubenol | 0.09 ± 0.11 a | 0.04 ± 0.06 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.02 a |
| Alcohol | Sequiterpene alcohol | Caryophyllenyl alcohol | 0.06 ± 0.1 a | 0.04 ± 0.07 a | - | - | - |
| Alcohol | Sequiterpene alcohol | β-Acorenol | 0.25 ± 0.22 a | 0.31 ± 0.54 a | - | 0.51 ± 0.59 a | - |
| Alcohol | Sequiterpene alcohol | Cubebol | - | - | 0.03 ± 0.05 | - | - |
| Alcohol | Sequiterpene alcohol | Epi-Cubebol | 0.03 ± 0.05 | - | - | - | - |
| Alcohol | Sequiterpene alcohol | Trans-Nerolidol | 0.02 ± 0.03 | - | - | - | - |
| Alcohol | Non-terpene alcohol | Cinnamyl Alcohol | 0.02 ± 0.03 a | 0.03 ± 0.06 a | 0.11 ± 0.2 a | - | - |
| Alcohol | Non-terpene alcohol | 2-Furanmethanol, 5-Ethenyltetrahydro-α,α,5-Trimethyl-, Trans- | - | - | 0.07 ± 0.13 | - | - |
| hydrocarbon Terpene | Sesquiterpene | E,E, alpha-farnesene * | 6.05 ± 2.9 ab | 2.32 ± 1.85 bcd | 2.41 ± 0.82 cd | 0.94 ± 0.71 de | 1.16 ± 0.89 e |
| hydrocarbon Terpene | Sesquiterpene | Beta-Caryophyllene * | 1.04 ± 0.11 a | - | - | - | - |
| hydrocarbon Terpene | Sesquiterpene | GermacreneD * | 3.22 ± 1.83 abc | 1.65 ± 1.62 bcde | 0.9 ± 0.74 cde | 0.07 ± 0.12 de | 0.26 ± 0.16 e |
| hydrocarbon Terpene | Sesquiterpene | Alpha-Humulene | 3.08 ± 4.81 a | 3.65 ± 1.15 a | 2.71 ± 2.71 a | 2.28 ± 0.94 a | 2.69 ± 1.39 a |
| hydrocarbon Terpene | Sesquiterpene | Delta-Elemene | 0.07 ± 0.01 a | 0.06 ± 0.08 b | - | - | - |
| hydrocarbon Terpene | Sesquiterpene | Delta-cadinene | 1.38 ± 0.63 a | 0.36 ± 0.32 bcde | 0.25 ± 0.21 cde | 0.14 ± 0.12 de | 0.26 ± 0.11 e |
| hydrocarbon Terpene | Sesquiterpene | Copaene | 0.33 ± 0.31 ab | 0.13 ± 0.14 bcde | 0.04 ± 0.04 cde | 0.02 ± 0.01 de | 0.04 ± 0.08 e |
| hydrocarbon Terpene | Sesquiterpene | Alpha-Cubebene | 0.12 ± 0.06 a | - | - | - | - |
| hydrocarbon Terpene | Sesquiterpene | Ylangene | 0.02 ± 0.02 a | 0.02 ± 0.02 a | 0.02 ± 0.04 a | - | - |
| hydrocarbon Terpene | Sesquiterpene | Beta-Elemene | 0.16 ± 0.14 a | 0.03 ± 0.05 a | - | - | - |
| hydrocarbon Terpene | Sesquiterpene | Alloaromadendrene | - | 0.04 ± 0.07 a | 0.38 ± 0.66 a | 1.3 ± 2.25 a | - |
| hydrocarbon Terpene | Sesquiterpene | α-Muurolene | 0.78 ± 0.7 a | 1.17 ± 2.03 a | 0.25 ± 0.44 a | - | - |
| hydrocarbon Terpene | Sesquiterpene | Gamma-Cadinene | 0.16 ± 0.28 a | 0.26 ± 0.46 a | - | 0.08 ± 0.14 a | - |
| hydrocarbon Terpene | Sesquiterpene | Zonarene | 0.06 ± 0.07 a | 0.18 ± 0.31 a | 0.1 ± 0.17 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| hydrocarbon Terpene | Sesquiterpene | Cubenene | 0.15 ± 0.21 a | 0.02 ± 0.03 a | - | - | - |
| hydrocarbon Terpene | Sesquiterpene | Alpha-Cadinene | 0.03 ± 0.06 a | 0.02 ± 0.04 a | - | - | - |
| hydrocarbon Terpene | Monoterpene | Alpha-Pinene | 0.94 ± 0.58 a | 0.6 ± 0.03 a | 0.47 ± 0.09 a | 0 ± 0.1 a | 0.43 ± 0.04 a |
| hydrocarbon Terpene | Monoterpene | Beta-Myrcene | 0.27 ± 0.12 a | 0.3 ± 0.02 a | 0.23 ± 0.07 a | 0.17 ± 0.15 a | 0.17 ± 0.15 a |
| hydrocarbon Terpene | Monoterpene | Beta-Pinene | 0.4 ± 0.25 a | 0.35 ± 0.01 a | 0.23 ± 0.19 a | 0.33 ± 0.1 a | 0.25 ± 0.08 a |
| hydrocarbon Terpene | Monoterpene | D-Limonene | 0.18 ± 0.04 a | 0.15 ± 0.02 a | 0.36 ± 0.49 a | 0.05 ± 0.09 a | 0.15 ± 0.1 a |
| hydrocarbon Terpene | Monoterpene | Gamma-terpinene | 0.02 ± 0.03 a | - | 0.07 ± 0.12 a | - | - |
| Ether-oxyde | - | P-cresyl methyl ether * | 2.27 ± 1.18 ab | 5.11 ± 0.5 bcde | 6.48 ± 0.58 cde | 8.29 ± 1.93 de | 6.27 ± 2.9 e |
| Ether-oxyde | - | Anethole | 0.07 ± 0.06 ab | 0.1 ± 0.09 bcde | 0.32 ± 0.17 cde | 0.19 ± 0.17 de | 0.29 ± 0.09 e |
| Oxide | Monoterpenic oxide | Eucalyptol | 0.83 ± 0.37 a | 1.25 ± 0.23 a | 0.38 ± 0.62 a | 0.63 ± 0.59 a | 0.54 ± 0.42 a |
| Phenol | - | Trans-Isoeugenol | 1.7 ± 2.94 a | 2.42 ± 0.55 bcde | 2.18 ± 0.72 cde | 1.89 ± 0.73 de | 1.82 ± 0.64 e |
| Phenol | - | p-Cresol | - | 0.04 ± 0.03 a | 0.22 ± 0.28 a | 0.2 ± 0.21 a | 0.32 ± 0.08 a |
| Aldehyde | Monoterpenic aldehyde | Citral | 0.07 ± 0.07 a | 0.06 ± 0.06 a | 0.11 ± 0.2 a | 0.01 ± 0.01 a | - |
| Aldehyde | - | Trans-Trans-Farnesal | 0.03 ± 0.05 a | 0.76 ± 1.31 a | - | - | - |
| Aldehyde | - | Benzene bytanal | - | - | 0.02 ± 0.03 | - | - |
| Alcane | - | 2-Phenylnitroethane | - | - | - | 0.04 ± 0.07 a | 0.14 ± 0.09 a |
| Alcane | - | Heptacosane | - | - | 0.04 ± 0.07 | - | - |
| Alcene | - | Bicyclo [4.1.0] Hept-3-Ene, 3,7,7-Trimethyl | - | - | - | 0.3 ± 0.36 | - |
| Alcene | - | 4-Isopropenyl-1-Methyl-1-Cyclohexene | - | - | - | 0.02 ± 0.03 a | 0.03 ± 0.05 a |
| Alcene | - | Cyclohexene, 3-Methyl-6-(1-Methylethylidene) - | - | - | 0.02 ± 0.03 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakira, A.; Garcia, C.; Soria, C.; Minier, J.; Chillet, M. Effect of Flower Development Stages on the Dynamics of Volatile Compounds in Ylang-Ylang (Cananga odorata) Essential Oil. Horticulturae 2022, 8, 986. https://doi.org/10.3390/horticulturae8110986
Chakira A, Garcia C, Soria C, Minier J, Chillet M. Effect of Flower Development Stages on the Dynamics of Volatile Compounds in Ylang-Ylang (Cananga odorata) Essential Oil. Horticulturae. 2022; 8(11):986. https://doi.org/10.3390/horticulturae8110986
Chicago/Turabian StyleChakira, Abacar, Cyrielle Garcia, Christian Soria, Jérôme Minier, and Marc Chillet. 2022. "Effect of Flower Development Stages on the Dynamics of Volatile Compounds in Ylang-Ylang (Cananga odorata) Essential Oil" Horticulturae 8, no. 11: 986. https://doi.org/10.3390/horticulturae8110986
APA StyleChakira, A., Garcia, C., Soria, C., Minier, J., & Chillet, M. (2022). Effect of Flower Development Stages on the Dynamics of Volatile Compounds in Ylang-Ylang (Cananga odorata) Essential Oil. Horticulturae, 8(11), 986. https://doi.org/10.3390/horticulturae8110986







