Comparative Study on Genome Size and Phytochemical Profile of Three Potential Species of Acacia: Threatened and Endemic to Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seeds Collection
2.2. Pot Experiment
2.3. Buffer Preparation for Nuclei Extraction
2.4. Nuclei Extraction and Staining
2.5. Flow Cytometric Analysis
2.6. Extraction of Phytochemicals
2.6.1. Determination of Total Phenolic Contents (TPCs)
2.6.2. Determination of the Total Flavonoid Content (TFCs)
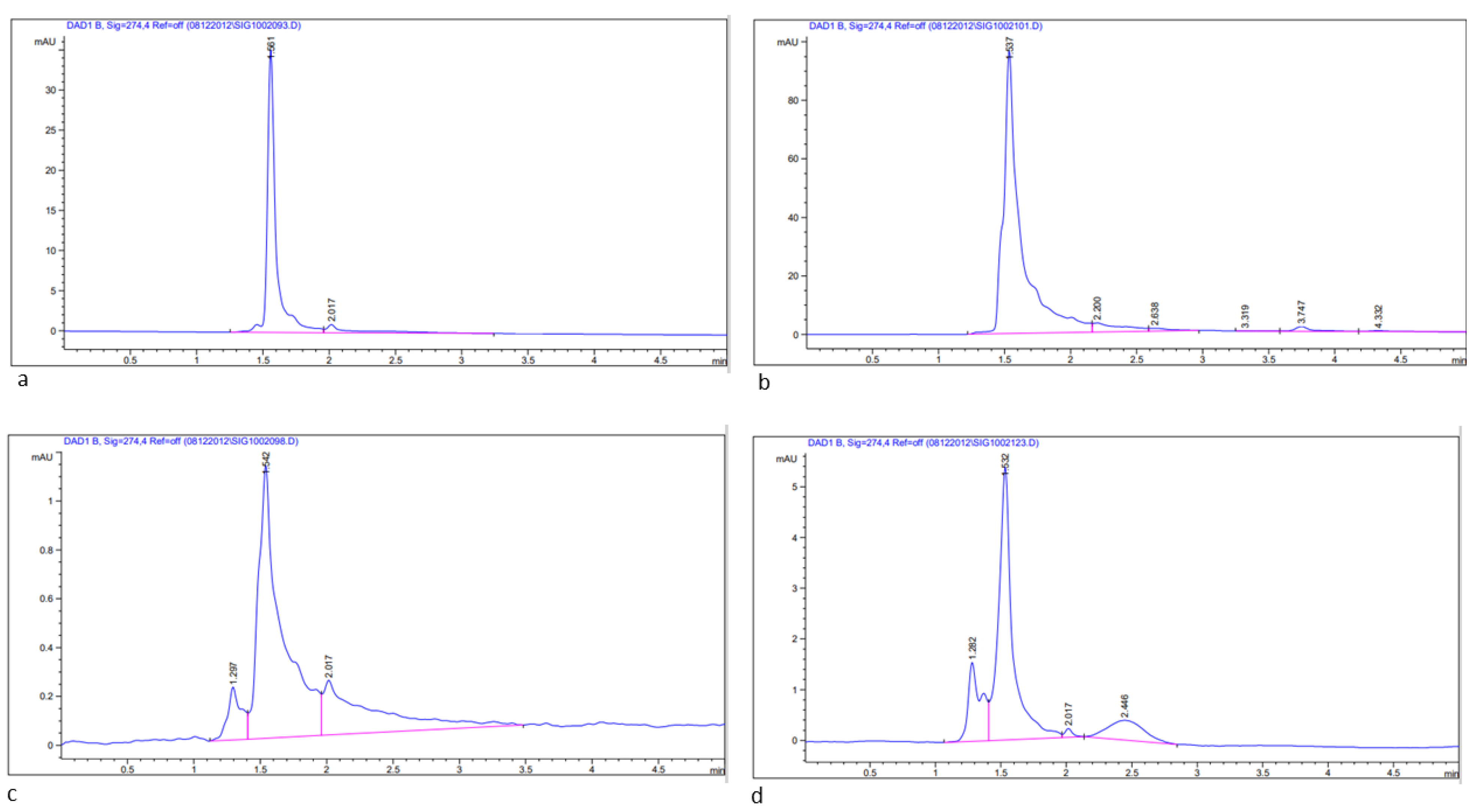
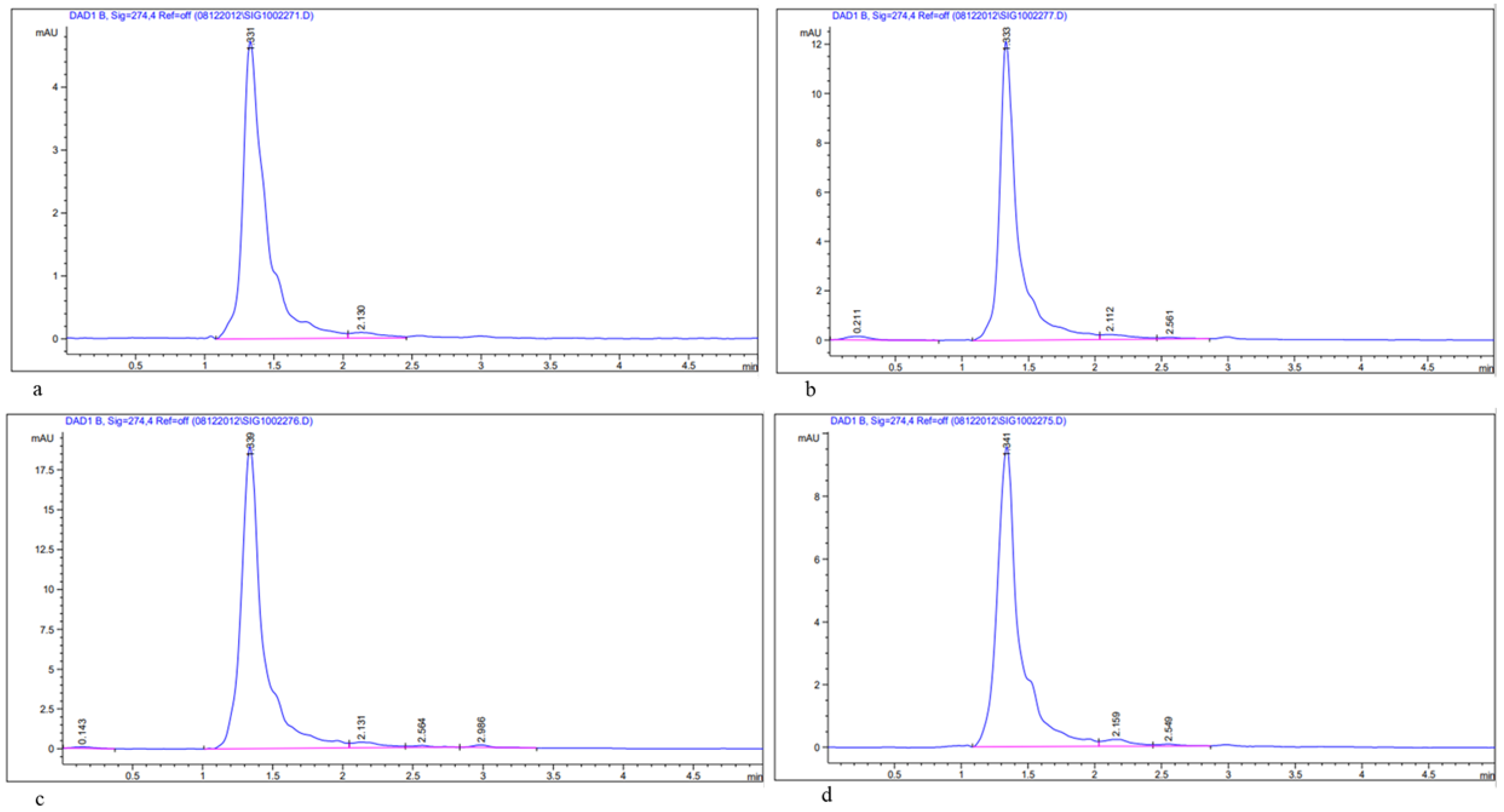
2.6.3. Estimation of Bioactive Compounds, Quercetin and Rutin in Methanolic Leaf Extract of Acacias Using HPLC
2.7. Functional Groups Characterization by FTIR
2.8. Statistical Analysis
3. Results
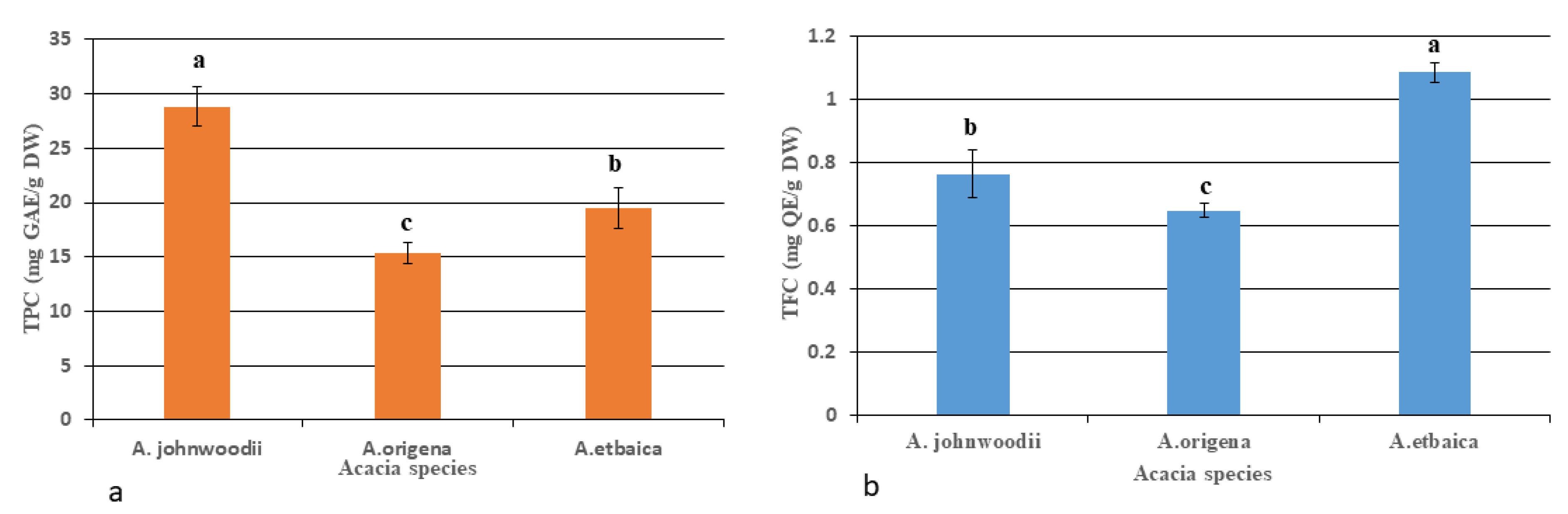
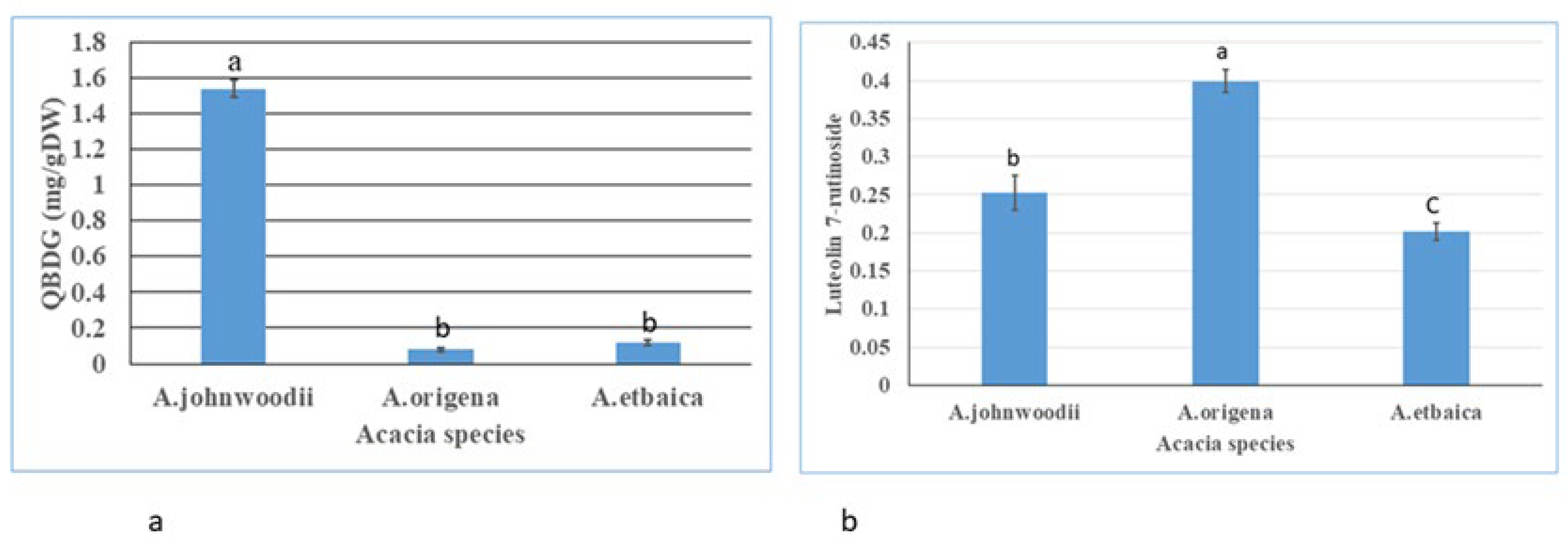
3.1. Determination of Total Flavonoids (TFCs), Total Phenols (TPCs), and Bioactive Compounds
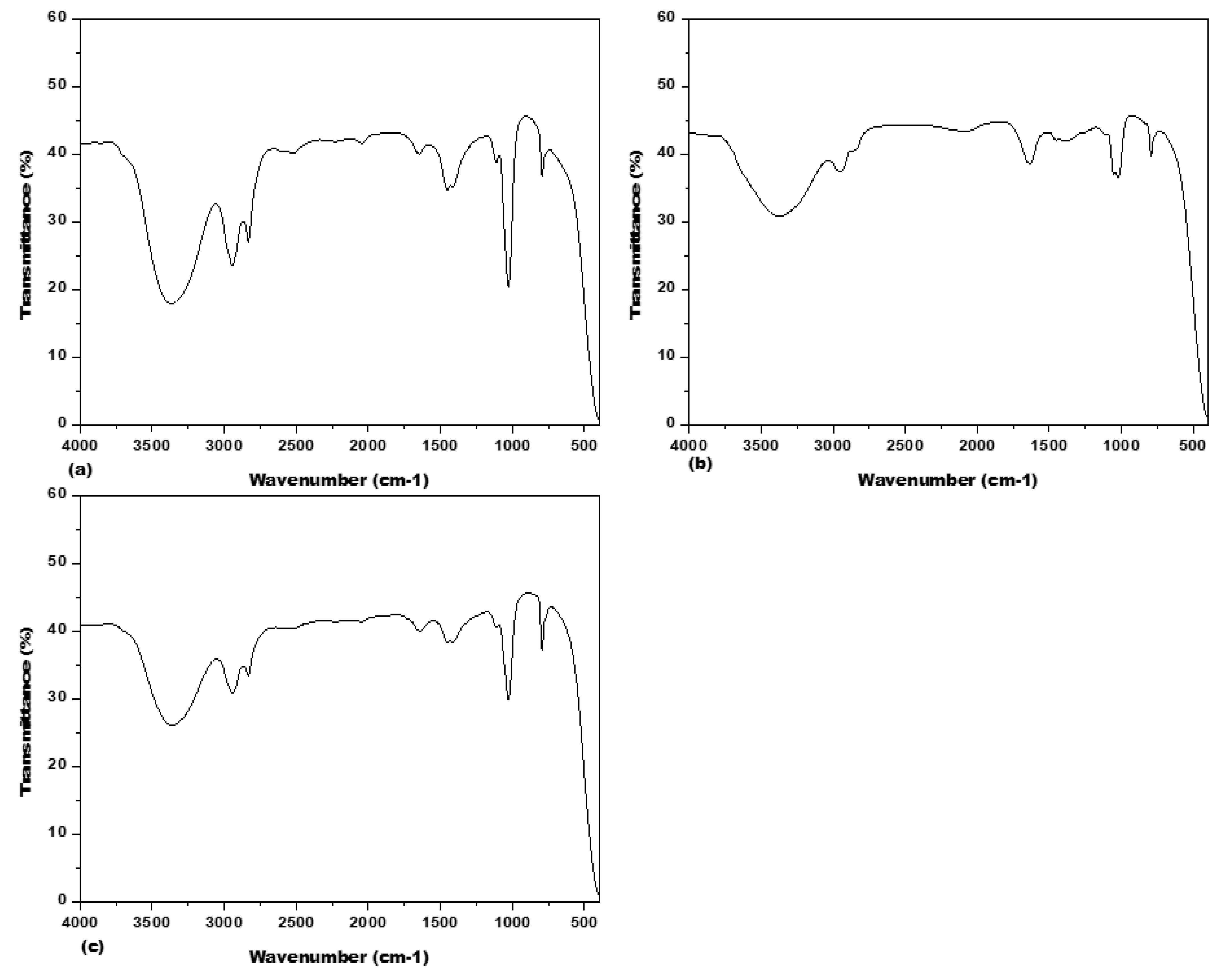
3.2. Fourier Transform Infrared Spectrometer (FTIR) Spectrum Analysis
4. Discussion
4.1. Genome Size (2C DNA Content) Variation within and among Acacias
4.2. Phytochemicals Profiling in Acacias
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orchard, A.E.; Maslin, B.R. (1584) Proposal to conserve the name Acacia (Leguminosae: Mimosoideae) with a conserved type. Taxon 2003, 52, 362–363. [Google Scholar] [CrossRef]
- Maslin, B.; Miller, J.; Seigler, D. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Austral. Syst. Botany 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Javed Muhammad, A.; Abdullah, M.Z.; Muhammad, N.; Ratnam, W. Detecting mislabeling and identifying unique progeny in Acacia mapping population using SNP markers. J. For. Res. 2017, 28, 1119–1127. [Google Scholar] [CrossRef]
- Muhammad, A.J.; Ong, S.S.; Ratnam, W. Characterization of mean stem density, fibre length and lignin from two Acacia species and their hybrid. J. For. Res. 2018, 29, 549–555. [Google Scholar] [CrossRef]
- Migahid, A.M. Flora of Saudi Arabia, 3rd ed.; Riyadh University Publication: Riyadh, Saudi Arabia, 1990. [Google Scholar]
- Collenette, S. Illustrated Guide to the Flowers of Saudi Arabia; Scorpion: Hampshire, UK, 1985. [Google Scholar]
- Al-Mefarrej, H. Diversity and frequency of Acacia spp. in three regions in the Kingdom of Saudi Arabia. Afr. J. Biotechnol. 2012, 11, 11420–11430. [Google Scholar] [CrossRef]
- Greilhuber, J.; Doležel, J.; Lysák, M.A.; Bennett, M.D. The origin, evolution and proposed stabilization of the terms ‘genome size’and ‘C-value’to describe nuclear DNA contents. Ann. Botany 2005, 95, 255–260. [Google Scholar] [CrossRef]
- Bennett, M.D.; Leitch, I.J. Nuclear DNA amounts in angiosperms: Targets, trends and tomorrow. Ann. Botany 2011, 107, 467–590. [Google Scholar] [CrossRef]
- Greilhuber, J.; Borsch, T.; Müller, K.; Worberg, A.; Porembski, S.; Barthlott, W. Smallest angiosperm genomes found in Lentibulariaceae, with chromosomes of bacterial size. Plant Biol. 2006, 8, 770–777. [Google Scholar] [CrossRef]
- Bennett, M.; Leitch, I. Plant DNA C-Values Database; (release 4.0, Dec. 2005); Royal Botanic Gardens: Kew, UK, 2005. [Google Scholar]
- Vinogradov, A.E. Selfish DNA is maladaptive: Evidence from the plant Red List. Trends Genet. 2003, 19, 609–614. [Google Scholar] [CrossRef]
- Knight, C.A.; Molinari, N.A.; Petrov, D.A. The large genome constraint hypothesis: Evolution, ecology and phenotype. Ann. Botany 2005, 95, 177–190. [Google Scholar] [CrossRef]
- Galbraith, D.W. Cytometry and plant sciences: A personal retrospective. Cytometry Part A J. Int. Soc. Anal. Cytol. 2004, 58, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, J.; Clermont, S.; Houston, L.; Rich, T.C.; Fay, M.F. Cytotype diversity in the Sorbus complex (Rosaceae) in Britain: Sorting out the puzzle. Ann. Botany 2012, 110, 1185–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żabicka, J.; Migdałek, G.; Słomka, A.; Sliwinska, E.; Mackiewicz, L.; Keczyński, A.; Kuta, E. Interspecific hybridization and introgression influence biodiversity—Based on genetic diversity of Central European Viola epipsila-V. palustris complex. Diversity 2020, 12, 321. [Google Scholar] [CrossRef]
- Levin, J.; Fay, M.F.; Pellicer, J.; Hedrén, M. Multiple independent origins of intermediate species between Sorbus aucuparia and S. hybrida (Rosaceae) in the Baltic region. Nordic J. Botany 2018, 36. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef]
- Pfosser, M.; Heberle-Bors, E.; Amon, A.; Lelley, T. Evaluation of sensitivity of flow cytometry in detecting aneuploidy in wheat using disomic and ditelosomic wheat–rye addition lines. Cytometry J. Int. So. Anal. Cytol. 1995, 21, 387–393. [Google Scholar] [CrossRef]
- Arumuganathan, K.; Earle, E. Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol. Biol. Report. 1991, 9, 229–241. [Google Scholar] [CrossRef]
- Doležel, J.; Binarová, P.; Lucretti, S. Analysis of nuclear DNA content in plant cells by flow cytometry. Biology 1989, 31, 113–120. [Google Scholar]
- Sadhu, A.; Bhadra, S.; Bandyopadhyay, M. Characterization of Tulbaghia violacea (Tulbaghieae, Allioideae, Amaryllidaceae) from India: A cytogenetic and molecular approach. Nucleus 2018, 61, 29–34. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- Arts, I.C.; Sesink, A.L.; Faassen-Peters, M.; Hollman, P.C. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. 2004, 91, 841–847. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Formica, J.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxidd Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Arroyo, S.; Camps, J.; Menendez, J.A.; Joven, J. Managing hypertension by polyphenols. Planta Med. 2015, 81, 624–629. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Libuy, M.; Feliu, F.; Hasson, D. Polyphenols in disease: From diet to supplements. Curr. Pharm. Biotechnol. 2014, 15, 304–317. [Google Scholar] [CrossRef]
- Mocanu, M.-M.; Nagy, P.; Szöllősi, J. Chemoprevention of breast cancer by dietary polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef] [PubMed]
- Al-Qurainy, F.; Tarroum, M.; Khan, S.; Nadeem, M.; Gaafar, A.-R.Z.; Alansi, S.; Alfarraj, N.S. Genome Estimation and Phytochemical Compound Identification in the Leaves and Callus of Abrus precatorius: A Locally Endangered Plant from the Flora of Saudi Arabia. Plants 2022, 11, 567. [Google Scholar] [CrossRef]
- Smith, J.; Heslop-Harrison, J. Nuclear DNA amounts in angiosperms. Philos. Trans. Roy. Soc. London B 1976, 274, 227–274. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protocols 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.; Gomez, J.; Vattuone, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Pyšek, P.; Lučanová, M.; Wigginton, S.; Tran, C.T.; Cronin, J.T. Plant genome size influences stress tolerance of invasive and native plants via plasticity. Ecosphere 2020, 11, e03145. [Google Scholar] [CrossRef]
- Pyšek, P.; Skálová, H.; Čuda, J.; Guo, W.Y.; Suda, J.; Doležal, J.; Kauzál, O.; Lambertini, C.; Lučanová, M.; Mandáková, T. Small genome separates native and invasive populations in an ecologically important cosmopolitan grass. Ecology 2018, 99, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Meyerson, L.A.; Cronin, J.T.; Bhattarai, G.P.; Brix, H.; Lambertini, C.; Lučanová, M.; Rinehart, S.; Suda, J.; Pyšek, P. Do ploidy level and nuclear genome size and latitude of origin modify the expression of Phragmites australis traits and interactions with herbivores? Biol. Invas. 2016, 18, 2531–2549. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Moles, A.T.; Leitch, I.J.; Bennett, M.D.; Dickie, J.B.; Knight, C.A. Correlated evolution of genome size and seed mass. New Phytol. 2007, 173, 422. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.M.; Leitch, I.J.; Knight, C.A. Genome size evolution in relation to leaf strategy and metabolic rates revisited. Ann. Botany 2007, 99, 495–505. [Google Scholar] [CrossRef]
- Morgan, H.D.; Westoby, M. The relationship between nuclear DNA content and leaf strategy in seed plants. Ann. Botany 2005, 96, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Ackerly, D.D. Variation in nuclear DNA content across environmental gradients: A quantile regression analysis. Ecol. Lett. 2002, 5, 66–76. [Google Scholar] [CrossRef]
- Greilhuber, J. Intraspecific variation in genome size in angiosperms: Identifying its existence. Ann. Botany 2005, 95, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lysák, M.A.; Rostková, A.; Dixon, J.M.; Rossi, G.; Doležel, J. Limited genome size variation in Sesleria albicans. Ann. Botany 2000, 86, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Cullis, C.A. Mechanisms and control of rapid genomic changes in flax. Ann. Botany 2005, 95, 201–206. [Google Scholar] [CrossRef]
- Pellicer, J.; López-Pujol, J.; Aixarch, M.; Garnatje, T.; Vallès, J.; Hidalgo, O. Detecting introgressed populations in the Iberian endemic Centaurea podospermifolia through genome size. Plants 2021, 10, 1492. [Google Scholar] [CrossRef]
- Chen, W.; Kao, Y.; Tang, C.; Tsai, C.; Lin, T. Estimating nuclear DNA content within 50 species of the genus Phalaenopsis Blume (Orchidaceae). Sci. Hortic. 2013, 161, 70–75. [Google Scholar] [CrossRef]
- Siljak-Yakovlev, S.; Stevanovic, V.; Tomasevic, M.; Brown, S.C.; Stevanovic, B. Genome size variation and polyploidy in the resurrection plant genus Ramonda: Cytogeography of living fossils. Environ. Exp. Botany 2008, 62, 101–112. [Google Scholar] [CrossRef]
- El Ferchichi Ouarda, H.; Walker, D.J.; Khouja, M.L.; Correal, E. Diversity analysis of Acacia tortilis (Forsk.) Hayne ssp. raddiana (Savi) Brenan (Mimosaceae) using phenotypic traits, chromosome counting and DNA content approaches. Gen. Resour. Crop Evol. 2009, 56, 1001–1010. [Google Scholar] [CrossRef]
- Bennett, M.D.; Leitch, I.J. Genome size evolution in plants. In The Evolution of the Genome; Elsevier: Amsterdam, The Netherlands, 2005; pp. 89–162. [Google Scholar]
- Soltis, P.S.; Marchant, D.B.; Van de Peer, Y.; Soltis, D.E. Polyploidy and genome evolution in plants. Curr. Opin. Gen. Dev. 2015, 35, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Carta, A.; Peruzzi, L. Testing the large genome constraint hypothesis: Plant traits, habitat and climate seasonality in L. iliaceae. New Phytol. 2016, 210, 709–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacho, N.I.; McIntyre, P.J.; Kliebenstein, D.J.; Strauss, S.Y. Genome size evolution is associated with climate seasonality and glucosinolates, but not life history, soil nutrients or range size, across a clade of mustards. Ann. Botany 2021, 127, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Guignard, M.S.; Crawley, M.J.; Kovalenko, D.; Nichols, R.A.; Trimmer, M.; Leitch, A.R.; Leitch, I.J. Interactions between plant genome size, nutrients and herbivory by rabbits, molluscs and insects on a temperate grassland. Proc. R. Soc. B 2019, 286, 20182619. [Google Scholar] [CrossRef] [Green Version]
- Edger, P.P.; Heidel-Fischer, H.M.; Bekaert, M.; Rota, J.; Glöckner, G.; Platts, A.E.; Heckel, D.G.; Der, J.P.; Wafula, E.K.; Tang, M. The butterfly plant arms-race escalated by gene and genome duplications. Proc. Natl. Acad. Sci. USA 2015, 112, 8362–8366. [Google Scholar] [CrossRef] [Green Version]
- Noirot, M.; Barre, P.; Duperray, C.; Louarn, J.; Hamon, S. Effects of caffeine and chlorogenic acid on propidium iodide accessibility to DNA: Consequences on genome size evaluation in coffee tree. Ann. Botany 2003, 92, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, J.; Rodriguez, E.; DOLEŽEL, J.; Santos, C. Flow cytometric and microscopic analysis of the effect of tannic acid on plant nuclei and estimation of DNA content. Ann. Botany 2006, 98, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Inbaraj, B.S.; Lu, H.; Kao, T.; Chen, B. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC–DAD–ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef]
- Bennett, M.D.; Price, H.J.; Johnston, J.S. Anthocyanin inhibits propidium iodide DNA fluorescence in Euphorbia pulcherrima: Implications for genome size variation and flow cytometry. Ann. Botany 2008, 101, 777–790. [Google Scholar] [CrossRef]
- Greilhuber, J.; Temsch, E.M.; Loureiro, J.C. Nuclear DNA content measurement. In Flow Cytometry with Plant Cells: Analysis of Genes, Chromosomes and Genomes; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 67–101. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Loureiro, J.; Rodriguez, E.; Doležel, J.; Santos, C. Two new nuclear isolation buffers for plant DNA flow cytometry: A test with 37 species. Ann. Botany 2007, 100, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Sun, S.; Zhao, Z.; Leung, H. A rapid method for identification of genus Lycium by FTIR spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2004, 24, 679–681. [Google Scholar] [PubMed]
- Cao, Z.; Liu, Y.; Zhao, J. Efficient discrimination of some moss species by fourier transform infrared spectroscopy and chemometrics. J. Spectr. 2014, 2014, 191796. [Google Scholar] [CrossRef] [Green Version]
- Depciuch, J.; Kasprzyk, I.; Drzymała, E.; Parlinska-Wojtan, M. Identification of birch pollen species using FTIR spectroscopy. Aerobiologia 2018, 34, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, Z.; Shang, Z.; Zhao, J. Classification and identification of Rhodobryum roseum Limpr. and its adulterants based on fourier-transform infrared spectroscopy (FTIR) and chemometrics. PLoS ONE 2017, 12, e0172359. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, T.; Rajasekaran, C.; Suthindhiran, K.; Mathew, L. Free radical scavenging, cytotoxic and hemolytic activities from leaves of Acacia nilotica (L.) Wild. ex. Delile subsp. indica (Benth.) Brenan. Evid.-Based Complem. Alter. Med. 2011, 2011, 274741. [Google Scholar] [CrossRef] [Green Version]
- Jelassi, A.; El Ayeb-Zakhama, A.; Nejma, A.B.; Chaari, A.; Harzallah-Skhiri, F.; Jannet, H.B. Phytochemical composition and allelopathic potential of three Tunisian Acacia species. Ind. Crops Prod. 2016, 83, 339–345. [Google Scholar] [CrossRef]
- Kaššák, P. Total flavonoids and phenolics content of the chosen genus Iris species. Acta Univ. Agric. Silviculturae Mendelianae Brunensis 2013, 60, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Drouet, S.; Garros, L.; Lorenzo, J.M.; Hano, C. Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (GX Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl. Antioxidants 2022, 11, 952. [Google Scholar]
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. J. Agri. Food Chem. 1999, 47, 4649–4652. [Google Scholar] [CrossRef]
- Shalashvili, A.; Rakviashvili, N. Rutin and luteolin 7-rutinoside from the leaves ofCitrus unshiu. Chem. Nat. Comp. 1984, 20, 621–622. [Google Scholar] [CrossRef]
- Ip, P.; Sharda, P.R.; Cunningham, A.; Chakrabartty, S.; Pande, V.; Chakrabartty, A. Quercitrin and quercetin 3-β-d-glucoside as chemical chaperones for the A4V SOD1 ALS-causing mutant. Protein Eng. Des. Selec. 2017, 30, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic composition of artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antognoni, F.; Zheng, S.; Pagnucco, C.; Baraldi, R.; Poli, F.; Biondi, S. Induction of flavonoid production by UV-B radiation in Passiflora quadrangularis callus cultures. Fitoterapia 2007, 78, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Rieger, G.; Muller, M.; Guttenberger, H.; Bucar, F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef]
- Yao, X.-H.; Zhang, Z.-B.; Song, P.; Hao, J.-Y.; Zhang, D.-Y.; Zhang, Y.-F. Different harvest seasons modify bioactive compounds and antioxidant activities of Pyrola incarnata. Ind. Crops Prod. 2016, 94, 405–412. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; de Macêdo, D.G.; Boligon, A.A.; Menezes, I.R.A.; de Almeida Souza, M.M.; da Costa, J.G.M. Influence of seasonality on the phenolic composition of Secondatia floribunda A. DC (Apocynaceae) during its phenological cycle. Acta Physiol. Plant. 2019, 41, 1–16. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Gabr, S.; Nikles, S.; Wenzig, E.M.P.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Sulaiman, C.; Balachandran, I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J. Pharm. Sci. 2012, 74, 258. [Google Scholar] [CrossRef] [Green Version]
- Verick Purba, B.A.; Sunarti, S.; Lukmandaru, G. Phenolics content and antioxidant activity of wood extractives from three clones of Acacia hybrid (Acacia mangium × Acacia auriculiformis). Maderas. Cien. Tecnol. 2021, 23. [Google Scholar] [CrossRef]

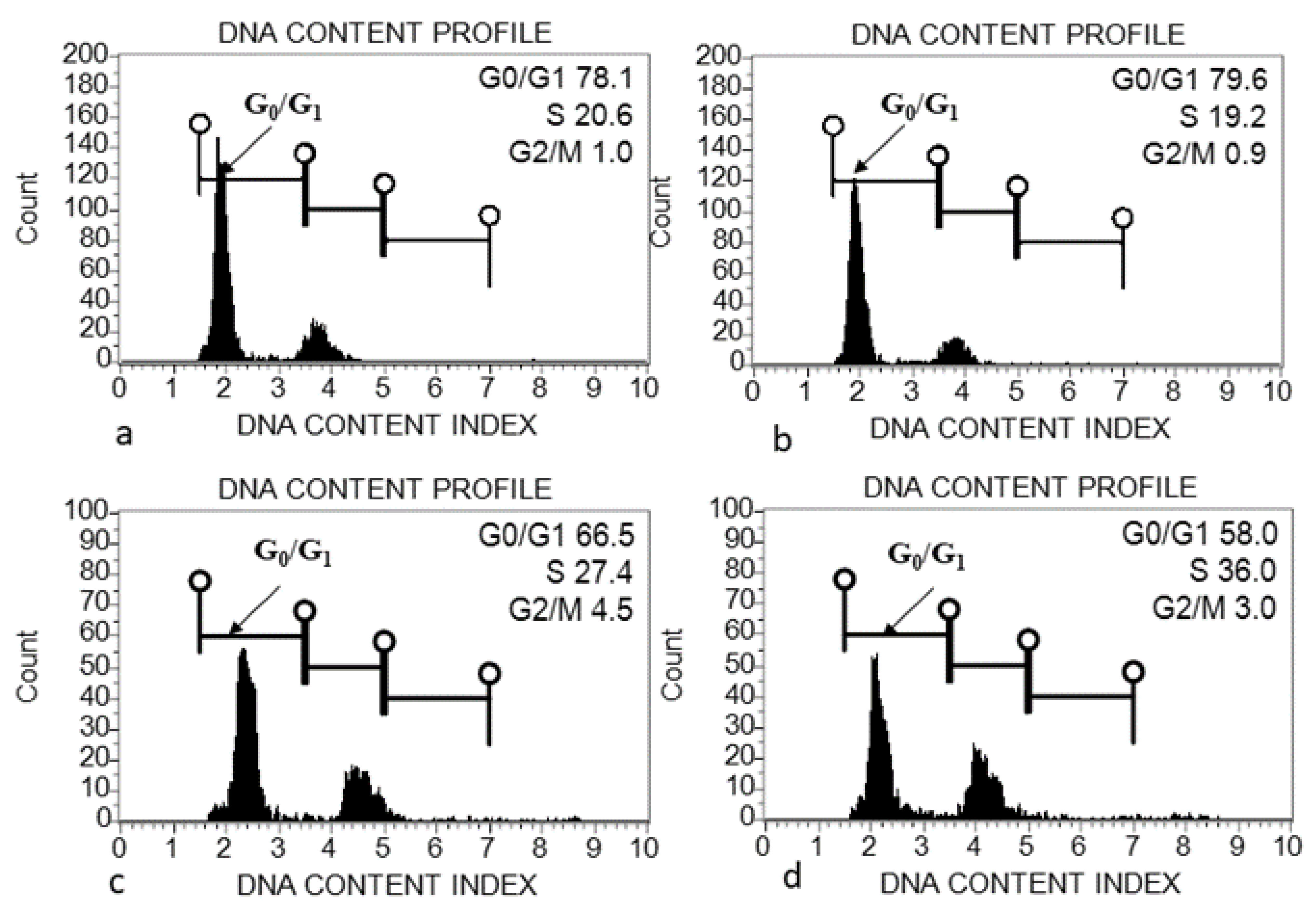
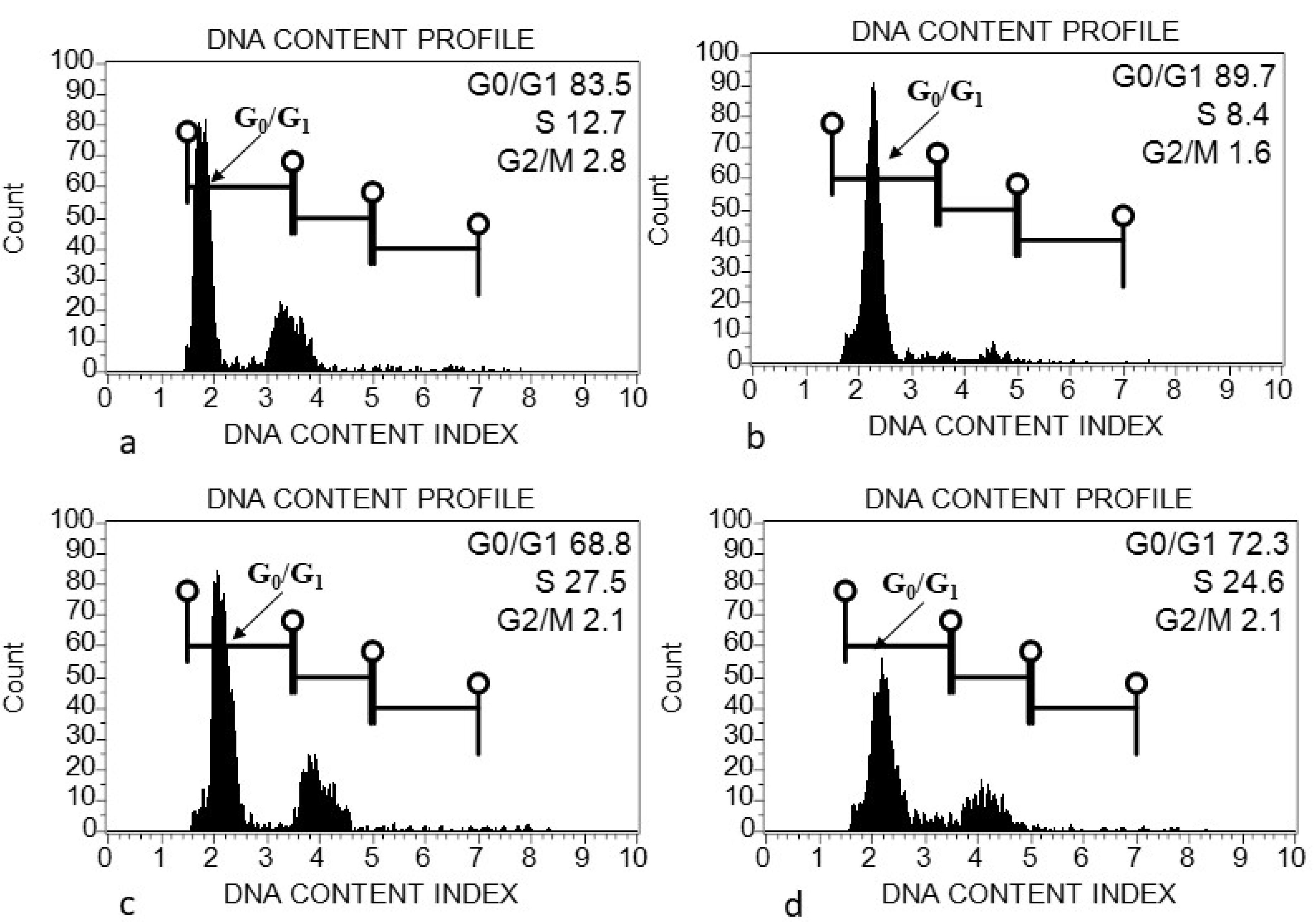
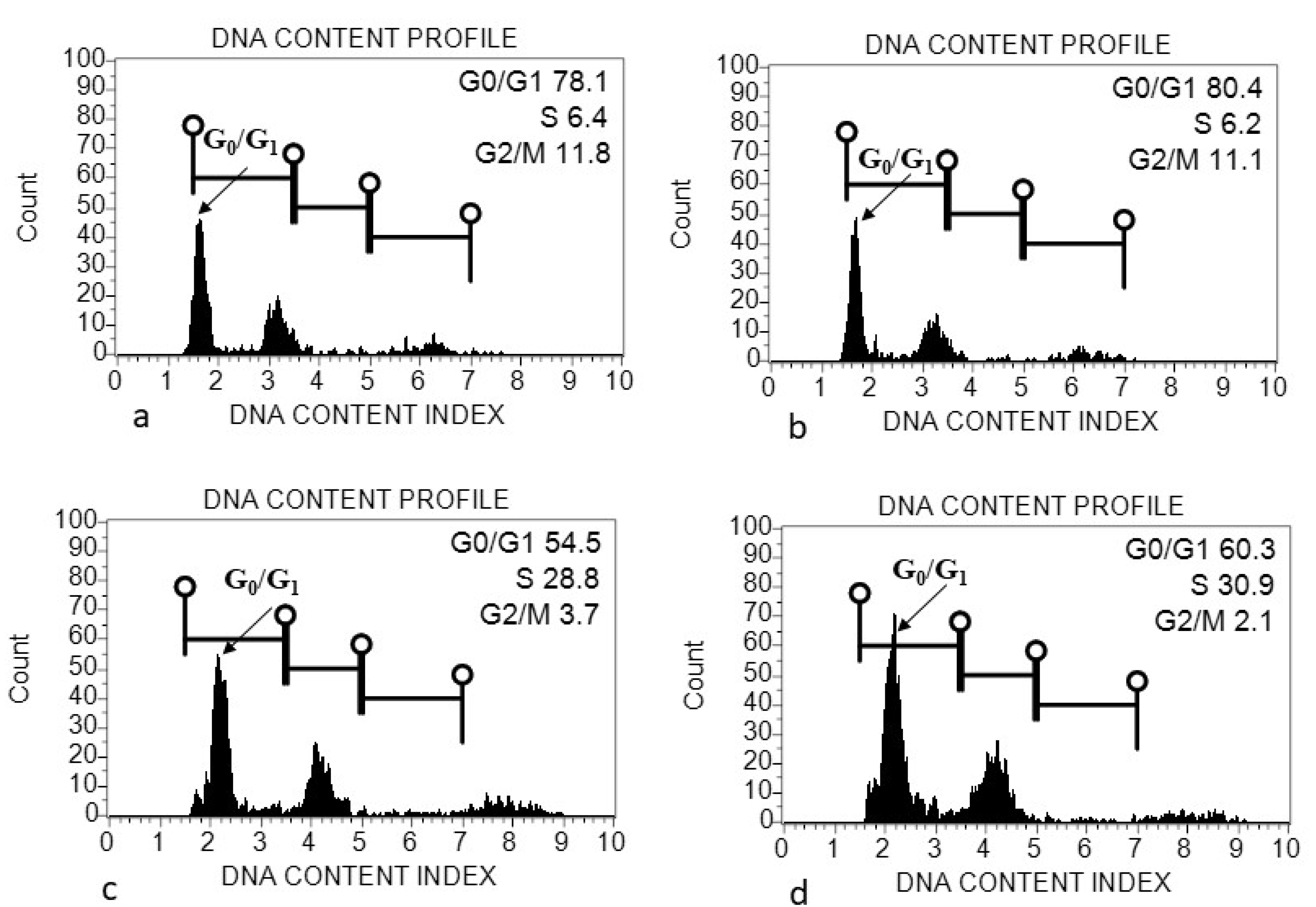
| Acacia Species | Accession Number | Species Status in Saudi Arabia | Collection Place | Seed Diameter (cm) (Mean ± SD) | Seed Length (cm) (Mean ± SD) | Leaf Length (cm) |
|---|---|---|---|---|---|---|
| Acacia johnwoodii (Boulos) Ragup., Seigler, Ebinger & Maslin | KSUSB95 | Threatened | Dhi Ain (Abha) | 0.62 ± 0.012 a | 1.1 ± 0.100 a | 4.7 ± 0.55 a |
| Acacia etbaica (Schweinf.) Kyal. & Boatwr | KSUSB99 | Near endemic | Wadi Khaytan (Abha) | 0.43 ± 0.007 c | 0.518 ± 0.01 c | 2.47 ± 0.34 b |
| Acacia origena (Hunde) Kyal. & Boatwr | KSUSB92 | Near endemic | Dhi Ain (Abha) | 0.52 ± 0.025 b | 0.630 ± 0.01 b | 1.53 ± 0.152 c |
| Plant Species | Extraction Buffers | |||
|---|---|---|---|---|
| GB Buffer (Mean ± SD) | MB01 Buffer (Mean ± SD) | Tris MgCl2 Buffer (Mean ± SD) | LB01 Buffer (Mean ± SD) | |
| Acacia etbaica (Schweinf.) Kyal. & Boatwr | 1.91 ± 0.00 c | 1.91 ± 0.02 c | 2.21 ± 0.02 a | 2.11 ± 0.02 b |
| Acacia johnwoodii (Boulos) Ragup., Seigler, Ebinger & Maslin | 2.19 ± 0.04 a | 2.19 ± 0.00 a | 2.07 ± 0.07 b | 2.12 ± 0.00 ab |
| Acacia origena (Hunde) Kyal. & Boatwr | 2.09 ± 0.00 a | 2.09 ± 0.01 a | 2.10 ± 0.18 a | 2.45 ± 0.05 b |
| Frequency (cm−1) | Intensity | Assignments | Compound Group |
|---|---|---|---|
| 794.26 | Medium | C=C bending | Alkene |
| 1028.52 | Strong | C-F stretching | Fluro compound |
| 1451.85 | Medium | C-H bending | Alkane |
| 1642.96 | medium medium medium | C-N stretching C=C stretching C=C stretching | Imine/oxime Alkene Conjugated alkene |
| 2834.06 | Strong, broad Weak, broad | O-H stretching O-H stretching | Carboxylic acid Alcohol |
| 2943.79 | Strong, broad Weak, broad Strong, broad Medium | O-H stretching O-H stretching N-H stretching C-H stretching | Carboxylic acid Alcohol Amine salt Alkane |
| 3363.62 | Strong, broad | O-H stretching | Alcohol |
| Frequency (cm−1) | Intensity | Assignments | Compound Group |
|---|---|---|---|
| 794.46 | Medium | C=C bending | Alkene |
| 1022.61 | Strong | C-F stretching | Fluro compound |
| 1398.89 | Strong | S=O stretching | sulfonyl chloride |
| 1637.10 | Medium Medium | C=C stretching C=C stretching | cyclic alkene Alkane |
| 2076.89 | Strong | N=C=S stretching | Isothiocyanate |
| 2947.10 | Weak, broad Medium Strong, broad | O-H stretching C-H stretching N-H stretching | Alcohol Alkane Amine salt |
| 3375.47 | Medium | N-H stretching | aliphatic primary amine |
| Frequency (cm−1) | Intensity | Assignments | Compound Group |
|---|---|---|---|
| 793.82 | Medium | C=C bending | Alkene |
| 1113.21 | Strong medium Strong | C-F stretching C-N stretching C-O stretching | Fluro compound amine aliphatic ether |
| 1452.43 | Medium | C=H bending | Alkane |
| 1648.33 | medium medium medium | C-N stretching C=C stretching C=C stretching | Imine/oxime Alkene Conjugated alkene |
| 2833.19 | Strong, broad Weak, broad | O-H stretching O-H stretching | Carboxylic acid Alcohol |
| 2944.93 | Strong, broad Weak, broad Strong, broad Medium | O-H stretching O-H stretching N-H stretching C-H stretching | Carboxylic acid Alcohol Amine salt Alkane |
| 3368.71 | Strong, broad | O-H stretching | Alcohol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Al-Qurainy, F.; Al-hashimi, A.; Nadeem, M.; Tarroum, M.; Salih, A.M.; Shaikhaldein, H.O. Comparative Study on Genome Size and Phytochemical Profile of Three Potential Species of Acacia: Threatened and Endemic to Saudi Arabia. Horticulturae 2022, 8, 994. https://doi.org/10.3390/horticulturae8110994
Khan S, Al-Qurainy F, Al-hashimi A, Nadeem M, Tarroum M, Salih AM, Shaikhaldein HO. Comparative Study on Genome Size and Phytochemical Profile of Three Potential Species of Acacia: Threatened and Endemic to Saudi Arabia. Horticulturae. 2022; 8(11):994. https://doi.org/10.3390/horticulturae8110994
Chicago/Turabian StyleKhan, Salim, Fahad Al-Qurainy, Abdulrahman Al-hashimi, Mohammad Nadeem, Mohamed Tarroum, Abdalrhaman M. Salih, and Hassan O. Shaikhaldein. 2022. "Comparative Study on Genome Size and Phytochemical Profile of Three Potential Species of Acacia: Threatened and Endemic to Saudi Arabia" Horticulturae 8, no. 11: 994. https://doi.org/10.3390/horticulturae8110994
APA StyleKhan, S., Al-Qurainy, F., Al-hashimi, A., Nadeem, M., Tarroum, M., Salih, A. M., & Shaikhaldein, H. O. (2022). Comparative Study on Genome Size and Phytochemical Profile of Three Potential Species of Acacia: Threatened and Endemic to Saudi Arabia. Horticulturae, 8(11), 994. https://doi.org/10.3390/horticulturae8110994








