Abstract
In vitro propagation of olive (Olea euorpea L.) always remained a challenging task due to its woody nature and oxidation of culture. The current study intended to optimize shoot induction and proliferation protocol for different cultivars (“Leccino”, “Gemlik”, “Moraiolo” and “Arbosana”) of olive-on-olive media (OM) provided with different concentrations (0, 0.5, 1.5, and 2.5 mgL−1) of 6-benzylaminopurine (BAP) by pre-exposing their explants (nodal segments) with different regimes (0, 24, and 48 h) of cooling. The impacts of treatments were evaluated on morphological (shoot induction percentage, primary shoot length, number of leaves shoot−1, and number of shoots per explant−1), physiological (total chlorophyll, carotenoids, CO2 assimilation, and proline), biochemical (primary and secondary metabolites) attributes of cultivars after 50 to 60 days of culture. Data recorded were subjected to statistical analysis. All traits depicted significant increases in all genotypes with increasing pre-cooling treatments and increasing supplementations of 6-benzylaminopurine (BAP). This increase was the highest for the interaction of 48 h pre-cooling and 2.5 mgL−1 BAP concentration. Moreover, correlation analysis of all traits revealed significant paired association among them in a positive direction, while principal component analysis (PCA) revealed the extent of association varied with types of treatments and the nature of genotypes. Among cultivars, Arbosana depicted more dramatic changes in morphological traits, physiological attributes, and biochemical contents due to varying interactions of pre-cooling and BAP treatments as compared to Moraiolo, Gemlik, and Leccino with in vitro systems.
1. Introduction
Olive (Olea euorpea L.) is an important plant of the Mediterranean region and an important source of traditional landscape, food, and oil. Various traditional methods of propagation such as grafting, cuttings, and leafy stem rooting are commonly used for olive multiplication [1]. These methods ensure the preservation of genetic traits but have numerous limitations such as season dependence, low success rate, nutrient requirement, the hygienic status of the mother plant, and variable response from cultivar to cultivar [2]. Moreover, these methods require large spaces and extensive areas to establish plant nurseries and do not guarantee the production of disease-free plants. From this perspective, the in vitro propagation method is one of the best choices for the commercial propagation of olives with a high survival rate throughout the season [3]. In addition, the multiplication of olives using tissue culture is not an easy task, owing to some challenges such as explants oxidation, high phenolic contents, the laborious establishment of shoot culture, cultivars dependency, and difficulties in disinfection [4]. Although in the past various shoot induction protocols of olives were optimized within micropropagation systems, all were cultivar dependent [1,5,6]. Successful in vitro propagation of olive is highly associated with type and culture medium composition. For example, Revilla et al. [7] successfully propagated olive using Kuniyuki walnut medium supplemented with 6-benzylaminopurine (BAP) as a growth regulator while sucrose was a carbohydrate source. Likewise, Piexe et al. [1] used BAP and coconut water for successful shoot induction in olive. Correspondingly, Chaari-Rkhis et al. [6] found olive medium (OM) [8] with supplementation of growth regulators is more effective in boosting the shooting and proliferation of different olive cultivars. In the same way, Hamooh and Shah [9] found zeatin as an active hormonal supplement in enhancing the shooting traits of different olive cultivars. Furthermore, Ali et al. [5] explicated that individual and combined application of BAP within in vitro olive medium significantly improves the growth-related traits of olive explants. Plant growth regulators such as BAP have a tendency to initiate intracellular processes that substantially improve related physiological and biochemical activities within the plant system [10]. Moreover, organic substances such as humic acids play an important role in plant tissue culture and micropropagation since they can act as growth by reducing the mutagenic effects of various chemical compounds [11,12,13,14]. Hence, a culture medium supplemented with proper hormonal concentrations is more effective in inducing growth and differentiation within in vitro culture system. The growth regulators such as BAP not only accelerate the differentiation processes of culture but also regulate the post-differentiation events [15]. Plantlets established within a nutrient medium containing a proper concentration of growth regulators show robust physiological and metabolic activities that consequently result in their early establishment within in vitro culture [16].
Hitherto, very limited studies have been conducted to elucidate the impacts of in vitro hormonal treatments on the dynamic association of physiological and metabolic traits with morphological traits of plant cultures [17]. Therefore, the effective role of hormones always remained a potential consideration while optimizing a protocol for any micropropagation system [10]. Apart from this, the choice of explants for olive propagation is also important for the successful establishment of culture [6]. Although in previous studies successful cultures of olive were established on OM using axillary buds as explants source, observed shoot induction percentage was comparatively low in explants that were not provided by proper pre-culturing treatments [6,18,19]. In addition, perennial plants including olives protect their delicate reproductive meristematic tissues in specialized structures known as buds, whose growth is strictly regulated by dormancy mechanisms imposing physiological constraints on their growth until optimum conditions return [20]. Dormancy is released in buds or primrodia by prolonged intervals of chilling. Moreover, axillary buds become dormant due to various endogenous factors such as the accumulation of ABA [21]. Infact, ABA masks intercellular communication during dormancy due to enhanced activity of callose synthase causing a high accumulation of callose that results in blockage of plasmodesmata [22]. Vernalin has the tendency to dissolve callose content that restores cellular communication and breaks bud dormancy by reciprocating the effect of ABA [23]. Moreover, chilling treatments break bud dormancy due to the increase in the production of vernalin, as reported by Leida et al. [19] in peach plants. In this context, the pre-cooling treatment is effective in breaking the dormancy owing to its tendency to counter the effect of ABA by stimulating the activity of vernalin [24]. Moreover, nodal segments are considered better explants than axillary buds due to their comparatively high resistance against oxidation due to the deposition of a high content of phenolic compounds [3]. The oxidation of phenolics is harmful in plant tissue culture as it leads to the browning of explants and medium that stops cell division and finally results in the failure of tissue culture [25]. Oxidative browning of explants can be prevented by controlling the leaching of phenolic compounds from plant tissue by providing them with some pre-treatments such as cooling [26]. Although high phenolic contents are a great obstacle in devising successful shooting protocols for woody plants, their effect can be nullified by pre-culturing treatments [27]. In this perspective, nodal explants can get rid of seasonal dormancy by properly exposing them to the proper durations of pre-cooling. To date, no comprehensive study has been conducted for the optimization of olive in vitro propagation protocol at a physiological, molecular, and biochemical level using different pre-cooling treatments for explants and varying concentrations of BAP. In this regard, the current study was conducted to optimize shoot induction protocol for olives using four different cultivars (“Leccino”, “Gemlik”, “Moraiolo” and “Arbosana”), by culturing their pre-cooled explants on OM using different concentrations of BAP. Furthermore, the effects of these treatments were assessed on the physiological, biochemical, and growth traits of plantlets grown within the micropropagation system.
2. Materials and Methods
In the current study, four different cultivars of olive such as “Leccino” (Italy), “Gemlik” (Turkey), “Moraiolo” (Italy), and “Arbosana” (Spain) were evaluated for the optimization of shoot induction protocol in a three replicate experiment using three factorial arrangements in randomized complete block design (RCBD) at plant tissue culture lab of Jilin Agricultural University, China.
2.1. Explant Disinfection, Media Preparation, and Treatments
The nodal segments obtained from soft, healthy, and lateral one-year-old branches of olive cultivars were used as explants. After detaching the leaves, branches were divided into 10 cm long pieces that were thoroughly washed for 30 min under running tap water. Subsequently, branch segments were cut into nodal segments of size 1–1.5 cm as explicated Chaari-Rkhis et al. [6]. The nodal segments were surface sterilized by treating them with 70% ethanol for 1 to 2 min. Afterward, they were dipped in 15% sodium hypochlorite solution for 10 min. Finally, the nodal segments were rinsed four times using sterilized distilled water and each step was conducted for 5 min. All sterilization steps were carried out under laminar air flow hood by sustaining sterilized atmosphere. In addition, Rugini [8] olive medium (OM) (PhytoTech labs, Lenexa, KS, USA) with optimized pH (5.75 ± 0.5) was prepared and supplemented with different concentrations (0, 0.5, 1.5, and 2.5 mgL−1) of 6-benzylaminopurine (BAP) (PhytoTech labs, USA) by following manufacturer’s instruction within 300 mL glass bottle. Before adding BAP, media was autoclaved as per method of Chaar-Rkhis et al. [6] at 121 °C under 15 psi pressure for 15 min. Afterward, media was cooled to 50 °C and filter sterilized BAP solution was added. Before culturing, explants were wrapped in aluminum foil were subjected to pre-cooling treatments of 0, 24, and 48 h at 4 to 8 °C in a chiller. Afterward, 3 to 4 explants (nodal segments) from each cultivar were cultured on Rugini [8] olive medium (OM) (PhytoTech labs, USA) in glass bottles under laminar flow chamber. For each treatment, three bottles were used.
2.2. In Vitro Propagation and Data Collection
The cultured bottles were kept in growth chamber under controlled conditions. The cultures were provided with 2500 lux light intensity, 25 °C temperature, and 16 h photoperiod. The data for growth, physiological, and biochemical parameters were collected from 50 to 60 days old plantlets of olives. For this purpose, we followed destructive sampling. Furthermore, cultures facing browning were immediately shifted within fresh medium.
2.3. Measurement of Growth Traits
The morphological parameters were evaluated on the basis of percentage of the induced shoots (PIS), length of primary shoot (cm) (LPS), number of leaves per shoot (NLPS), and number of shoots per explant (NSPE). The data for PIS were recorded for each cultivar by applying percentage formula while the data obtained for LPS and NLPS were estimated on average basis from randomly selected five primary shoots from each treatment before subjecting to statistical analysis. In the same way, data were recorded for NSPE.
2.4. Assessment of Physiological Parameters
Among physiological parameters such as total chlorophyll, carotenoids, and proline were estimated following the methods used by Mahmood et al. [28]. In addition, proline content was estimated based on reactivity with ninhydrin using UV–Vis spectrophotometer (DeNovix, Wilmington, DE, USA). On the other hand, assimilation rate of CO2 (ACO2) was measured using specific apparatus IRGA (ADC BioScientific, Hoddesdon, UK).
2.5. Assessment ofBiochemical Contents
2.5.1. Quantification of Primary Metabolites
Leaf carbohydrates were quantified using the method of Boussadia et al. [29]. The carbohydrate contents such as fructose, glucose, and sucrose were extracted using ethanol reagent and extract was subjected to centrifugation at 5000 rpm for 10 min. Afterward, metabolites were quantified using high pH anion-exchange chromatography. On the other hand, starch content was estimated using acid hydrolysis method used by Chow and Landhausser [30]. For this purpose, dried 50 mg leaf samples were extracted using 80% hot ethanol that subsequently followed enzymatic digestion. Afterward, quantification was performed at 525 after adding H2SO4.
2.5.2. Quantification of Secondary Metabolites
Among secondary metabolites, total phenols were estimated following the method used by Siddiqui et al. [31]. For this purpose, Folin–Ciocalteu reagent was used, with gallic acid as the standard. In addition, total flavonoids were calculated following the procedure by Zhao et al. [32] and quantified using colorimetric assay method, with rutin as standard. Total tannins content was determined by following the protocol of Fadda and Mulas [33]. For this purpose, 2 mL of extracted sample was treated with 10 mL vanillin hydrochloride and for quantification; absorbance was recorded at 500 nm. Total alkaloid contents were determined by following the procedure optimized by Li et al. [34]. In this regard, the prepared standard solution of leaf extract was quantified using UV–Vis spectrophotometer (DeNovix, USA), and absorbance was recorded as 418 nm.
2.6. Statistical Analysis
A tri-replicate experiment was conducted in randomized complete block design (RCBD) using three factorial arrangements with pre-cooling as one factor, varieties as second factor, and BAP concentrations as third factor. The data collected were evaluated statistically by applying analysis of variance (ANOVA) at a 5% probability level, with the help of computer-based software Statistix ver. 8.1 (McGraw-Hill 2008). Furthermore, correlation and principal component analysis (PCA) were conducted with the help of computer-based statistical tool RStudio version 1.3.959 (RStudio Team 2020) using the PerformaceAnalytics, FactoMineR, factoextra, devtools, ggplot2, ggpubr, gplots, and pheatmap packages of R version 4.1.0 (R Core Team 2021).
3. Results
3.1. Growth Traits
All individual factors of treatments significantly (p ≤ 0.01) affected the mean values of growth-related traits such as LPS, PIS, NLPS, and NSPE in all olive cultivars (Table 1). All growth traits showed statistically significant (p ≤ 0.01) increase with changing regimes of pre-cooling and increasing concentrations of BAP (Table 1). Moreover, among cultivars “Arbosana” (V4) plantlets recorded a maximum improvement in the aforementioned growth traits followed by “Moraiolo” (V3), “Leccino” (V2), and “Gemlik” (V1) as indicated in Table 1. In addition, V4 revealed a maximum mean value for LPS (3 cm) that was significantly (p ≤ 0.01) different from the means of other varieties. Correspondingly, V4 exhibited significantly (p ≤ 0.01) high PIS (85%) as compared to V3 (75%), V2 (70%), and V1 (60%) as shown in Table 1. Similarly, under the same conditions, V4 depicted statistically (p ≤ 0.01) distinct improvement in NLPS (5) and NSPE (4). In addition, among two-way interactions, the interaction of BAP with pre-cooling (T × LB) and cultivars (V × LB) illustrated statistically distinct (p ≤ 0.05) change in all growth traits (Tables 5 and 6). At all regimes of pre-cooling, BAP manifested maximum improvements in growth-related traits of olive cultivars at concentration 2.5 mgL−1 (L4) (Table 6); however, this improvement was more dramatic at 48 h (T3) interval of pre-cooling (Table 5). Overall, the performance of explants from all cultivars was notably different under varying concentrations of BAP and changing regimes of pre-cooling. Likewise, the two-way interaction T × V, and the three-way interaction T × V × LB showed no significant effect on the growth traits of cultivars.

Table 1.
Effect of different pre-cooling treatments and varying concentrations of BAP on growth traits of different olive (Olea euorpea L.) genotypes cultured within micropropagation system using OM.
3.2. Physiological Parameters
All traits except proline depicted a significant increase with increasing regimes of pre-cooling and BAP concentrations (Table 2). In addition, among cultivars “Arbosana” (V4) illustrated a maximum increase in all physiological traits followed by “Moraiolo” (V3), “Gemlik” (V2) and “Leccino” (V1). Furthermore, V4 exhibited maximum chlorophyll content (50 μg cm−2) that was slightly higher than the mean chlorophyll contents of other varieties (Table 2). Correspondingly, V4 recorded the highest (8 μmol m−2s−1) while V1 recorded the lowest mean value (7 μmol m−2s−1) for ACO2 at 2.5 mgL−1 (L4) concentration of BAP in plantlets whose explants were treated with 48 h interval of pre-cooling. In addition, under analogous conditions, V4 depicted a statistically significant (p ≤ 0.01) increase in carotenoids (5.29 μg cm−2) and a decline in proline (38.7 μg g−1 FW) contents. Among physiological traits, chlorophyll and carotenoids showed significant (p ≤ 0.05) variation due to the two-way interaction of BAP with pre-cooling treatments (T × LB) and cultivars (V × LB) (Tables 5 and 6). All cultivars pre-treated with a 48 h regime of cooling illustrated maximum chlorophyll and carotenoid contents at a BAP concentration of 2.5 mgL−1 (Tables 5 and 6). In general, explants from all cultivars pre-treated with different cooling intervals responded differently under provided in vitro conditions due to varying concentrations of BAP. All traits showed no significant variation due to two-way interaction T × V and three-way interaction T × V × LB.

Table 2.
Effect of different pre-cooling treatments and varying concentrations of BAP on physiological parameters of different olive (Olea euorpea L.) genotypes cultured within micropropagation system using OM.
3.3. Biochemical Contents
3.3.1. Primary Metabolites
All primary metabolic contents including fructose, glucose, sucrose, and starch varied significantly (p ≤ 0.01, p ≤ 0.05) due to the individual effect of olive cultivars’ pre-cooling regimes and varying concentrations of BAP within OM (Table 3). All metabolites depicted statistically significant (p ≤ 0.01) increase at the pre-cooling treatment of 48 h (T3) and BAP concentration of 2.5 mgL−1 (L4) as shown in Table 3. On the other hand, among cultivars, “Arbosana” (V4) revealed the highest rise in all primary metabolites followed by “Moraiolo” (V3), “Gemlik” (V2), and “Leccino” (V1). Moreover, V4 recorded maximum fructose (0.04 mg g−1 DW), sucrose (0.5 mg g−1 DW), and starch (0.7 mg g−1 DW) contents as compared to other cultivars. In the same way, V3 demonstrated the maximum (1.42 mg g−1 DW) and V1 recorded the minimum (1 mg g−1 DW) increase in the amount of glucose. In addition, among two-way interactions T × LB significantly (p ≤ 0.05) altered the mean values of glucose, sucrose, and fructose contents while V × LB significantly (p ≤ 0.05) altered the mean values of fructose and sucrose content (Tables 5 and 6). All pre-cooled olive cultivars showed a maximum rise in the quantities of primary metabolites when OM augmented with BAP at 2.5 mgL−1 (L4), however, this incline was more dramatic in all cultivars that followed the pre-cooling treatment of 48 h (T3) as concluded by Tables 5 and 6. As a whole, explants from all cultivars pre-treated with different cooling regimes behaved differently under provided in vitro conditions due to varying levels of BAP. On the other hand, all primary metabolites showed no significant variation due to two-way interaction T × V and three-way interaction p× V × LB.

Table 3.
Effect of different pre-cooling treatments and varying concentrations of BAP on primary metabolites of different olive genotypes cultured within micropropagation system using OM.
3.3.2. Secondary Metabolites
Among individual treatment factors, pre-cooling regimes significantly (p ≤ 0.05, p ≤ 0.01) increased alkaloids and phenolic contents (Table 4). On the other hand, varying BAP concentrations significantly (p ≤ 0.05) impacted the amount of secondary metabolic contents including alkaloids, phenols, tannins, and flavonoids (Table 4). Moreover, among cultivars “Arbosana” (V4) manifested a more dramatic increase in the concentrations of alkaloids, phenols, and flavonoids followed by “Moraiolo” (V3), “Gemlik” (V2) and “Leccino” (V1). In addition, V4 illustrated the maximum alkaloids (2.5 mg g−1 DW) that were a bit higher than the alkaloid content of other cultivars (Table 4). Following the analogous trend, V4 revealed a more dramatic rise in flavonoid (1.54 mg g−1 DW) and phenol content (1.5 mg g−1 DW) unlike other cultivars (Table 4). Among two-way interactions T × LB and V × LB significantly altered the mean values of alkaloids and flavonoids (Table 5 and Table 6). The BAP made a statistically distinct (p ≤ 0.05) rise in alkaloids and flavonoid contents of all olive cultivars at a concentration of 2.5 mgL−1 (L4) under all pre-cooling treatments; however, this rise was at the maximum at 48 h (Table 5 and Table 6). Generally, plantlets from all cultivars whose explants followed different pre-cooling treatments before culturing showed variable responses within in vitro conditions on OM augmented with different concentrations of BAP. In addition, two-way interaction T × V and three-way interaction T × V × LB showed no significant effect on the concentration of secondary metabolites.

Table 4.
Effect of different pre-cooling treatments and varying concentrations of BAP on secondary metabolites of different olive genotypes cultured within micropropagation system using OM.
Table 4.
Effect of different pre-cooling treatments and varying concentrations of BAP on secondary metabolites of different olive genotypes cultured within micropropagation system using OM.
| Treatments | Alkaloids mg (g DW)−1 | Flavonoids mg (g DW)−1 | Tannins mg (g DW)−1 | Phenols mg (g DW)−1 |
|---|---|---|---|---|
| Pre-cooling (T) (Hours) | ||||
| T1 (0 h) | 1.52 ± 0.074 c | 1.35 ± 0.067 b | 0.63 ± 0.015 a | 0.70 ± 0.025 a |
| T2(24 h) | 1.75 ± 0.077 b | 1.44 ± 0.077 ab | 0.65 ± 0.014 a | 0.85 ± 0.026 a |
| T3 (48 h) | 2.00 ± 0.09 a | 1.60 ± 0.082 a | 0.72 ± 0.015 a | 0.93 ± 0.026 a |
| LSD | 0.18 | 0.15 | 0.17 | 0.14 |
| Varieties (V) | ||||
| V1 (Leccino) | 1.5 ± 0.093 d | 1.39 ± 0.084 c | 1.45 ± 0.084 a | 1.00 ± 0.049 d |
| V2 (Gemlik) | 1.70 ± 0.10 c | 1.42 ± 0.080 bc | 1.43 ± 0.080 a | 1.03 ± 0.055 bc |
| V3 (Morailo) | 1.84 ± 0.10 b | 1.50 ± 0.095 ab | 1.49 ± 0.095 a | 1.08 ± 0.059 ab |
| V4 (Arbosana) | 2.5 ± 0.01 a | 1.54 ± 0.098 a | 1.50 ± 0.098 a | 1.5 ± 0.064 a |
| LSD | 0.045 | 0.09 | 0.1 | 0.06 |
| BAP (LB) (mgL−1) | ||||
| L1 (0) | 1.02 ± 0.031 d | 0.84 ± 0.035 d | 0.68 ± 0.017 d | 0.73 ± 0.026 d |
| L2 (0.5) | 1.64 ± 0.054 b | 1.20 ± 0.049 c | 0.80 ± 0.027 c | 1.04 ± 0.035 c |
| L3 (1.5) | 1.48 ± 0.054 c | 1.42 ± 0.054 b | 1.01 ± 0.032 b | 1.27 ± 0.031 a |
| L4(2.5) | 1.85 ± 0.062 a | 1.62 ± 0.068 a | 1.11 ± 0.027 a | 1.16 ± 0.033 b |
| LSD | 0.023 | 0.040 | 0.055 | 0.028 |
| Significance | ||||
| T | * | ** | ns | ns |
| V | ** | ** | ns | * |
| LB | ** | ** | ** | ** |
| T × V | ns | ns | ns | ns |
| T × LB | * | * | ns | * |
| V × LB | * | * | ns | ns |
| T × V × LB | ns | ns | ns | ns |
Means with same letter (s) in each column indicate no significant difference in traits due to treatments. *, ** represent significant differences at p ≤ 0.05 and p ≤ 0.01, respectively, while “ns” represents non-significant difference.

Table 5.
Traits showing statistically significant (p ≤ 0.05) difference due to interaction effect of pre-cooling regimes and BAP concentrations (T × LB).
Table 5.
Traits showing statistically significant (p ≤ 0.05) difference due to interaction effect of pre-cooling regimes and BAP concentrations (T × LB).
| PCT (T) (hours) | BAP (LB) (mgL−1) | LPS (cm) | PIS | NLPS | NSPE | Chlorophyll (μg cm−2) | Carotenoids (μg cm−2) | Glucose mg g−1 DW | Fructose mg g−1 DW | Sucrose mg g−1 DW | Proline μg g−1 FW | Alkaloids mg g−1 DW | Flavanoids mg g−1 DW | Phenols mg g−1 DW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 (0 h) | L1 (0) | 1.00 ± 0.08 | 36.7 ± 1.41 | 2.45 ± 0.08 | 1.06 ± 0.03 | 18.5 ± 0.37 | 2.62 ± 0.07 | 0.16 ± 0.01 | 0.005 ± 0.0007 | 0.02 ± 0.001 | 46.7 ± 0.3 | 0.85 ± 0.03 | 0.75 ± 0.03 | 0.69 ± 0.02 |
| L2 (0.5) | 1.69 ± 0.12 | 46.0 ± 2.32 | 3.15 ± 0.17 | 1.74 ± 0.10 | 23.8 ± 0.39 | 3.60 ± 0.11 | 0.47 ± 0.009 | 0.012 ± 0.001 | 0.10 ± 0.007 | 44.7 ± 0.44 | 1.22 ± 0.052 | 0.94 ± 0.05 | 0.85 ± 0.034 | |
| L3 (1.5) | 1.94 ± 0.01 | 52.4 ± 2.94 | 3.79 ± 0.14 | 2.12 ± 0.11 | 27.5 ± 0.41 | 4.22 ± 0.9 | 0.58 ± 0.010 | 0.020 ± 0.002 | 0.21 ± 0.003 | 42.0 ± 0.90 | 1.420 ± 0.058 | 1.13 ± 0.05 | 1.001 ± 0.050 | |
| L4 (2.5) | 2.19 ± 0.07 | 57.0 ± 2.77 | 4.22 ± 0.15 | 2.40 ± 0.12 | 33.5 ± 0.52 | 4.81 ± 0.14 | 0.74 ± 0.011 | 0.028 ± 0.002 | 0.25 ± 0.004 | 22.8 ± 0.37 | 1.58 ± 0.06 | 1.28 ± 0.06 | 1.11 ± 0.04 | |
| T2 (24 h) | L1 (0) | 1.04 ± 0.02 | 42.0 ± 0.73 | 2.49 ± 0.05 | 1.21 ± 0.02 | 19.5 ± 0.31 | 2.78 ± 0.06 | 0.22 ± 0.005 | 0.008 ± 0.0003 | 0.05 ± 0.008 | 48.5 ± 0.52 | 1.08 ± 0.03 | 1.001 ± 0.04 | 0.87 ± 0.034 |
| L2 (0.5) | 2.04 ± 0.06 | 55.0 ± 1.88 | 3.25 ± 0.08 | 2.23 ± 0.12 | 27.4 ± 0.30 | 4.03 ± 0.10 | 0.56 ± 0.005 | 0.020 ± 0.001 | 0.21 ± 0.011 | 45.4 ± 0.72 | 1.44 ± 0.04 | 1.20 ± 0.03 | 1.07 ± 0.04 | |
| L3 (1.5) | 2.29 ± 0.01 | 61.2 ± 1.88 | 4.25 ± 0.16 | 2.57 ± 0.12 | 33.3 ± 0.55 | 4.94 ± 0.12 | 0.68 ± 0.006 | 0.034 ± 0.001 | 0.26 ± 0.01 | 42.8 ± 0.48 | 1.58 ± 0.05 | 1.40 ± 0.05 | 1.22 ± 0.03 | |
| L4 (2.5) | 2.57 ± 0.07 | 65.3 ± 1.54 | 4.70 ± 0.16 | 2.98 ± 0.12 | 37.4 ± 0.67 | 5.40 ± 0.9 | 0.85 ± 0.001 | 0.043 ± 0.0021 | 0.31 ± 0.010 | 24.7 ± 0.31 | 1.77 ± 0.05 | 1.61 ± 0.07 | 1.31 ± 0.03 | |
| T3 (48 h) | L1 (0) | 1.13 ± 0.03 | 41.3 ± 0.59 | 2.50 ± 0.01 | 1.20 ± 0.01 | 20.8 ± 0.29 | 2.72 ± 0.10 | 0.23 ± 0.008 | 0.007 ± 0.0004 | 0.07 ± 0.005 | 50.5 ± 0.51 | 1.12 ± 0.03 | 1.04 ± 0.03 | 0.93 ± 0.025 |
| L2 (0.5) | 2.37 ± 0.11 | 62.0 ± 2.15 | 3.79 ± 0.16 | 2.33 ± 0.17 | 31.4 ± 0.72 | 4.87 ± 0.13 | 0.63 ± 0.009 | 0.025 ± 0.001 | 0.27 ± 0.007 | 49.5 ± 0.74 | 1.77 ± 0.04 | 1.46 ± 0.03 | 1.20 ± 0.02 | |
| L3 (1.5) | 2.67 ± 0.13 | 68.4 ± 2.50 | 4.53 ± 0.12 | 2.93 ± 0.20 | 36.5 ± 0.67 | 5.37 ± 0.13 | 0.75 ± 0.009 | 0.037 ± 0.002 | 0.32 ± 0.008 | 45.0 ± 0.84 | 1.94 ± 0.04 | 1.69 ± 0.02 | 1.28 ± 0.01 | |
| L4 (2.5) | 2.89 ± 0.14 | 72.8 ± 2.58 | 4.83 ± 0.11 | 3.57 ± 0.28 | 41.8 ± 0.63 | 6.12 ± 0.17 | 0.91 ± 0.011 | 0.050 ± 0.002 | 0.36 ± 0.011 | 22.5 ± 0.39 | 2.20 ± 0.03 | 1.97 ± 0.04 | 1.39 ± 0.01 | |
| LSD | 0.1388 | 2.2443 | 0.1944 | 0.1422 | 1.0253 | 0.2206 | 0.0289 | 0.0028 | 0.0102 | 2.1257 | 0.0421 | 0.0700 | 0.0500 |
* Only traits showing significant difference are indicated in table, while means with difference greater than LSD are significantly different at p ≤ 0.05. (LPS, length of primary shoot; PIS, percentage of induced shoots; NLPS, number of leaves per shoot; NSPE, number of shoots per explant).

Table 6.
Traits showing statistically significant (p ≤ 0.05) difference due to interaction effect of varieties and BAP concentrations (V × LB).
Table 6.
Traits showing statistically significant (p ≤ 0.05) difference due to interaction effect of varieties and BAP concentrations (V × LB).
| Varieties (V) | BAP (LB) (mgL−1) | LPS | PIS | NLPS | NSPE | Chlorophyll (μg cm−2) | Carotenoids (μg cm−2) | Fructose mg g−1 DW | Sucrose mg g−1 DW | Alkaloids mg g−1 DW | Flavanoids mg g−1 DW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leccino (V1) | L1 (0) | 0.87 ± 0.07 | 39.1 ± 2.16 | 2.27 ± 0.07 | 1.11 ± 0.04 | 11.1 ± 0.30 | 2.64 ± 0.10 | 0.006 ± 0.0008 | 0.03 ± 0.008 | 0.93 ± 0.06 | 0.81 ± 0.04 |
| L2 (0.5) | 1.67 ± 0.15 | 47.1 ± 2.95 | 2.91 ± 0.15 | 1.64 ± 0.12 | 18.1 ± 1.18 | 3.87 ± 0.23 | 0.016 ± 0.002 | 0.17 ± 0.02 | 1.29 ± 0.10 | 1.06 ± 0.07 | |
| L3 (1.5) | 1.92 ± 0.12 | 53.1 ± 3.08 | 3.66 ± 0.14 | 1.94 ± 0.01 | 22.4 ± 1.37 | 4.62 ± 0.1 | 0.026 ± 0.003 | 0.24 ± 0.01 | 1.48 ± 0.10 | 1.24 ± 0.07 | |
| L4 (2.5) | 2.21 ± 0.10 | 57.2 ± 3.38 | 4.07 ± 0.15 | 2.26 ± 0.11 | 25.6 ± 1.44 | 5.10 ± 0.1 | 0.035 ± 0.005 | 0.28 ± 0.01 | 1.65 ± 0.13 | 1.41 ± 0.07 | |
| Gemlik (V2) | L1 (0) | 1.9 ± 0.03 | 39.4 ± 1.27 | 2.49 ± 0.04 | 1.17 ± 0.04 | 11.6 ± 0.20 | 2.87 ± 0.04 | 0.006 ± 0.0007 | 0.05 ± 0.001 | 0.97 ± 0.05 | 0.94 ± 0.05 |
| L2 (0.5) | 1.97 ± 0.13 | 54.6 ± 2.41 | 3.22 ± 0.08 | 2.01 ± 0.14 | 18.7 ± 1.39 | 3.97 ± 0.25 | 0.015 ± 0.002 | 0.19 ± 0.02 | 1.43 ± 0.08 | 1.17 ± 0.06 | |
| L3 (1.5) | 2.22 ± 0.11 | 60.2 ± 2.49 | 4.11 ± 0.18 | 2.46 ± 0.1 | 23.1 ± 1.57 | 4.62 ± 0.24 | 0.029 ± 0.003 | 0.26 ± 0.01 | 1.54 ± 0.09 | 1.34 ± 0.05 | |
| L4 (2.5) | 2.51 ± 0.10 | 65.0 ± 2.40 | 4.49 ± 0.14 | 3.01 ± 0.2 | 27.0 ± 1.52 | 5.27 ± 0.23 | 0.038 ± 0.004 | 0.29 ± 0.01 | 1.79 ± 0.13 | 1.53 ± 0.05 | |
| Morailo (V3) | L1 (0) | 1.12 ± 0.04 | 42.4 ± 1.42 | 2.59 ± 0.03 | 1.16 ± 0.02 | 11.2 ± 0.32 | 2.69 ± 0.07 | 0.007 ± 0.002 | 0.05 ± 0.001 | 1.02 ± 0.03 | 0.94 ± 0.05 |
| L2 (0.5) | 2.19 ± 0.12 | 56.1 ± 3.40 | 3.61 ± 0.16 | 2.24 ± 0.11 | 19.6 ± 1.53 | 4.34 ± 0.20 | 0.020 ± 0.003 | 0.20 ± 0.02 | 1.58 ± 0.09 | 1.23 ± 0.04 | |
| L3 (1.5) | 2.42 ± 0.16 | 62.4 ± 3.61 | 4.32 ± 0.11 | 2.76 ± 0.16 | 24.1 ± 1.91 | 4.80 ± 0.17 | 0.017 ± 0.004 | 0.27 ± 0.01 | 1.73 ± 0.07 | 1.47 ± 0.02 | |
| L4 (2.5) | 2.62 ± 0.16 | 67.0 ± 2.71 | 4.81 ± 0.14 | 3.23 ± 0.27 | 28.2 ± 1.73 | 5.70 ± 0.25 | 0.040 ± 0.003 | 0.32 ± 0.02 | 1.92 ± 009 | 1.71 ± 0.03 | |
| Arbosana (V4) | L1 (0) | 1.12 ± 0.04 | 41.2 ± 0.94 | 2.57 ± 0.11 | 1.19 ± 0.03 | 11.6 ± 0.20 | 2.62 ± 0.13 | 0.007 ± 0.0006 | 0.06 ± 0.001 | 1.13 ± 0.05 | 1.03 ± 0.03 |
| L2 (0.5) | 2.29 ± 0.14 | 59.7 ± 4.06 | 3.86 ± 0.15 | 2.51 ± 0.15 | 20.6 ± 1.52 | 4.50 ± 0.2 | 0.023 ± 0.002 | 0.21 ± 0.02 | 1.60 ± 0.09 | 1.35 ± 0.07 | |
| L3 (1.5) | 2.62 ± 0.16 | 67.0 ± 4.48 | 4.67 ± 0.13 | 3.01 ± 0.17 | 25.2 ± 1.95 | 5.24 ± 0.2 | 0.037 ± 0.002 | 0.28 ± 0.01 | 1.82 ± 0.09 | 1.57 ± 0.06 | |
| L4 (2.5) | 2.86 ± 0.17 | 71.1 ± 4.45 | 4.96 ± 0.14 | 3.44 ± 0.26 | 28.7 ± 2.11 | 5.61 ± 0.34 | 0.048 ± 0.004 | 0.33 ± 0.02 | 2.04 ± 0.08 | 1.84 ± 0.05 | |
| LSD | 0.1610 | 2.588 | 0.2234 | 0.1644 | 1.2170 | 0.2659 | 0.0045 | 0.0118 | 0.0482 | 0.0809 |
* Only traits showing significant difference are indicated in table, while means with difference greater than LSD are significantly different at p ≤ 0.05. (LPS, length of primary shoot; PIS, percentage of induced shoots; NLPS, number of leaves per shoot; NSPE, number of shoots per explant.
3.4. Correlation and Principal Component Analysis (PCA)
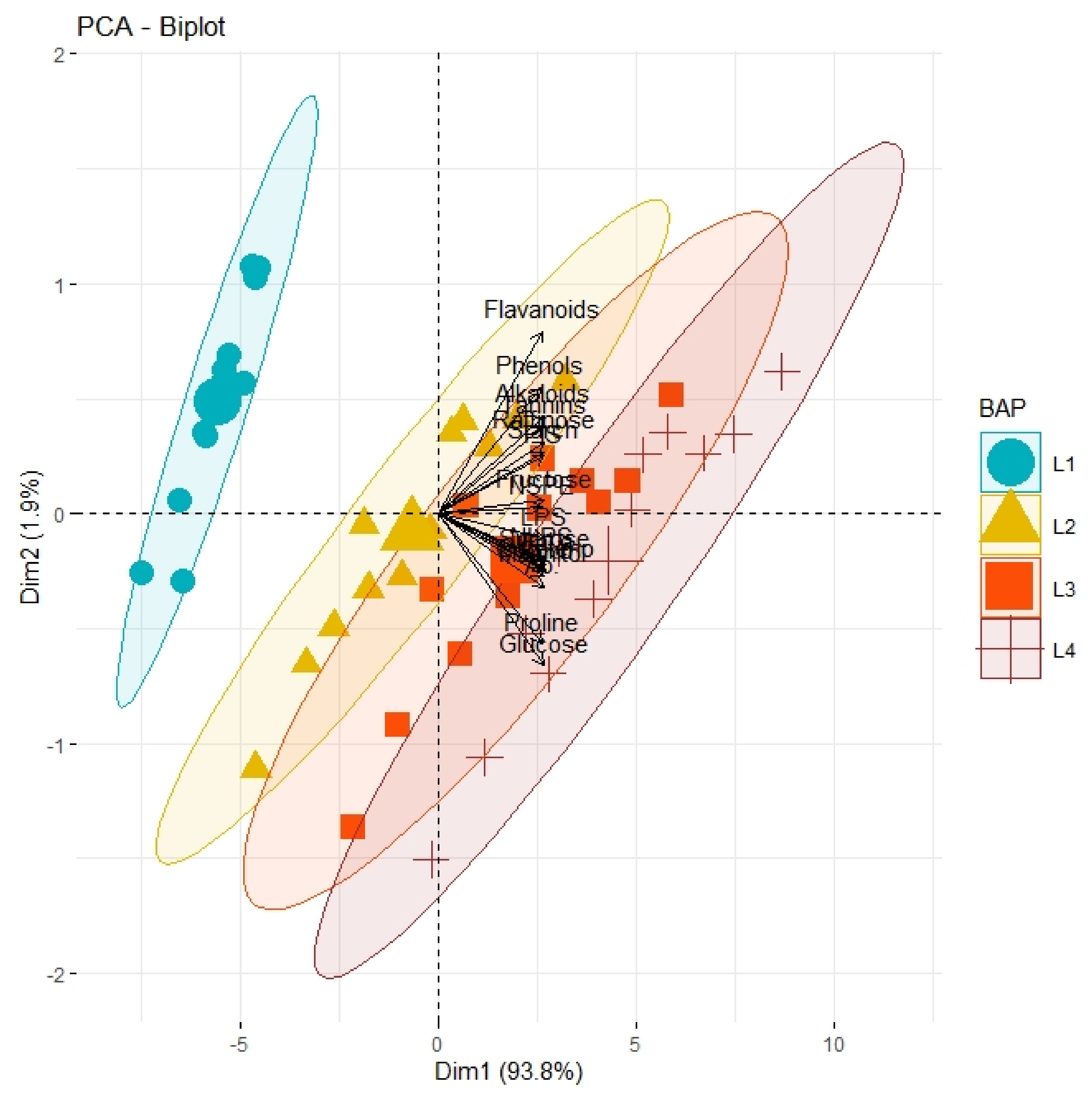
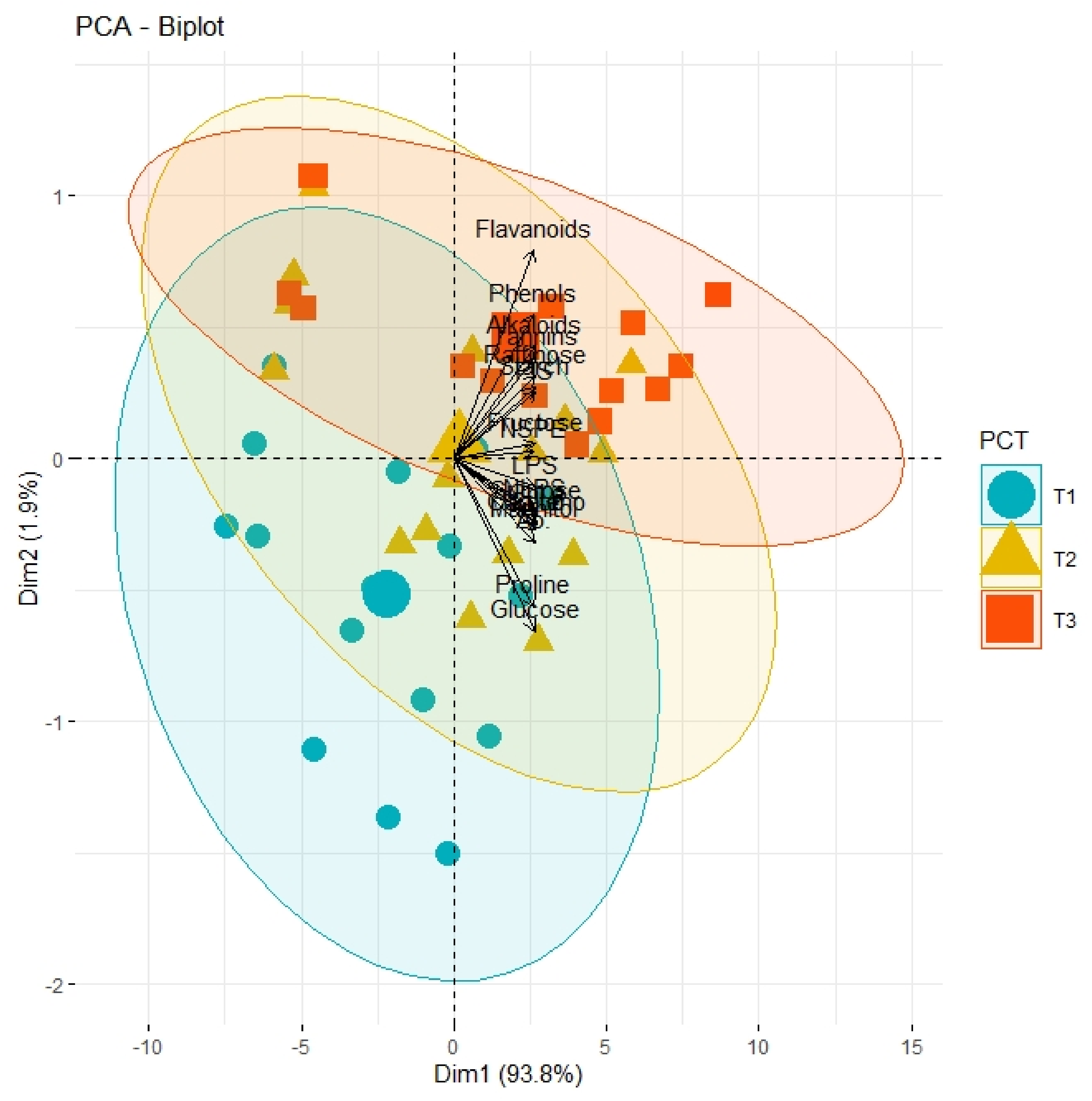
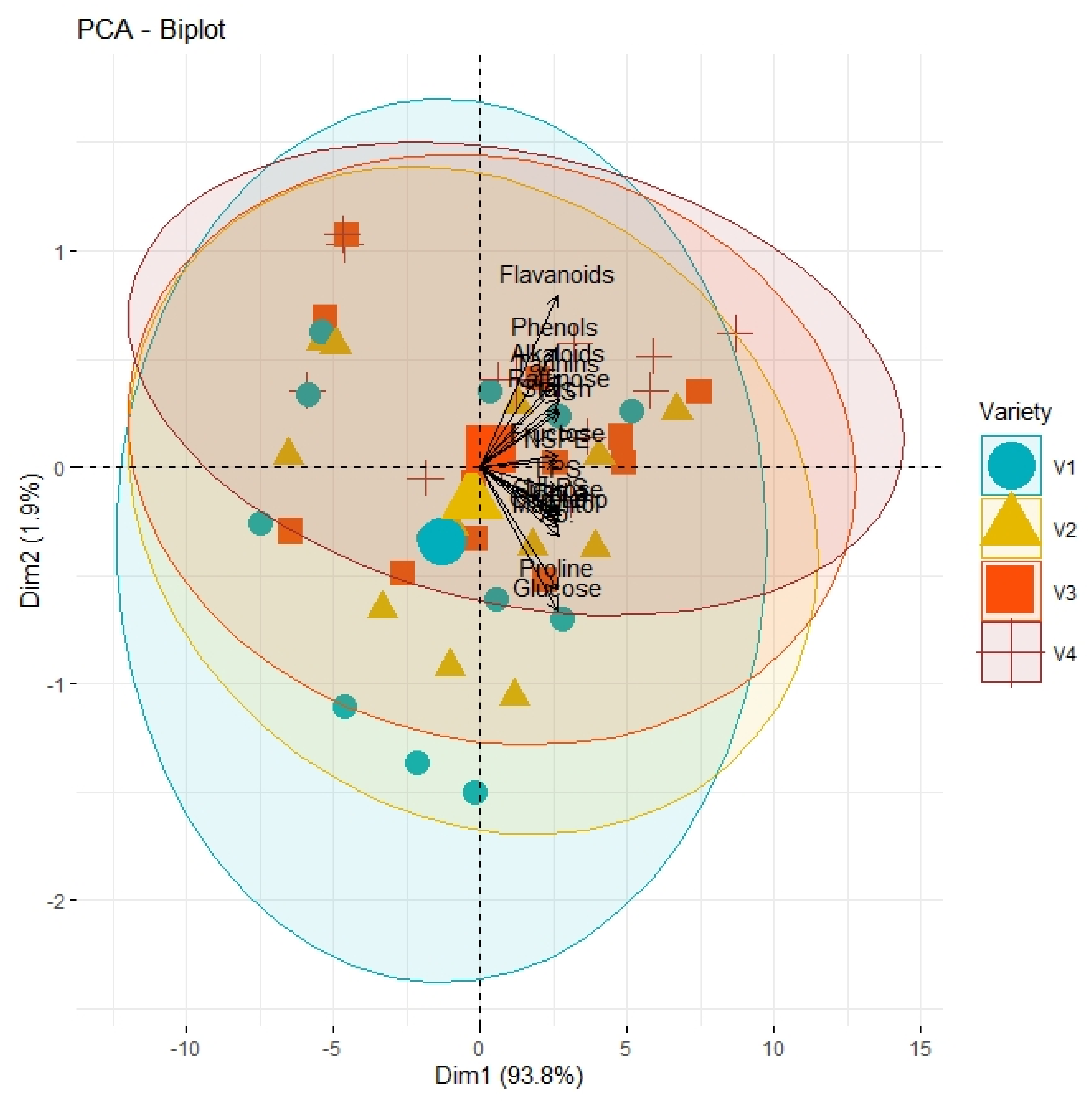
Correlation analysis illustrated a significantly high paired association among growth traits, physiological parameters, and biochemical contents of all olive cultivars subjected to different pre-cooling regimes and varying in vitro concentrations of BAP (Table 7). All primary and secondary metabolites revealed a significantly high paired association with physiological traits such as chlorophyll and ACO2. Furthermore, all growth traits of plantlets depicted a strong paired association between themselves, and physiological traits as illustrated in Table 1. On the other hand, principal component analysis (PCA) deciphered varying responses of traits with respect to varying concentrations of BAP, different regimes of pre-cooling, and types of cultivars. The response of all traits under study revealed analogous behavior at varying doses of BAP such as L2 (0.5 mgL−1), L3 (1.5 mgL−1), and L4 (2.5 mgL−1) as compared to control (Figure 1). However, the divergence of trait clusters from graph origin was different at all applied concentrations of BAP which revealed a differential extent of association of traits at each concentration. Overall, this divergence of traits cluster from origin was the highest for 2.5 mgL−1 (L4), which was an indicator of a promising increase in the strength of traits association. In addition, the PCA graph for pre-cooling treatments revealed the analogous response of all traits at treatments T2 and T3 as compared to control (T1) as illustrated in Figure 2. Correspondingly, the differential divergence of trait clusters from the origin at each pre-cooling treatment showed the varying extent of association among traits that was maximum at T3. This indicated variable associated response of traits with changing regimes of pre-cooling. Furthermore, the PCA graph for all olive cultivars revealed the complementary response of all traits as explicated by the merged circles of clusters (Figure 3). However, the divergence cultivars with respect to the proximity of traits from origin were different, which indicated the differential response of each cultivar for the same sort of traits. Among cultivars, “Arbosana” (V4) manifested a response of traits in close proximity followed by “Moraiolo” (V3), “Gemlik” (V2), and “Leccino” (V1).

Table 7.
Correlation table describing the significance of association between different metabolic, physiological, and growth traits of different cultivars of olives due to different regimes of pre-cooling and BAP levels.
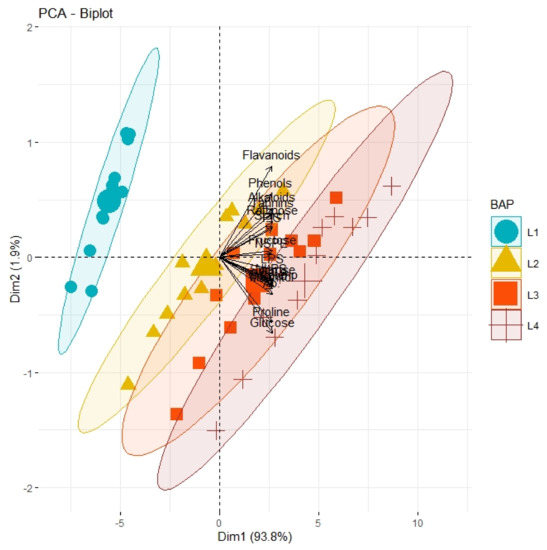
Figure 1.
PCA graph demonstrating the extent of association and divergence of physiological, biochemical, and growth-related traits of different olive genotypes from origin under varying concentrations (L1, control; L2, 0.5 mgL−1; L3, 1.5 mgL−1; L4, 2.5 mgL−1) of BAP with respect to control.
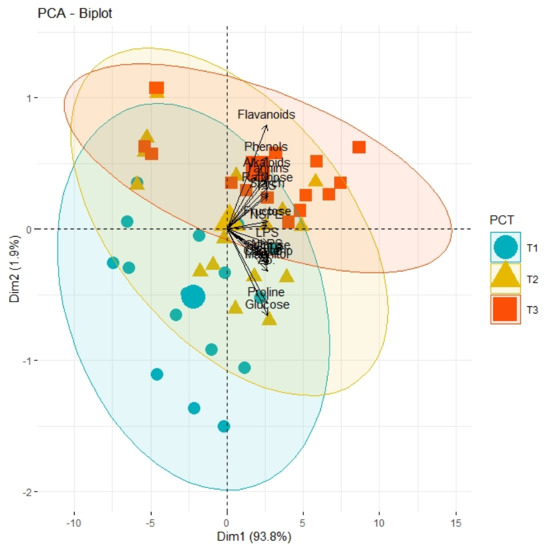
Figure 2.
PCA graph demonstrating the extent of association and divergence of physiological, biochemical, and growth-related traits of different olive genotypes from origin under varying pre-cooling treatments (T1, control; T2, 24 h; T3, 48 h).
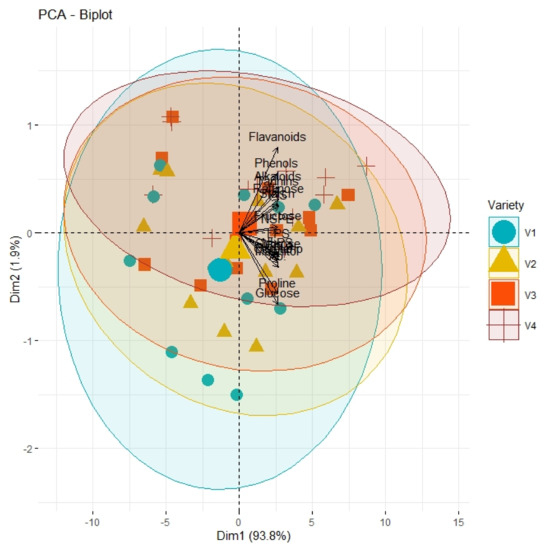
Figure 3.
PCA graph explicating the varying extent of association and divergence of physiological, biochemical, and growth-related traits from origin with respect to type of genotypes (V1, Leccino; V2, Gemlik; V3, Moraiolo; V4, Arbosana).
4. Discussion
The current study intended to elucidate the impacts of various combinations of pre-cooling treatments and BAP concentrations on morphological, physiological, and biochemical traits of different olive genotypes cultured on OM during in vitro conditions. Despite incessant efforts, the delineation of optimized protocols for shoot induction in olive always remained a case of potential concern. In this context, the current study deeply assessed the impacts of different pre-cooling and BAP treatments on different olive cultivars for setting appropriate shoot induction protocol. For BAP, the present study noticed that for the induction of shooting and proliferation activity, at least its supplementation is mandatory at a concentration of 0.5 mgL−1. Likewise, in the past, various studies proved that the addition of zeatin in OM is mandatory for inducing shooting in axillary buds of olive, however, its concentration at 2.5 mgL−1 depicted comparatively better results [1,6]. However, the supplementation of zeatin was variable in terms of effectivity for different olive cultivars [9]. Therefore, BAP supplementation in combination with pre-cooling treatments of explants was used as an alternative strategy. Apart from this pre-cooling treatment of explants such as axillary buds and nodal segments is an effective tool to increase their shoot induction percentage, as it has a tendency to break their dormancy by triggering the activity of vernalin [26]. In fact, vernalin has a tendency to reciprocate the effect of ABA whose deposition results in the synthesis of callose that blocks intercellular transport occurring through plasmodesmata [21]. Likewise, the present study recorded that integration of an extended span of pre-cooling and high concentration of BAP made significant improvements in growth traits such as LPS, SIP, NLPS, and NSPE of all olive cultivars (Table 1). These findings were inconsistent with the findings of Peixe et al. [1] Chaari-Rkhis et al. [6] and Zuccherelli and Zuccherelli [35] who reported a significant increase in LPS, SIP, NLPS, and NSPE under increased concentrations of growth regulators within OM. Furthermore, internodal segments cultured after 24 and 48 h of pre-cooling also recorded consistent increases in physiological processes and metabolic activities with increasing concentrations of BAP as evident from high accumulation pigments and primary metabolites (Table 2, Table 3, Table 5 and Table 6). Perhaps, plant growth is strongly associated with the synthesis of structural and regulatory enzymes in addition to the synthesis of enzymes participating in photosynthesis [36]. Proline has a tendency to protect the essential enzymes and to balance the subcellular machinery that facilitates the specific function of carbon and nitrogen [37]. Proline serves as an osmoprotectant in the cell; therefore, it prevents plantlets from dehydration within the micropropagation system when they sense any sort of abiotic stress [36]. With increasing BAP concentrations, proline content depicted a significant reduction in all genotypes that is because of potential role of BAP in cohering metabolic activities as reported by Hamooh and Shah [9]. Furthermore, the increase in chlorophyll is also associated with dramatic increase in the levels of various metabolites including both primary and secondary [38]. High chlorophyll content is directly connected with enhanced CO2 assimilation due to increase in quantity of photosynthetic pigments such as chlorophyll and carotenoids [39]. Perhaps due to these reasons all genotypes of olive depicted strong positive correlation between physiological and growth traits within micropropagation system due to increasing BAP concentrations (Table 7). In addition, high content of carbohydrate may increase the production of substrates involved in shikimic acid pathway that ultimately trigger the production of secondary metabolites [40]. Therefore, increased production of secondary metabolites is directly associated with the high production of carbohydrates as reported by Ghasemzadeh et al. [41]. Correspondingly, the current study recorded complete parallelism between the increment of primary and secondary metabolic contents (Table 3, Table 4 and Table 7). Moreover, the current study noticed a significant change in the levels of both primary (fructose, glucose, sucrose, and starch) and secondary (alkaloids, phenols, tannins, and flavonoids) metabolites due to optimization of BAP concentrations with pre-cooling treatments within in vitro micropropagation system (Table 3, Table 4, Table 5 and Table 6). Various protocols are optimized for different olive cultivars using different concentrations of growth regulators such as BAP and zeatin within OM by testing their impacts on growth traits. However, the current study recorded dynamic impacts of changing hormonal concentrations on physiological and metabolic activities in addition to growth traits. Hormones, being signaling entities, are potentiate enough to establish the coherence between physiological and biochemical activities for triggering the growth and differentiation process at the cellular level [42]. The current study validated these views by recording strong paired association between physiological, biochemical, and growth traits through correlation analysis (Table 7). Although all cultivars under study responded in an analogous way to the varying conditions of the micropropagation system, the extent of response was different with respect to pre-cooling treatments, BAP concentration, and cultivars (Figure 1, Figure 2 and Figure 3). This could be attributed to the different genetic makeup of all genotypes due to which they responded differently to treatments. As a whole, on olive media, during both optimizations, the performance of “Arbosana” was exceptionally different at the morphological, physiological, and biochemical levels.
5. Conclusions
Overall, those cultivars whose explants were pre-treated at 48 h cooling treatments before culturing depicted noteworthy performance at a BAP concentration of 2.5 mgL−1. The current study remained fruitful in optimizing shoot induction and proliferation protocol for olive cultivars by comprehensively evaluating the effects of all treatments at physiological, biochemical, and morphological levels. Moreover, this study proved that BAP is a good hormonal supplement to OM that can increase the effectiveness of the culture medium for olive propagation within in vitro conditions.
Author Contributions
W.L., C.-b.D., and X.L. came up with the idea and supervised the study; S.K. and Z.H.S. conducted experiments and wrote the manuscript; M.R. and Z.H.S. analyzed the data statistically; Y.Z. (Yinan Zheng), Y.Z. (Yue Zhang), F.A., and X.C. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peixe, A.; Raposo, A.; Lourenço, R.; Cardoso, H.; Macedo, E. Coconut water and BAP successfully replaced zeatin in olive (Olea europaea L.) micropropagation. Sci. Hortic. 2007, 113, 1–7. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lambardi, M.; Ozudogru, E.A.; Roncasaglia, R. In vitro propagation of olive (Olea europaea L.) by nodal segmentation of elongated shoots. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Humana Press: Totowa, NJ, USA, 2012; pp. 33–44. [Google Scholar]
- Benelli, C.; De Carlo, A. In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (Plantform™). 3 Biotech 2018, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmad, T.; Abbasi, N.A.; Hafiz, I.A. Effect of different media and growth regulators on in vitro shoot proliferation of olive cultivar ‘Moraiolo’. Pak. J. Bot. 2009, 41, 783–795. [Google Scholar]
- Chaari-Rkhis, A.; Maalej, M.; Drira, N.; Standardi, A. Micropropagation of olive tree Olea europaea L. ‘Oueslati’. Turk. J. Agric. For. 2011, 35, 403–412. [Google Scholar]
- Revilla, M.A.; Pacheco, J.; Casares, A.; Rodriguez, R. In vitro reinvigoration of mature olive trees (Olea europaea L.) through micrografting. In Vitro Cell. Dev. Biol.-Plant 1996, 32, 257. [Google Scholar] [CrossRef]
- Rugini, E. In vitro propagation of some olive (Olea europaea L.) cultivars with different root ability, and medium development using analytical data from developing shoots and embryos. Sci. Hortic. 1984, 24, 123–134. [Google Scholar] [CrossRef]
- Hamooh, B.T.; Shah, Z.H. In vitro evaluation of shoot induction and proliferation protocol for olive cultivars by assessing morpho-physiologic effects of pre-cooling and growth regulators. Int. J. Biol. 2017, 11, 126–139. [Google Scholar]
- Ahmadi-Lahijani, M.J.; Kafi, M.; Nezami, A.; Nabati, J.; Erwin, J.E. ABA and BAP improve the accumulation of carbohydrates and alter carbon allocation in potato plants at elevated CO2. Physiol. Mol. Biol. Plants 2021, 27, 313–325. [Google Scholar] [CrossRef]
- Tahiri, A.; Destain, J.; Thonart, P.; Druart, P. In vitro model to study the biological properties of humic fractions from landfill leachate and leonardite during root elongation of Alnus glutinosa L. Gaertn and Betula pendula Roth. Plant Cell Tiss. Organ Cult. 2015, 122, 739–749. [Google Scholar] [CrossRef]
- Ferrara, g.; Loffredo, E.; Simeone, R.; Senesi, N. Evaluation of antimutagenic and desmutagenic effects of humic and fulvic acids on root tips of Vicia faba. Environ. Toxicol. 2000, 15, 513–517. [Google Scholar] [CrossRef]
- Ferrara, G.; Loffredo, E.; Senesi, N.; Marcos, R. Humic acids reduce the genotoxicity of mitomycin C in human lymphoblastoid cell line TK6. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 603, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Loffredo, E.; Senesi, N. Anticlastogenic, antitoxic and sorption effects of humic substances on the mutagen maleic hydrazide tested in leguminous plants. Eur. J. Soil Sci. 2004, 55, 449–458. [Google Scholar] [CrossRef]
- Salem, J.; Hassanein, A.; El-Wakil, D.A.; Loutfy, N. Interaction between Growth Regulators Controls In Vitro Shoot Multiplication in Paulownia and Selection of NaCl-Tolerant Variants. Plants 2022, 11, 498. [Google Scholar] [CrossRef]
- Lebedev, V.; Arkaev, M.; Dremova, M.; Pozdniakov, I.; Shestibratov, K. Effects of Growth Regulators and Gelling Agents on Ex Vitro Rooting of Raspberry. Plants 2018, 8, 3. [Google Scholar] [CrossRef]
- Leva, A.; Petruccelli, R.; Rinaldi, L. Somaclonal Variation in Tissue Culture: A Case Study with Olive. In Recent Advances in Plant in vitro Culture; Leva, A., Rinaldi, L.M.R., Eds.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Antonopoulou, C.; Dimassi, K.D.; Therios, I.T.; Hatzissavvidis, C. Does dikegulac affect in vitro shoot proliferation and hyperhydricity incidence in olive explants? Hortic. Sci. 2018, 45, 125–130. [Google Scholar] [CrossRef]
- Brunner, A.M.; Evans, L.M.; Hsu, C.Y.; Sheng, X. Vernalization and the chilling requirement to exit bud dormancy: Shared or separate regulation? Front. Plant Sci. 2014, 5, 732. [Google Scholar] [CrossRef]
- Pan, W.; Liang, J.; Sui, J.; Li, J.; Liu, C.; Xin, Y.; Zhang, Y.; Wang, S.; Zhao, Y.; Zhang, J.; et al. ABA and Bud Dormancy in Perennials: Current Knowledge and Future Perspective. Genes 2021, 12, 1635. [Google Scholar] [CrossRef]
- Liu, J.; Sherif, S.M. Hormonal orchestration of bud dormancy cycle in deciduous woody perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Alexandre, C.; Hennig, L. FLC-independent vernalization responses. Int. J. Plant Dev. Biol. 2007, 1, 202–211. [Google Scholar]
- Leida, C.; Conejero, A.; Arbona, V.; Gómez-Cadenas, A.; Llácer, G.; Badenes, M.L.; Ríos, G. Chilling-dependent release of seed and bud dormancy in peach associates to common changes in gene expression. PLoS ONE 2012, 10, 35777. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Cazzonelli, C.I. Prolonged cold exposure to Arabidopsis juvenile seedlings extends vegetative growth and increases the number of shoot branches. Plant Signal. Behav. 2020, 15, 1789320. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Saxena, P.K. Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: A novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE 2013, 8, 76802. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Vemmos, S.N. Season and explant origin affect phenolic content, browning of explants, and micropropagation of× Malosorbus florentina (Zucc.) Browicz. HortScience 2013, 48, 102–107. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, P.; Xue, J.; Xue, Y.; Wang, S.; Zhang, X. Advances in phenolic substances and their effects on browning in woody plant tissue culture. Acta Hortic. Sin. 2019, 46, 1645–1654. [Google Scholar]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant Growth Promoting Rhizobacteria and Silicon Synergistically Enhance Salinity Tolerance of Mung Bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed]
- Boussadia, O.; Steppe, K.; Zgallai, H.S.; Ben El-Hadj, S.; Braham, M.; Lemeur, R.; Van-Labeke, M.C. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteate Benth). J. Taibah Univ. Med. Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef]
- Zhao, L.J.; Liu, W.; Xiong, S.H.; Tang, J.; Lou, Z.H.; Xie, M.X.; Xia, B.H.; Lin, L.M.; Liao, D.F. Determination of total flavonoids contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int. J. Anal. Chem. 2018, 2018, 8195784. [Google Scholar] [CrossRef]
- Fadda, A.; Mulas, M. Chemical changes during myrtle (Myrtus communis L.) fruit development and ripening. Sci. Hortic. 2010, 125, 477–485. [Google Scholar] [CrossRef]
- Li, L.; Long, W.; Wan, X.; Ding, Q.; Zhang, F.; Wan, D. Studies on quantitative determination of total alkaloids and berberine in five origins of crude medicine “Sankezhen”. J. Chromatogr. Sci. 2015, 53, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Zuccherelli, G.; Zuccherelli, S. In vitro propagation of fifty olive cultivars. Acta Hortic. 2002, 586, 931–934. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Ejaz, B.; Sajid, Z.A.; Aftab, F. Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of Saccharum spp., hybrid cv. HSF-under salt stress. Turk. J. Biol. 2012, 36, 630–640. [Google Scholar] [CrossRef]
- Aslam, M.; Sultana, B.; Anwar, F.; Munir, H. Foliar spray of selected plant growth regulators affected the biochemical and antioxidant attributes of spinach in a field experiment. Turk. J. Agric. For. 2016, 40, 136–145. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules 2010, 15, 7907–7922. [Google Scholar] [CrossRef]
- El Sabagh, A.; Islam, M.S.; Hossain, A.; Mubeen, M.; Iqbal, M.A.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 8, 68. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).