Classification, Distribution, Biosynthesis, and Regulation of Secondary Metabolites in Matricaria chamomilla
Abstract
:1. Introduction
2. Biological Characteristics
3. Active Constituents of M. chamomilla
4. Terpenoids
4.1. Types and Functions of Terpenoids in M. chamomilla
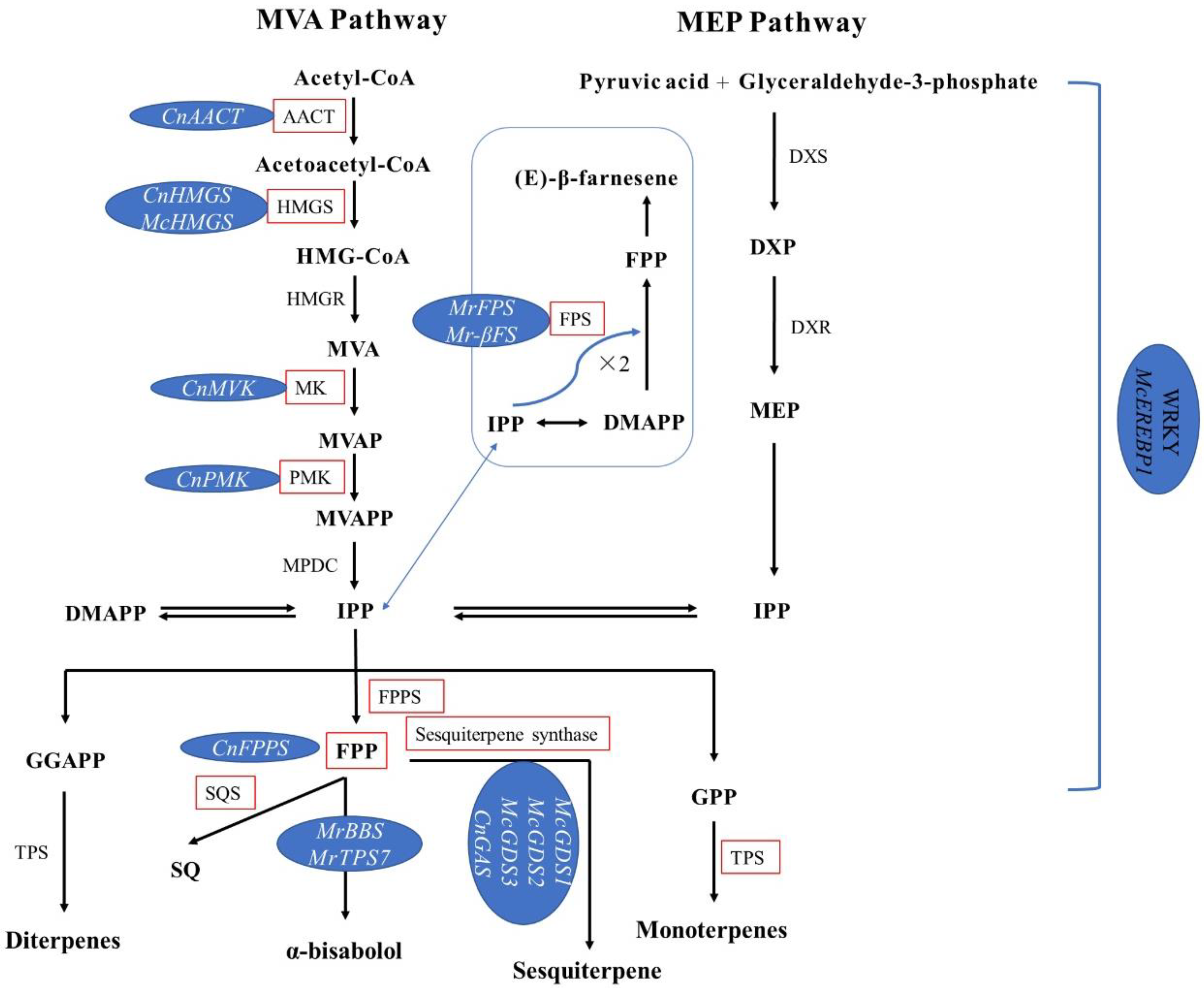
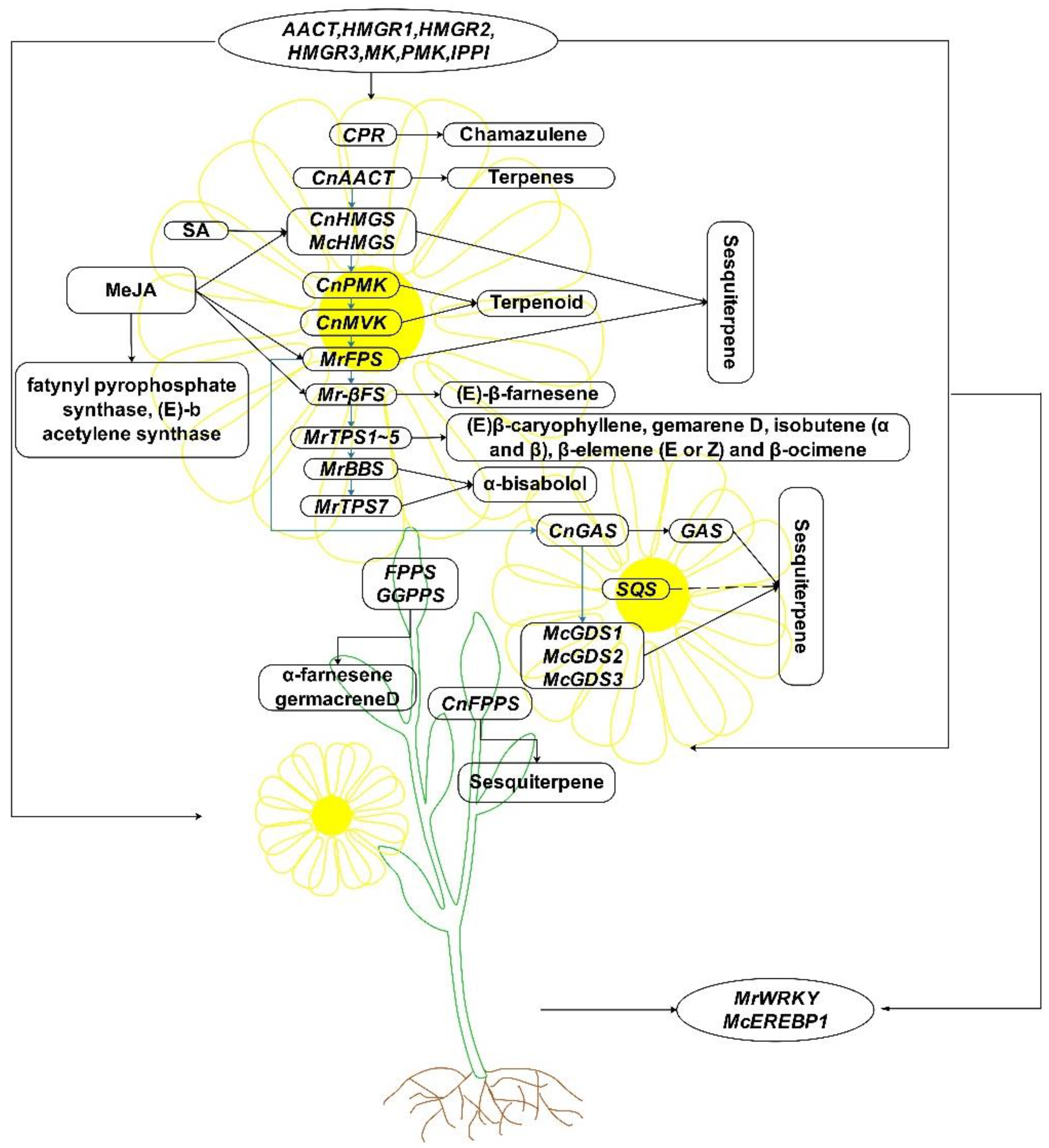
4.2. Research Progress in Terpene Biosynthesis
5. Biosynthesis of M. chamomilla Flavonoids
6. Other Research Advances
6.1. Effects of Biological and Abiotic Stress on Essential Oil Composition
6.2. Effect of Geographical Origin on Composition of M. chamomilla
6.3. Effect of Seedling Age on Chamomile Composition
6.4. Metabolic Differences in Mature Varieties from Different Regions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.M.; Meng, X.X.; Zhang, W.W.; Liao, Y.L.; Chang, J.; Xu, F. Tissue culture technology of Matricaria chamomilla L. North. Hortic. 2018, 2, 72–76. [Google Scholar]
- Han, S.L.; Li, X.X.; Mian, Q.H.; Lan, W.; Liu, Y. Comparison of antioxidant activity between two species of chamomiles produced in Xinjiang by TLC-bioautography. China J. Chin. Mater. Med. 2013, 38, 193–198. [Google Scholar]
- Wan, W.T.; Song, Y.J.; Xu, L.J.; Xiao, P.G.; Miao, J.H. An overview on modern research and application potency of Chamomile. Mod. Chin. Med. 2019, 21, 260–265. [Google Scholar]
- Bhattacharjee, S.K. Handbook of Aromatic Plants; Pointer Publishers: Jaipur, India, 2005; pp. 277–279. [Google Scholar]
- Xia, Q.X.; Bai, H.T.; Sun, L.C.; Gao, T.G.; Jiang, C.D.; Shi, L. Research progress on active composition and practical application of medicinal plants of Matricaria recutita. Acta Hortic. Sin. 2012, 39, 1859–1864. [Google Scholar]
- Shiva, M.P.; Lehri, A.; Shiva, A. Matricaria-Chamomilla/Iaromatic and Medicinal Plants; International Book Distributors: New Delhi, India, 2002; pp. 223–228. [Google Scholar]
- Xu, L. United States Pharmacopeia (24th Edition): Chamomile. World Notes Plant Med. 2002, 2, 82–83. [Google Scholar]
- Wu, Z.Y. Flora of China; Science Press: Beijing, China, 2010; pp. 49–50. [Google Scholar]
- Yang, Y.S.; Pan, L.S. Isolation and structural determination of flavones from Matricaria chamomilla L. Appl. Chem. Ind. 2008, 37, 697–698. [Google Scholar]
- Zhou, B.T.; Li, X.Z. Study of the chemical composition of chamomile. J. Hunan Coll. Tradit. Chin. Med. 2001, 21, 27–28. [Google Scholar]
- Zhao, Y.F. Chemical Constituents and Quality Standard of Matricaria chamomilla L. as Uygur Medicine. Master’s Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2018. [Google Scholar]
- Krüger, H. Characterisation of Chamomile Volatiles by Simultaneous Distillation Solid-Phase Extraction in Comparison to Hydrodistillation and Simultaneous Distillation Extraction. Planta Med. 2010, 76, 843–846. [Google Scholar] [CrossRef]
- Schilcher, H. Die Kamille: Handbuch für Ärzte, Apotheker und Andere Naturwissenschaftler; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1987; pp. 59–61. [Google Scholar]
- Kazemi, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita. Int. J. Food Prop. 2015, 18, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Wesolowska, A.; Grzeszczuk, M.; Kulpa, D. Propagation Method and Distillation Apparatus Type Affect Essential Oil from Different Parts of Matricaria recutita L. Plants. J. Essent. Oil Bear. Plants 2015, 18, 179–194. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, D.; Liang, X.X.; Yang, L.X.; Sun, P.; Ma, Y.; Wang, K.; Chang, X.Q.; Yang, L. Chemical constituents from Matricaria chamomilla L. (I). J. Chin. Pharm. Sci. 2018, 27, 324–331. [Google Scholar]
- Cordero, C.; Sgorbini, B.; Rubiolo, P.; Belliardo, F.; Liberto, E.; Bicchi, C. Headspace–solid-phase microextraction fast GC in combination with principal component analysis as a tool to classify different chemotypes of chamomile flower-heads (Matricaria recutita, L.). Phytochem. Anal. 2006, 17, 217–225. [Google Scholar]
- Xu, Y.B.; Tang, H.; Zhu, S.Y.; Zhe, W.; Wang, K.; Mao, D.S.; Fu, L.; Chen, R.R. Analysis of Volatile Components of Chamomile Oil of Different Origins by Gas Chromatography Time-of-Flight Mass Spectrometry. Sci. Technol. Food Ind. 2015, 36, 6. [Google Scholar]
- Das, M.; Ram, G.; Singh, A.; Mallavarapu, G.R.; Ramesh, S.; Ram, M.; Kumar, S. Volatile constituents of different plant parts of Chamomilla recutita L. Rausch grown in the Indo-Gangetic plains. Flavour. Fragr. J. 2002, 17, 9–12. [Google Scholar] [CrossRef]
- Povh, N.P.; Garcia, C.A.; Marques, M.O.M.; Meireles, M.A.A. Extraction of essential oil and oleoresin from chamomile (Chamomila recutita [L.] Rauschert) by steam distillation and extraction with organic solvents: A process design approach. Rev. Bras. Plantas Med. 2001, 4, 1–8. [Google Scholar]
- McKay, D.L.; Blimiberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Russell, K.; Jacob, S.E. Bisabolol. Dermatitis 2010, 21, 57–58. [Google Scholar] [CrossRef]
- Uno, M.; Kokuryo, T.; Yokoyama, Y.; Senga, T.; Nagino, M. α-Bisabolol Inhibits Invasiveness and Motility in Pancreatic Cancer Through KISS1R Activation. Anticancer Res. 2016, 36, 583–589. [Google Scholar] [PubMed]
- Yan, H.B.; Xu, R.X. Effect of α-bisabolol on migration and invasion of glioblastoma cells. Acad. J. Chin. Pla Med. Sch. 2018, 39, 699–706. [Google Scholar]
- Sharkey, T.D.; Gray, D.W.; Pell, H.K.; Breneman, S.R.; Topper, L. Isoprene synthase genes form a monophyletic clade of acyclic terpene synthases in the TPS-B terpene synthase family. Evol. Int. J. Org. Evol. 2013, 67, 1026–1040. [Google Scholar] [CrossRef]
- Schilcher, H.; Imming, P.; Goeters, S. Active Chemical Constituents of Matricaria chamomilla L. syn. Chamomilla recutita (L.) Rauschert. Chamomile Ind. Profiles 2005, 1, 56–76. [Google Scholar]
- Farhoudi, R. Chemical constituents and antioxidant properties of Matricaria Recutita and Chamaemelum nobile essential oil growing wild in the south west of Iran. J. Essent. Oil Bear. Plants 2013, 16, 531–537. [Google Scholar] [CrossRef]
- Schnee, C.; Köllner, T.G.; Held, M.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef]
- Köllner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.; Gershenzon, J.; Degenhardt, J. A Maize (E)-β-Caryophyllene Synthase Implicated in Indirect Defense Responses against Herbivores Is Not Expressed in Most American Maize Varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.T.; Cheng, C.L.; Cheng, X.W.; Huan, H.W.; Ling, S.; Lin, Y.; Wei, J.; Xiao, R.Y.; Lu, J.Z.; Zhan, F.; et al. Analysis of terpenoid biosynthesis pathways in german chamomile (Matricaria recutita) and roman chamomile (Chamaemelum nobile) based on co-expression networks. Genomics 2020, 112, 1055–1064. [Google Scholar]
- Wright, G.A.; Schiestl, F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 2009, 23, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.W.; Tao, T.T.; Liu, X.M.; Xu, F.; Chang, J.; Liao, Y.L. De novo assembly and comparative transcriptome analysis: Novel insights into sesquiterpenoid biosynthesis in Matricaria chamomilla L. Acta Physiol. Plant. 2018, 40, 129. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.D.; Chen, Y.C.; Lin, L.Y.; Wu, Q.K.; Christian, S. Transcriptome Sequencing and Expression Analysis of Terpenoid Biosynthesis Genes in Litsea cubeba. PLoS ONE 2013, 8, e76890. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.J.; Chen, X.; Zhang, M.; Ping, S.; Liu, Y.J.; Tong, Y.R.; Wang, X.J.; Huang, L.Q.; Gao, W. Molecular cloning and characterisation of farnesyl pyrophosphate synthase from Tripterygium wilfordii. PLoS ONE 2015, 10, e0125415. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Acetylenes: Cytochrome P450 oxidation and mechanism-based enzyme inactivation. Drug Metab. Rev. 2019, 51, 162–177. [Google Scholar] [CrossRef]
- Ling, S.P.; Zhang, H.M.; Su, S.S.; Zhang, X.S.; Liu, X.Y.; Pan, G.F.; Yuan, Y. Molecular Cloning and characterization of a Cytochrome P450 reductase gene (CPR) full-length in Matricaria recutita. J. Agric. Biotechnol. 2014, 22, 580–589. [Google Scholar]
- Fu, M.; Liu, X.; Meng, X.; Wang, L.; Tan, J.; Zhou, X.; Xu, F. Cloning and Sequence Analysis of an Acetyl-CoA C-Acetyltransferase Gene (AACT) from Chamaemelum nobile. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Meng, X.; Xu, F.; Chang, J. Molecular cloning and sequence analysis of a phosphomevalonate kinase gene (CnPMK) from Chamaemelum nobile. Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.X.; Zhang, W.; Xu, F.; Yan, J.; Liu, X.; Liao, Y.L.; Chang, J. Cloning and sequence analysis of mevalonate kinase gene (CnMVK) from Chamaemelum nobile. Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3, 23–28. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, X.H.; Xu, F.; Chen, Q.W.; Tao, T.T.; Lei, J.; Zhang, W.W.; Liao, Y.L.; Chang, J.; Li, X.X.; et al. Cloning, Expression Profiling and Functional Analysis of CnHMGS, a Gene Encoding 3-hydroxy-3-Methylglutaryl Coenzyme A Synthase from Chamaemelum nobile. Molecules 2016, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Su, S.S.; Liu, X.Y.; Pan, G.F.; Hou, X.J.; Zhang, H.M.; Yuan, Y. In vitro characterization of a (E)-β-farnesene synthase from Matricaria recutita L. and its up-regulation by methyl jasmonate. Gene 2015, 571, 58–64. [Google Scholar] [CrossRef]
- Tao, T.T.; Chen, Q.W.; Meng, X.X.; Yan, J.P.; Xu, F.; Chang, J. Molecular cloning, characterization, and functional analysis of a gene encoding 3-hydroxy-3-methylglutaryl-coenzyme A synthase from Matricaria chamomilla. Genes Genom. 2016, 38, 1179–1187. [Google Scholar] [CrossRef]
- Su, S.S.; Zhang, H.M.; Liu, X.Y.; Pan, G.F.; Ling, S.P.; Zhang, X.S.; Yang, X.M.; Tai, Y.L.; Yuan, Y. Cloning and characterization of a farnesyl pyrophosphate synthase from Matricaria recutita L. and its upregulation by methyl jasmonate. Genet. Mol. Res. 2015, 14, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.X.; Yan, J.P.; Liao, Y.L.; Chang, J.; Xu, F. Cloning and expression analysis of HMGR gene from Chamaemelum nobile. Acta Agric. Boreal. Sin. 2016, 31, 68–75. [Google Scholar]
- Sun, J.M. Omics-Based Study on Genes Related to Volatile Terpenoids from Matricaria chamomilla L. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2018. [Google Scholar]
- Tai, Y.L. Molecular Cloning, Expression of FPS and Analysis of Salt Stress in Matrucarua chamomilla L. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2012. [Google Scholar]
- Yang, X.M. Study on Rapid Propagation of Matricaria chamomile L and Genetic Transformation of FPS Gene in Tobacco. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2013. [Google Scholar]
- Gupta, P.; Akhtar, N.; Tewari, S.K.; Sangwan, R.S.; Trivedi, P.K. Differential expression of farnesyl diphosphate synthase gene from Withania somnifera in different chemotypes and in response to elicitors. Plant Growth Regul. 2011, 65, 93–100. [Google Scholar] [CrossRef]
- Lan, J.B.; Yu, R.C.; Yu, Y.Y.; Fan, Y.P. Molecular cloning and expression of Hedychium coronarium farnesyl pyrophosphate synthase gene and its possible involvement in the biosynthesis of floral and wounding/herbivory induced leaf volatile sesquiterpenoids. Gene 2013, 518, 360–367. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, K.; Chen, L.Q. Molecular cloning and expression of Chimonanthus praecox farnesyl pyrophosphate synthase gene and its possible involvement in the biosynthesis of floral volatile sesquiterpenoids. Plant Physiol. Biochem. 2010, 48, 845–850. [Google Scholar] [CrossRef]
- Su, S.S. Cloning and Characterization of (E)-β-Farnesene Synthase from Matricaria recutita L. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2015. [Google Scholar]
- Son, Y.J.; Kwon, M.; Ro, D.K.; Kim, S.U. Enantioselective microbial synthesis of the indigenous natural product (−)-α-bisabolol by a sesquiterpene synthase from chamomile (Matricaria recutita). Biochem. J. 2014, 463, 239–248. [Google Scholar] [CrossRef]
- Irmisch, S.; Krause, S.T.; Kunert, G.; Gershenzon, J.; Degenhardt, J.; Köllner, T.G. The organ-specific expression of terpene synthase genes contributes to the terpene hydrocarbon composition of chamomile essential oils. BMC Plant Biol. 2012, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.X. Cloning and Characterization of α-Bisabolol Synthase Gene (MrBBS) from Matricaria chamomilla L. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2018. [Google Scholar]
- Mondal, P. Biosynthesis and Regulation of Terpene Production in Accessions of Chamomile (Matricaria recutita L.). Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2020. [Google Scholar]
- Ling, C.C.; Zheng, L.J.; Yu, X.R.; Wang, H.H.; Wang, C.X.; Wu, H.Y.; Zhang, J.; Yao, P.; Tai, Y.L.; Yuan, Y. Cloning and functional analysis of three aphid alarm pheromone genes from German chamomile (Matricaria chamomilla L.). Plant Sci. 2020, 294, 110463. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.P.; Meng, X.X.; Zhu, L.; Zhang, W.W.; Chang, J.; Xu, F. Molecular cloning and expression analysis of germacrene A synthase gene in Chamaemelum nobile. Chin. Tradit. Herb. Drugs 2017, 48, 1851–1859. [Google Scholar]
- Ling, S.P. Molecular Cloning and Characterization of Squalene Synthase Gene and Analysis of Essential Oil from Different Organs of Matricaria recutita. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2014. [Google Scholar]
- Liu, X.M.; Tao, T.T.; Meng, X.X.; Zhang, W.W.; Chang, J.; Xu, F. Cloning and expression analysis of a farnesyl diphosphate synthase (FPPS) gene from Chamaemelum nobile. Not. Bot. Horti Agrobot. 2017, 45, 358–364. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.Q.; Wu, G.Q. Role of WRKY Transcription Factor in Plant Response to Stresses. Biotechnol. Bull. 2021, 37, 203–215. [Google Scholar]
- Xiang, L.; Zhang, W.W.; Qu, J.W.; Han, H.; Xu, F.; Liao, Y.L. Identification and Expression Analysis of the WRKY Transcription Factor in Matricaria recutita L. Mol. Plant Breed. 2020, 18, 2127–2137. [Google Scholar]
- Izumi, S.; Takashima, O.; Hirata, T. Geraniol Is a Potent Inducer of Apoptosis-like Cell Death in the Cultured Shoot Primordia of Matricaria chamomilla. Biochem. Biophys. Res. Commun. 1999, 259, 519–522. [Google Scholar] [CrossRef]
- Ashida, Y.; Nishimoto, M.; Matsushima, A.; Watanabe, J.; Hirata, T. Molecular Cloning and mRNA Expression of Geraniol-inducible Genes in Cultured Shoot Primordia of Matricaria chamomilla. Biosci. Biotechnol. Biochem. 2002, 66, 2511–2514. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y. The main composition and affecting factors of aroma volatiles in flowers. North. Horticult. 2012, 6, 184–189. [Google Scholar]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; Del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Sagi, S.; Avula, B.; Wang, Y.H.; Zhao, J.; Khan, I.A. Quantitative determination of seven chemical constituents and chemo-type differentiation of chamomiles using high-performance thin-layer chromatography. J. Sep. Sci. 2014, 37, 2797–2804. [Google Scholar] [CrossRef]
- Mobasheri, L.; Khorashadizadeh, M.; Safarpour, H.; Mohammadi, M.; Anani Sarab, G.; Askari, V.R. Anti-inflammatory activity of ferula assafoetida Oleo-Gum-Resin (Asafoetida) against TNF-α-stimulated Human Umbilical Vein Endothelial Cells (HUVECs). Media Inflamm. 2022, 31, 5171525. [Google Scholar] [CrossRef]
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Schreier, P.; Baraldi, M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000, 59, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Švehlíková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert). Phytochemistry 2004, 65, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Babenko, N.A.; Shakhova, E.G. Effects of Chamomilla recutita flavonoids on age-related liver sphingolipid turnover in rats. Exp. Gerontol. 2006, 41, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Herrmann, M.; Hartmann, B.; Joppe, H.; Schmidt, C.O.; Bertram, H.J. HPLC/MS and HPLC/NMR as hyphenated techniques for accelerated characterization of the main constituents in Chamomile (Chamomilla recutita [L.] Rauschert). Eur. Food Res. Technol. 2008, 226, 755–760. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Song, Q.; Tian, Y.; Liu, X.; Wang, L.; Mao, D.; Zhang, W.; Xu, F. Characterization and expression analysis of four members genes of flavanone 3-hydroxylase families from chamaemelum nobile. Not. Bot. Horti Agrobot. 2020, 48, 102–115. [Google Scholar] [CrossRef]
- Badi, H.N.; Yazdani, D.; Ali, S.M.; Nazari, F. Effects of spacing and harvesting time on herbage yield and quality/quantity of oil in thyme, Thymus vulgaris L. Ind. Crops Prod. 2004, 19, 231–236. [Google Scholar] [CrossRef]
- Banchio, E.; Zygadlo, J.; Valladares, G.R. Quantitative variations in the essential oil of Minthostachys mollis (kunth.) griseb. in response to insects with different feeding habits. J. Agric. Food Chem. 2005, 53, 6903–6906. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Babaeian, J.N.; Modarresi, M.; Bagheri, N.; Jamali, A. Increase of Chamazulene and α-Bisabolol Contents of the Essential Oil of German chamomile (Matricaria chamomilla L.) Using Salicylic Acid Treatments under Normal and Heat Stress Conditions. Foods 2016, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhu, L.; Song, Q.; Chang, J.; Ye, J.; Zhang, W.; Liao, Y.; Xu, F. Effects of 5-aminolevulinic Acid on the Photosynthesis, Antioxidant System, and α-Bisabolol Content of Matricaria recutita. Not. Bot. Horti Agrobot. 2018, 46, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Matt, P.; Krapp, A.; Haake, V.; Mock, H.P.; Stitt, M. Decreased Rubisco activity leads to dramatic changes of nitrate metabolism, amino acid metabolism and the levels of phenylpropanoids and nicotine in tobacco antisense RBCS transformants. Plant J. 2002, 30, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Repčák, M.; Imrich, J.; Franeková, M. Umbelliferone, a stress metabolite of Chamomilla recutita (L.) Rauschert. J. Plant Physiol. 2001, 158, 1085–1087. [Google Scholar] [CrossRef]
- Maksymiec, W. Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 2007, 29, 177–187. [Google Scholar] [CrossRef]
- Kováčik, J.; Tomko, J.; Bačkor, M.; Repčák, M. Matricaria chamomilla is not a hyperaccumulator, but tolerant to cadmium stress. Plant Growth Regul. 2006, 50, 239–247. [Google Scholar] [CrossRef]
- Prokop’ev, I.A.; Filippova, G.V.; Shein, A.A.; Gabyshev, D.V. Impact of urban anthropogenic pollution on seed production, morphological and biochemical characteristics of chamomile, Matricaria chamomila L. Russ. J. Ecol. 2014, 45, 18–23. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef]
- Raal, A.; Kaur, H.; Orav, A.; Arak, E.; Kailas, T.; Muurisepp, M. Content and composition of essential oils in some Asteraceae species. Proc. Natl. Acad. Sci. USA 2011, 60, 55–63. [Google Scholar] [CrossRef]
- Orav, A.; Kailas, T.; Ivask, K. Volatile constituents of Matricaria recutita L. from Estonia. Est. A Sci. Chem. 2001, 50, 39–45. [Google Scholar]
- Mohammad, R.; Hamid, S.; An, A.; Norbert, D.K.; Patrick, V.D. Effects of planting date and seedling age on agro-morphological characteristics, essential oil content and composition of German chamomile (Matricaria chamomilla L.) grown in Belgium. Ind. Crops Prod. 2010, 31, 145–152. [Google Scholar] [CrossRef]
- Mertens, D.; Boege, K.; Kessler, A.; Koricheva, J.; Thaler, J.S.; Whiteman, N.K.; Poelman, E.H. Predictability of biotic stress structures plant defence evolution. Trends Ecol. Evol. 2021, 36, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite profiling and antioxidative activity of Sage (Salvia fruticosa Mill.) under the influence of genotype and harvesting period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Yang, K.; Dong, L.; Ye, J.; Xu, F. Classification, Distribution, Biosynthesis, and Regulation of Secondary Metabolites in Matricaria chamomilla. Horticulturae 2022, 8, 1135. https://doi.org/10.3390/horticulturae8121135
Wu H, Yang K, Dong L, Ye J, Xu F. Classification, Distribution, Biosynthesis, and Regulation of Secondary Metabolites in Matricaria chamomilla. Horticulturae. 2022; 8(12):1135. https://doi.org/10.3390/horticulturae8121135
Chicago/Turabian StyleWu, Hanbin, Ke Yang, Liwei Dong, Jiabao Ye, and Feng Xu. 2022. "Classification, Distribution, Biosynthesis, and Regulation of Secondary Metabolites in Matricaria chamomilla" Horticulturae 8, no. 12: 1135. https://doi.org/10.3390/horticulturae8121135




